2021 Volume 46 Issue 1 Pages 23-28
2021 Volume 46 Issue 1 Pages 23-28
Ecdysteroids are a class of steroid hormones in arthropods that control molting and metamorphosis through interaction with intracellular nuclear receptors. In contrast to the extensive literature describing their biosynthetic pathways and signaling components, little has been known about how these hormones are traveling into and out of the cells through lipid bilayers of the cell membranes. Recently, a series of studies conducted in the fruit fly Drosophila melanogaster revealed that membrane transporters have critical functions in trafficking ecdysteroids across cell membranes, challenging the classical simple diffusion model of steroid hormone transport. Here we summarize recent advances in our understanding of membrane transporters involved in ecdysteroid signaling in Drosophila, with particular focus on Ecdysone Importer (EcI) that is involved in ecdysteroid uptake in peripheral tissues. We then discuss the potential advantage of EcI blockers as a novel pest management tool as compared to classical insect growth regulators.
As insects and other arthropods have a semi-rigid exoskeleton, they need to shed their old cuticle through a process called molting during growth. It has been long known that insect molting and metamorphosis are under endocrine control,1,2) and the first “molting hormone” termed ecdysone was isolated in 1954.3) Ecdysteroids, ecdysone and related compounds with molting hormone activities, are a group of steroid hormones that play a central role in the regulation of various developmental and physiological processes in arthropods including insects.4–6) Because biosynthetic processes and signaling pathways of ecdysteroids have multiple levels of similarities with those of mammalian steroid hormones, studies of insect ecdysteroids have significantly contributed to the advancement of the basic steroid hormone research, as exemplified by the first description of the genomic action of steroid hormones in dipteran flies.7) More recently, application of the molecular genetic tools in the fruit fly Drosophila melanogaster has particularly contributed to the accumulation of knowledge about physiological processes that are both upstream and downstream of ecdysteroid signaling.8–10) Moreover, besides basic biological studies, applied aspects of ecdysteroid signaling have also been extensively investigated in various insect species, in order to develop novel insect growth regulators (IGRs).11,12) For example, diacylhydrazine (DAH)-based non-steroidal ecdysone agonists that target the ecdysone receptor (EcR) have been developed and used as IGRs for over 30 years.13–15)
As steroid hormones are small lipophilic molecules, it has been widely accepted that they can simply diffuse across lipid bilayers of the plasma membranes. However, recent studies using Drosophila have challenged this simple diffusion model for ecdysteroids. Here we review recent findings of membrane transporter-mediated ecdysteroid trafficking across cell membranes and discuss the possibility of developing a novel class of IGRs that manipulate ecdysteroid transport for pest control.
During insect development, ecdysteroids are mainly synthesized from dietary sterols such as cholesterol or phytosterols in a specialized steroidal gland called the prothoracic gland (PG).9,16) After transport of dietary sterols into the PG, biosynthesis of ecdysone (an immediate precursor of a biologically active ecdysteroid) is achieved through hydroxylation and oxidation steps mediated by a series of enzymes that are highly substrate-selective and predominantly expressed in the PG.16) After secreted into the hemolymph, ecdysone is transported through the circulation to the peripheral tissues such as the gut and fat body, where it is converted to 20-hydroxyecdysone (20E; a biologically active ecdysteroid) by a cytochrome P450 monooxygenase named Shade.17) 20E is released back into the hemolymph and re-enters its target cells, where it binds to the intracellular nuclear receptor complex composed of EcR and Ultraspiracle (USP).14,18,19) The EcR/USP complex functions as a ligand-dependent transcription factor, which initiates the gene expression cascade that eventually leads to molting and metamorphosis.2,18,19)
In contrast to the extensive literature on ecdysteroid biosynthesis, little has been known about the mechanisms that regulate ecdysteroid secretion from the PG. This is due to the widely accepted notion that all steroid hormones are able to pass directly through lipid membranes because they are small lipophilic compounds. This is what is written in major biology textbooks today, and we generally believe that all steroid hormones can enter and exit cells freely across lipid bilayers by simple diffusion. Despite this widespread assumption, however, there are only a few reports where such simple diffusion of steroid hormones across cell membranes has been analyzed either in vitro or in silico.20,21) The available evidence is, therefore, far too limited to be generalized to all steroid hormones.
