2022 年 47 巻 3 号 p. 101-110
2022 年 47 巻 3 号 p. 101-110
Parasitic plants in the Orobanchaceae family include devastating weed species, such as Striga, Orobanche, and Phelipanche, which parasitize major crops, drastically reduces crop yields and cause economic losses of over a billion US dollars worldwide. Advances in basic research on molecular and cellular processes responsible for parasitic relationships has now achieved steady progress through advances in genome analysis, biochemical analysis and structural biology. On the basis of these advances it is now possible to develop chemicals that control parasitism and reduce agricultural damage. In this review we summarized the recent development of chemicals that can control each step of parasitism from strigolactone biosynthesis in host plants to haustorium formation.
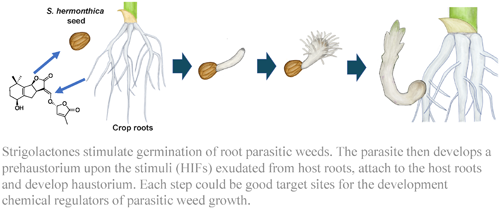
The various events of parasitism relevant to this review are summarized in Fig. 1. Strigolactones (SLs) produced by and released from host plants induces ethylene production in Striga seeds, stimulating germination. After germination, a haustorium forms upon the perception of haustorium induction factors (HIFs) in host plants.
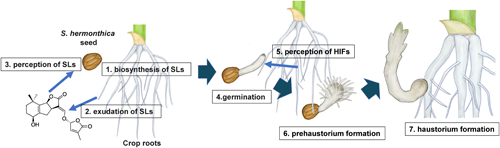
Parasitic plants in the Orobanchaceae family include devastating weed species, such as Striga, Orobanche, and Phelipanche, which parasitize major crops, drastically reducing crop yields and causing economic losses of over a billion US dollars worldwide. To solve this problem, intensive research efforts have given rise to a range of biological and/or chemical methods, including the breeding of weed-resistant crops, the utilization of cover crops, the use of selective herbicides, and the development of suicidal germination inducers. However, a definitive solution has not yet been found. Research on the molecular and cellular processes responsible for such parasitic relationships has now achieved steady progress thanks to recent advances in genome analysis, biochemical analysis, and structural biology. As a result, it is now possible to develop chemical control agents given the knowledge of the corresponding parasitic mechanisms. This review briefly introduces the current state-of-the-art in parasitic mechanisms, outlines the development of chemical control methods currently being attempted, including inhibitors of SL biosynthesis, suicidal germination inducers, Striga receptor inhibitors and inhibitors of haustorium formation, and discusses the advantages and disadvantages of these methods.
SLs act as gemination stimulants of root-parasitic weeds. Therefore, reducing the exudation of SLs from roots can lead to a reduction in the germination of root-parasitic weeds. As the amount of SLs released from the roots of SL biosynthesis mutants is lower than that of the wild type, SL biosynthesis mutants can reduce the damage caused by root parasitic weeds. However, a decrease in crop yield has been reported due to decreases in the endogenous level of SLs.1) This induces excessive branching, which leads to a decrease in crop yield. SL biosynthesis inhibitors also decrease the amount of SLs from the roots of treated plants and induce morphological changes in plants; therefore, the practical application of SL biosynthesis inhibitors involves difficulties similar to those observed in SL-deficient mutants. However, in the case of SL biosynthesis inhibitors, it is possible to control the levels of SLs in plants by adjusting the amount of inhibitor applied, without inducing significant morphological changes. This could be an advantage of SL biosynthesis inhibitors over mutants. The structures of the SL biosynthesis inhibitors are shown in Fig. 2.