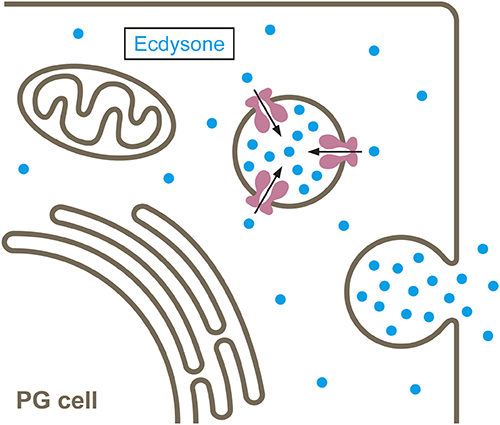
In 2015, it was reported that in Drosophila, ecdysone is secreted into the hemolymph from the PG through a vesicle-mediated process, rather than simple diffusion.22) Once synthesized in the PG, ecdysone is incorporated into secretory vesicles at high concentrations by an ATP-binding cassette (ABC) transporter called Atet and released into the hemolymph through exocytosis (Fig. 1).22) Although this ecdysone transport happens against the concentration gradient through an ATP-dependent active transporter, the fact that ecdysone can be contained within vesicles suggested that ecdysteroids may not be able to freely traverse lipid bilayers. This opened up an intriguing possibility that the cellular uptake of ecdysteroids by peripheral tissues through lipid bilayers down the concentration gradient (termed facilitated diffusion) may also be controlled by a transporter-mediated process.
In 2018, two independent in vivo and in vitro genetic screens identified a membrane transporter that mediates cellular uptake of ecdysteroids in Drosophila.23) The first screen conducted transgenic RNAi of >500 putative transporter-encoding genes to test for their requirement in ecdysteroid-dependent developmental events. The second screen utilized a recently developed pooled CRISPR screening approach in Drosophila S2 cells, which enables to generate cell pools of CRISPR mutants.24) Using this method, >25,000 sgRNAs targeting approximately 4,000 genes were screened for resistance in 20E-dependent cell cycle arrest. These two independent screens identified the same transporter-encoding gene, Oatp74D (CG7571), which was renamed as Ecdysone Importer (EcI).23)
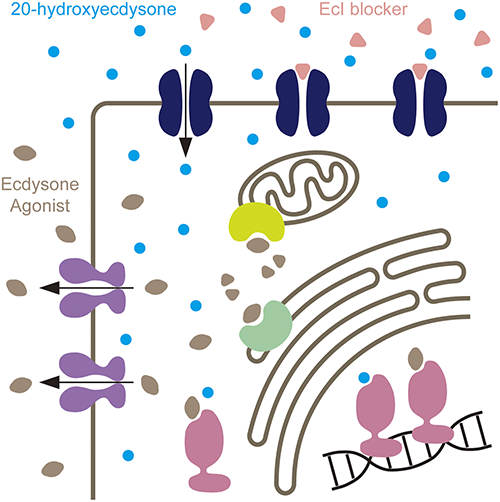
EcI is required for 20E-dependent events in the salivary gland and fat body, and the EcI protein is localized on the plasma membrane in these tissues. Importantly, fat body-specific knockdown of EcI significantly reduced ecdysteroid levels within the fat body cells, indicating that EcI is essential for importing ecdysteroids into the cells. EcI null mutants developed normally as first instar larvae, but they stopped growing at the end of the first instar and failed to molt into the second instar. This indicates that EcI is required for the canonical function of 20E as the “molting hormone” in insects. It has been well known that the developmental arrest phenotype caused by defects in ecdysone production or secretion can be rescued by oral administration of 20E.25,26) However, feeding 20E to the EcI mutants did not rescue their first instar arrest phenotype, further suggesting that EcI is required for 20E functions in vivo. In contrast, feeding a DAH-based non-steroidal ecdysone agonist chromafenozide (CF) to the EcI mutant larvae rescued the developmental arrest phenotype. Non-steroidal ecdysone agonists are a class of insecticides that mimic the action of 20E by binding directly to EcR, despite their structural differences with 20E.14,15) It is therefore likely that CF enters cells through a molecular machinery distinct from EcI (Fig. 2). Overall, these results indicate that EcI is a transporter that mediates ecdysteroid uptake in insects.
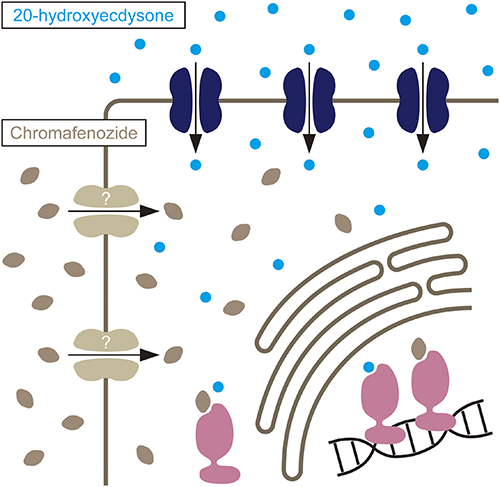
The function of EcI was further analyzed using Drosophila S2 cells and mammalian HEK293 cells transfected with EcR and a luciferase reporter construct with ecdysone response element (EcRE). Knockdown of EcI in S2 cells significantly reduced the response to 20E. Conversely, when EcI was overexpressed in S2 cells, their response to 20E was significantly increased. Importantly, neither EcI RNAi nor overexpression affected the cells’ response to CF, further suggesting that CF enters cells independently of EcI. Although the activity was significantly lower than that of 20E, the effect of ecdysone was also affected by EcI RNAi and overexpression, suggesting that both 20E and ecdysone are substrates for EcI. Indeed, overexpression of EcI with Shade, the gene encoding a P450 enzyme that converts ecdysone to 20E, significantly increased the efficiency of the ecdysone-to-20E conversion in S2 cells (unpublished result), suggesting that ecdysone likely enters peripheral tissues through EcI. Most importantly, heterologous expression of EcI and EcR in mammalian HEK293 cells makes them responsive to 20E, while expression of EcR alone does not. Collectively, these results demonstrate that EcI is both necessary and sufficient for inducing ecdysteroid-dependent gene expression in EcR-expressing animal cells. Ecdysteroid-inducible gene expression systems, where EcRE-driven gene expression is induced in a ligand-dependent manner, have been developed in EcR-expressing mammalian cells.27,28) Interestingly, 20E is known to be incapable of inducing EcRE-driven gene expression in mammalian cells, but the reason why the endogenous ligand cannot activate EcR in mammalian cells has been elusive.29) However, it is now clear that it is because mammalian cells do not express EcI. Considering that some other ecdysteroids have been used as potent ligands for the same gene expression systems,28) it is likely that mammalian cells also possess high selectivity for the incorporation of different steroidal compounds. Altogether, this study clearly challenges the simple diffusion model of steroid hormone transport across cell membranes and instead indicates that a transporter-mediated, facilitated diffusion mechanism is required for ecdysteroid entry into target cells.23)
In conjunction with extensive reorganization of the body structure during metamorphosis, insects need to transform the central nervous system (CNS) to adapt to adult-specific behaviors such as mating and flight.30,31) 20E serves as a master regulator that evokes such transformation of the CNS including remodeling, differentiation, and programmed cell death in the CNS during metamorphosis.32–35) Like mammals, the insect CNS is also physically separated from circulation by the blood-brain barrier (BBB), which acts as a highly selective gatekeeper to control the incorporation of molecules including hormones, to ensure normal neuronal functions.36–38) Thus, in order to act on its target neurons, circulating 20E needs to enter the CNS by crossing the BBB. In Drosophila, the BBB consists of surface glial cell layers that cover the entire CNS.39–41) Again, the conventional simple diffusion model does not indicate that such cell layers have a critical function in regulating steroid hormone entry into the CNS, whereas our transporter-mediated facilitated diffusion model gives those cell layers a critical gatekeeper role.