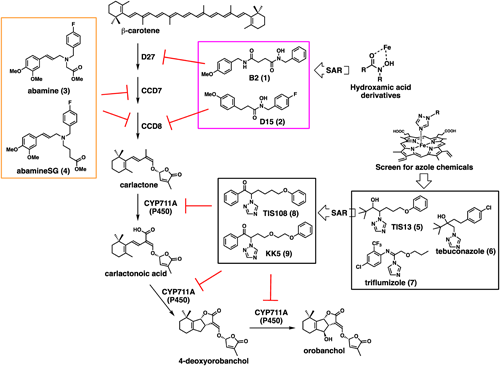
Several iron-containing enzymes, such as carotenoid isomerase, carotenoid cleavage dioxygenases (CCD), and cytochrome P450s (P450), are involved in SL biosynthesis. Sergeant et al. focused on hydroxamic acid, which can chelate iron ions, and reported that hydroxamic acid derivatives induced a more branching like SL-deficient mutant in Arabidopsis.2) Harrison et al. performed a structure–activity relationship (SAR) study of hydroxamic acid derivatives and found that the hydroxamic acids B2 and D15 exhibited selective inhibition against D27 of carotenoid isomerase and CCD8, respectively.3) Abamine4) and abamine SG5) are abscisic acid (ABA) biosynthesis inhibitors targeting NCED, a family of CCD, in the ABA biosynthesis pathway. Kitahata et al. reported that abamine reduces the levels of SL in several plant species, as well as reducing the germination rate of Orobanche minor seeds grown with tobacco.6) Based on the structure of abamine, several chemicals could be designed to inhibit CCDs specifically during SL synthesis.
The SL biosynthesis pathway includes at least one cytochrome P450 enzyme. Because an electron-deficient nitrogen-heterocycle can inhibit enzymatic activity by coordinating with a heme iron in the active center of cytochrome P450s, several SL biosynthesis inhibitors have been identified by screening for chemicals with nitrogen-heterocycles. For instance, azole chemicals, such as tebuconazole,7) TIS13,8) and triflumizole,9) reduce 4-deoxyorobanchol (4DO) production in rice. However, these chemicals induce plant dwarfism, likely caused by the inhibition of P450s involved in other plant hormone biosynthesis pathways. To develop a selective SL biosynthesis inhibitor, Ito et al. performed a SAR study of TIS13 and developed TIS108 as a potent SL biosynthesis inhibitor that could target CYP711A of P450.10) TIS108 reduced the levels of SL in root exudates and roots in various plants. In addition, TIS108 effectively suppressed S. hermonthica and O. minor parasitism in rice and tomato plants in a pot test. Interestingly, no morphological changes were observed after TIS108 treatment, and the yield of the rice plants in this pot test was comparable to that of the wild type rice grown in normal soil. Finally, the target of TIS108 was identified as CYP711A2, and knockout rice mutants of this gene showed phenotypes comparable to the wild type, indicating that canonical strigolactone production is not required for the normal rice phenotype.11) Although TIS108 was also valid against root parasitic weeds in the tomato pot test,12) whether SL biosynthesis inhibitors are sufficiently effective in fields contaminated by root parasitic weeds remains unclear and is an issue for future research.
Recently, SAR studies on TIS108 have led to the development of KK5, which has a higher inhibitory activity on 4DO biosynthesis in rice.13) This indicates that the further development of SL biosynthesis inhibitors could be effective in controlling root parasitic weeds. In summary, these results indicate that specific SL biosynthesis inhibitors can be effective tools for controlling root parasitic weed damage without inducing morphological changes in the host plants. If inhibitors that do not induce any significant morphological changes in treated plants could be obtained, the resulting knockout mutants of the inhibitor target genes would be very useful for the preparation of resistant plants against attack from root-parasitic weeds.
1.2. Gibberellins, cytokinins and selective SL agonistsThe structures of the plant hormones and their mimics that can control SL biosynthesis are shown in Fig. 3. Gibberellins (GAs) and cytokinins are plant hormones that also suppress SL biosynthesis.14) Ito et al. reported that GA signaling negatively regulates the endogenous levels of SLs.15) The application of an inactive GA metabolite (GA8) to wild type plants or that of active GA (GA3) to GA signaling mutants (gid1-3 and gid2-2) did not induce this regulation, whereas the application of active GA (GA3) to the wild type and a GA biosynthesis mutant (Tanginbozu) reduced their levels of SLs. No SLs were detected in the constitutive GA response mutant (slr1-5). These results indicate that the regulation of endogenous SL levels by GA signaling depends on DELLA protein activity. The increased levels of SLs in gid2-2 were similar to those in Tanginbozu, suggesting that a reduction in GA signaling induces an increase in SL levels. Therefore, GA regulates SL biosynthesis. To explore the effects of GA treatment on the interaction of plants with root-parasitic weeds, Striga germination and infection assays were performed. Consistent with the results of the SL levels in GA-treated rice, the root exudates of rice seedlings treated with 50 nM GA exhibited less germination-stimulating activity than the control plants. As a result of the reduced germination frequency, statistically fewer Striga species established parasitism on 100 nM GA-treated rice. Although GA application is not practical due to its high cost, some GA agonists have been reported.16–19) Thus, these results strongly suggest that GA agonists could be used to control Striga germination.
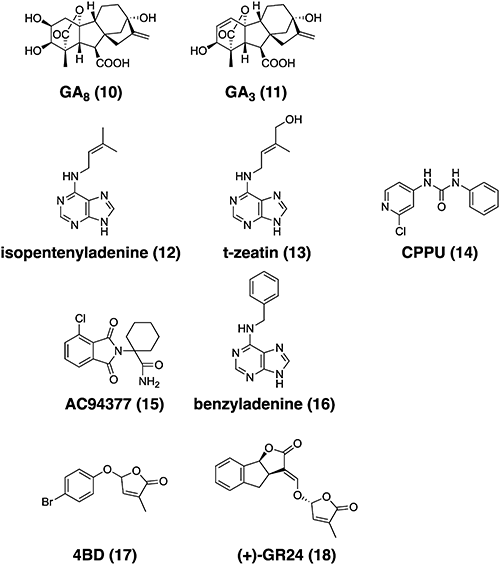
Cytokinins (CKs) also suppress SL biosynthesis. Yoneyama et al. reported that cytokinins (CKs) significantly suppressed 4DO levels in the root tissues and root exudates of rice plants, even those grown under Pi-deficient conditions.20) CPPU [N-(2-chloro-4-pyridyl)-N′-phenylurea], a phenylurea-type CK structurally distinct from adenine-type CKs, also suppressed the 4DO levels. This result strongly suggests that the inhibitory effect on 4DO production was attributable to CK activity. Even in the SL signaling mutant d3, CK application suppressed 4DO production and exudation. This inhibitory effect of CK on 4DO production and exudation was completely and inversely dependent on the CK concentration. The 4DO levels in both root tissues and root exudates decreased with an increasing t-zeatin concentration. This decrease in SL production following CK application was due to the suppression of SL biosynthesis gene expression. The negative effects of CK (t-zeatin and CPPU) application have also been observed in dicotyledonous tomato plants.21)
Although GA and cytokinins affect the morphology of plants and may decrease crop yield, a suitable method for the application of these hormones to reduce Striga damage could be used for crop fields as the GA agonist AC94377 and cytokinin agonist benzyladenine are currently registered as plant growth regulators.
Selective SL agonists are also powerful candidates for the control of root parasitic weeds by suppressing SL biosynthesis.22) Fukui et al. reported an interesting SL agonist, 4BD, which shows activity similar to that of the major strigolactone (SL) analog GR24 in many aspects, in a biological assay on plants.23) 4BD strongly inhibited tiller bud outgrowth in the SL-deficient rice mutant d10 at the same concentration as GR24. The same result was also observed in the Arabidopsis thaliana SL-deficient mutants max1, max3, and max4. However, the application of 4BD to the Arabidopsis SL-insensitive mutant max2 induced no morphological changes. The expression of SL biosynthetic genes was also reduced by 4BD treatment, most likely via negative feedback regulation to reduce the SL levels in the treated plants.24) Interestingly, in a seed germination assay on Striga hermonthica, 4BD showed far less activity than GR24.25) These results suggest that 4BD is the first plant-specific SL mimic, which could be used for reducing the SL levels in host plants and decreasing damage from root parasitic weeds. As several 4BD derivatives have been reported to be more potent than 4BD,26–28) their potency to control root-parasitic weeds is of great interest.