Importantly, immunohistochemical and gene expression analyses revealed that EcI is highly expressed in the BBB.42,43) Indeed, EcI knockdown in the BBB severely impaired the CNS transformation, and the CNS retained its larval morphology even in the pupal stage. Furthermore, BBB-specific knockdown of EcI suppressed 20E signaling within the entire CNS and blocked 20E-mediated neuronal events such as remodeling, differentiation, and programmed cell death. Interestingly, BBB-specific knockdown of EcI caused a defect in wandering behavior, and the larvae mostly pupated on or in the food. Wandering behavior is an innate behavior induced by 20E through which larvae exit their food and prepare for pupation.44–46) Furthermore, the majority of EcI RNAi prepupae failed to perform head eversion during pupal ecdysis, another innate behavior known to be induced by 20E.47) These results, therefore, suggest that EcI in the BBB is required for circulating 20E to access neurons within the CNS that are responsible for 20E-mediated innate behaviors. The EcI function in the BBB was further investigated by using an ex vivo CNS culture system, where BBB-specific knockdown of EcI significantly reduced the response of the CNS to 20E in the culture media. This study thus demonstrates that EcI is required for ecdysteroid uptake into the CNS and provides important implications in controlling brain function by regulating ecdysteroid permeability across the BBB.43)
As ecdysteroids play a pivotal role in regulating development and physiology in insects and other arthropods, components of the ecdysteroid signaling pathways have been selected as targets for the development of IGRs.11,12) In particular, DAH-based non-steroidal ecdysone agonists targeting EcR have been used as effective IGRs for various insect species. Currently, five DAH-based IGRs are widely used as commercial insecticides, namely CF, tebufenozide, methoxyfenozide, fufenozide, and halofenozide.14,15)
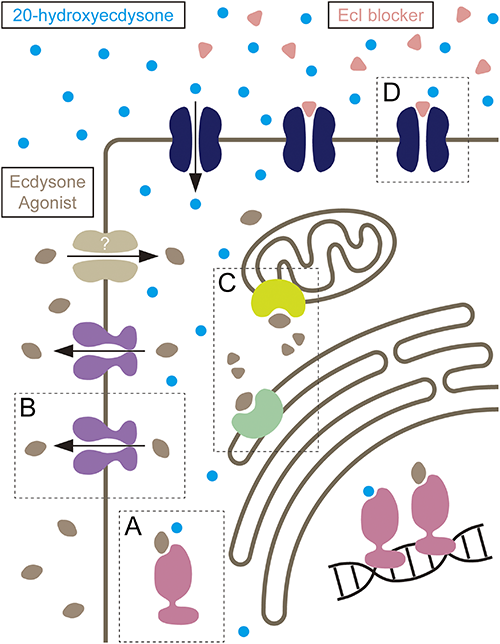
When these effective IGRs that disrupt ecdysteroid signaling are already available, what is the advantage of developing new IGRs targeting novel players in ecdysteroid signaling, such as EcI? EcI is a member of the solute carrier organic anion transporter gene superfamily that encodes an organic anion transporting polypeptide (OATP) with 12 predicted transmembrane domains.23) OATPs are widely present in the animal kingdom, and multiple chemicals with OATP-blocking activities have already been reported.48,49) Importantly, our molecular phylogenetic analysis revealed that a clear ortholog of EcI is present only in arthropods that utilize ecdysteroids as the primary molting hormone.23) It is, therefore, possible that arthropod-selective ecdysone antagonists can be developed by screening for potential EcI blockers. As compared to ecdysone agonists such as DAH-based IGRs, ecdysone antagonists have not been widely used as pest control reagents, and EcI blockers may broaden the target pest species that can be treated by IGRs.
Recently, resistance to DAH-based ecdysone agonists has been reported in field populations of several insect species.15) This is partly because these IGRs need to enter the cells to interact with their target proteins, and therefore they become easy targets for intracellular detoxification enzymes such as cytochrome P450 monooxygenases, as well as multidrug ABC transporters that eliminate various pesticides out of the cells.50–53) In this regard, EcI may become an ideal target for developing novel types of IGRs that are less likely to develop such insecticide resistance, as EcI is localized on the cell surface and its potential blockers can access their target protein directly from the hemolymph without entering the cytoplasm (Fig. 3). For the same reason, ecdysteroid efflux transporters in peripheral tissues, such as E23,54) may also be an attractive target for developing similar types of IGRs.
Recent findings of ecdysteroid transporters in fruit flies critically challenged the simple diffusion model of ecdysteroid trafficking across cell membranes. Evidence is now accumulating in other systems that supports the facilitated diffusion mechanism of ecdysteroid transport, and the effort to identify effective EcI blockers is currently underway. As evidenced by the effective use of CF in the EcI research, identification of novel IGRs can not only provide novel methods of pest control, but also promote basic scientific studies. Considering that EcI is highly conserved in arthropods, EcI-blocking ecdysone antagonists are expected to promote our understanding of steroid hormone signaling in a diverse array of insects and beyond.