Phosphate or nitrogen deficiency promotes SLs biosynthesis. Thus, chemicals that stimulate phosphate or nitrogen signaling are powerful tools for suppressing SL biosynthesis.29,30)
Since the discovery of SLs as seed germination inducers of root-parasitic weeds, many attempts have been made to use SL or SL-like active compounds for the control of root-parasitic weeds. Root-parasitic weeds, especially obligate root-parasitic weeds, cannot survive without a host plant. As a result, after germination, in response to SLs, they must parasitize the host plant before the nutrients stored in the seeds are exhausted. In contrast, if root-parasitic weeds are forced to germinate in the absence of host plants, with germination stimulants, such as SLs, they will be unable to grow and eventually die. This method of control, which uses the propensity of root parasitic weeds to germinate seeds by SL, is known as “suicidal germination” induction. The induction of suicidal germination using SL or SL-like active compounds in the absence of the host is effective in reducing the number of root parasite seeds in the soil. This method does not directly affect the host plant because it is conducted before the host plant is grown and therefore does not alter the morphology of the host plant. To this end, compounds must have strong germination-inducing activity in the soil and a high degree of chemical stability. The structures of SLs and their mimics are shown in Fig. 4.
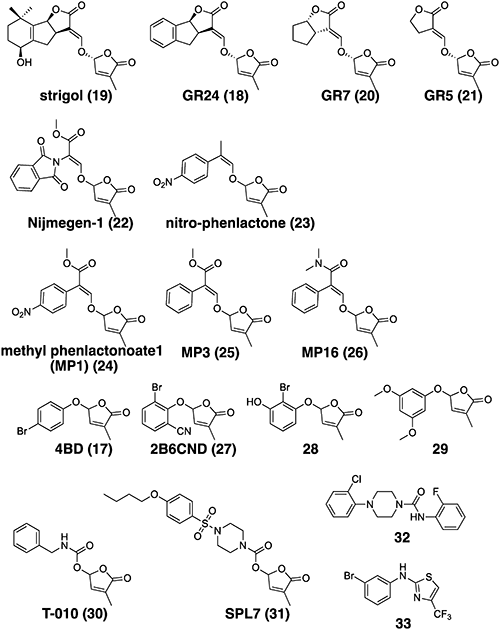
Since the isolation of strigol in 196631) and the determination of its relative stereo configuration in 1972,32) strigol and its synthetic analogs have been actively studied. As a result, several strigol analogs, such as GR24, GR7, and GR5, have been developed and confirmed to act as germination inducers for Striga.33,34) At present, GR24 is used as a standard substance in SL physiology and biochemistry research. The tricyclic structure of a canonical SL is referred to as the ABC ring, while the other lactone ring connected to the C-ring, the lactone ring, via an enol ether structure is referred to as the D-ring. Early SAR studies showed that the structure of the C- and D-rings linked via an enol ether is essential for the expression of seed germination-inducing activity in root-parasitic weeds.
Based on the SAR analysis, SL agonists with improved stability and easy chemical synthesis have been developed. Nijmegen-1, which does not have a structure corresponding to the C-ring, has also been shown to induce germination of root parasitic weeds.35) Nijmegen-1 was demonstrated to be a suicidal germination inducer that reduced Phelipanche ramosa parasitism in tobacco in field trials.36)
The structural requirements found in this series of compounds, including Nijmegen-1, are consistent with the fact that non-canonical SLs, defined as SLs without the ABC tricycle, also possess seed germination-inducing activity in root-parasitic weeds. This has led to the discovery of effective suicidal germination inducers from non-canonical SL analogs. Al-Babilli et al. reported that nitro phenlactone and methyl phenlactonoates (MPs), analogs of carlactone, and carlactonoic acid (CLA) induce seed germination in S. hermonthica and P. aegyptiaca.37–39) In particular, the half-maximal effective concentration (EC50) value for germination-inducing activity against seeds of S. hermonthica of MP16 is known to be in the range of GR24.39) MP1, 3, and 16 showed their efficiencies in Striga-infested field in Burkina Faso.40,41) Kountche et al. demonstrated that a suicidal germination treatment with MP1 or its analogs applied after rainfall reduced the number of S. hemonthica parasitic plants on subsequently grown sorghum by 55%.40) Chemical stability is an important factor for suicidal germination induction activity in soil. The stability of MP1 and MP16 in aqueous solution is comparable to that of GR24. However, some compounds have been found to be more stable than GR24. MP3 is more stable in aqueous solutions than GR24, and appears to be as effective as GR24 and more active than other MP compounds in the evaluation of SL-like bioactivity in rice, which requires long-term treatment compared to the evaluation of seed germination induction activity. Additionally, these compounds have the advantage of low preparation costs compared to GR24 because they can be chemically synthesized in a short step.
Research has prompted a further simplification of the required structures, showing that an ether bond between the D-ring and phenol or enol is sufficient for SL activity, suggesting that the structural constraints in the design of SL-like active compounds are fewer than expected. Fukui et al. found that phenoxyfuranone analogs linked to a substituted phenol and D-ring restored the excessive tillering morphology in a SL-deficient rice mutant, and named a series of compounds containing 4-bromo debranone (4BD) as “debranone”.23,25) Although the first generation of debranones, such as 4BD, shows a similar activity to that of GR24 in many aspects of a biological assay on non-parasitic plants, 4BD showed far less activity than GR24 in a seed germination assay on S. hermonthica.25) Subsequently, debranone was found to have the potential to be designed selectively for specific SL functions depending on the position and type of substituent on the phenyl ring.27,28) For instance, although 2B6CND, 28, and 29 exert a stimulatory effect on seed germination of S. hermonthica and P. ramosa, even at concentrations where GR24 is clearly bioactive, they only slightly restore rice hyper tillering phenotype and have no effect on Arabidopsis morphology.26,42) The EC50 for the seed germination of S. hermonthica of 2B6CND and 28 was 10 to 1000 times higher than that of GR24. Further structural modifications are required to develop debranones as effective seed germination inducers.
SL agonists that combine a simple structure with germination activity against Striga and Orobanche could be applied to induce the suicidal germination of root-parasitic weeds in the field. Samejima et al. reported that the application of the carbamate derivative T-010 to soil contaminated with S. hermonthica resulted in a reduction in S. hermonthica infestation of sorghum and a significant increase in the biomass and yield in the field.43)
Nevertheless, the structural requirement of the D-ring for stimulating the seed germination of root-parasitic weeds could be a bottleneck in obtaining more easily synthesized and chemically stable SL agonists. Uraguchi et al. reported that SPL7 is the most active SL agonist that has seed germination-inducing activity in S. hermonthica at concentrations of femtomoles/L comparable to naturally occurring SLs.44) One of the factors responsible for the strong biological activity of SPL7 is its role as an agonist that selectively acts on ShHTL7, which has a high affinity for SL among the paralogs of the SL receptor D14, which has been shown to be present in at least 11 in S. hermonthica.45,46) SPL7 is a structural analog of a hit compound obtained by a chemical screen conducted to discover agonists of ShHTLs, using seed germination stimulation of S. hermonthica as an indicator. Since the minimum effective concentration is 1/100 million of that of GR24, it can overcome the chemical instability caused by the D-ring.
From this perspective, the future development of SL agonists will involve the screening of compounds without a D-ring. In vitro compound screening and screening using transgenic plants have already led to the discovery of SL-like active compounds, such as 32 and 33, without a D-ring as SL agonists with phytohormone activity.47,48) Although debate continues regarding the molecular mechanism of SL perception, to D14 and paralogs, the conformational changes in the receptor induced by ligand binding are thought to drive signal transduction. Therefore, finding compounds that have low synthetic costs and that can induce structural changes without hydrolysis by D14 and paralogs would facilitate the practical agricultural use of SL as a suicidal germination inducer.
2.2. Ethylene and its mimicsAs mentioned above, one ideal solution to deplete the Striga seed bank is suicidal germination by SLs. However, because of the instability of SL analogs, this procedure has been thought to be impractical, despite the fact that many field trials have yielded positive results. The most notable success of this procedure was the use of ethylene to induce germination of S. asiatica in the United States49) because ethylene produced upon treatment with SLs is an active ingredient for stimulating germination.50) The structures of ethylene, its inhibitors and mimics are shown in Fig. 5. This method is also attractive for the control of S. hermonthica, although it has been reported that exogenously applied ethylene does not induce high levels of S. hermonthica germination.51,52) Egley also reported that 2-chloroethylphosphonic acid (ethephon) stimulated germination of aged, pretreated but still dormant witchweed (Striga asiatica Lour.) seed.53) Ethephon and ethylene biosynthesis intermediate 1-aminocyclopropane-1-carboxylic acid (ACC) more effectively stimulated germination than ethyene at concentrations of 0.01 and 1 mM, respectively in a concentration-dependent manner.50,54) The study also demonstrated that germination induced by synthetic strigolactone GR24 was inhibited by the ethylene biosynthesis inhibitor aminoethoxyvinylglycine (AVG) and an ethylene receptor inhibitor 1-methylcyclopropene. However, this approach cannot be applied in sub-Saharan Africa, where the income of subsistence farmers is usually too low to afford such technology.55) Therefore, the application of cost conscious and stable ethylene mimics appears to be an attractive solution. However, reports on the development of ethylene mimics as inducers of suicidal germination are lacking, although there have been two reports on the development of chemicals that act as mimics in Arabidopsis.
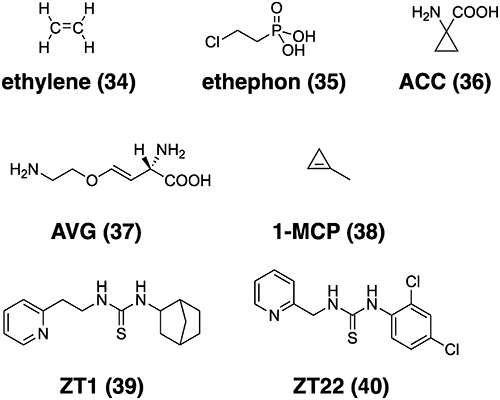
In this context, Koyama et al. screened chemicals from a chemical library that stimulated the germination of Striga hermonthica and showed ethylene-like activity.56) ZT1 was thus selected, and through SAR studies, ZT22 was ultimately selected as the germination promoter. In the pot test, ZT22 showed good potency in suppressing the emergence of Striga hermonthica, indicating that ethylene mimics can be used as suicidal germination inducers (16th WCPP meeting). Since ethylene is important for germination, the use of ethylene biosynthesis inhibitors is also promising for controlling Striga germination, and the development of these chemicals is expected in this field in the future. Recently, Cui et al. reported that the application of ethylene signaling inhibitors also caused haustorial defects, indicating that ethylene signaling regulates the cell proliferation and differentiation of parasite cells.57) Genetic disruption of host ethylene production also perturbs parasite invasion. They proposed that parasitic plants use ethylene as a signal to invade the host roots. Based on this report, the development of efficient ethylene signaling or biosynthesis inhibitors could provide powerful tools for controlling damage caused by root-parasitic plants.
Numerous studies have been conducted to identify inhibitors of SL receptors (Fig. 6). SL, a plant hormone, binds to the ligand-binding pocket of receptor D14 and its orthologs, and is then hydrolyzed by D14.58,59) In a previous study, we obtained rice D14 in crystal form and soaked it in a solution of GR24 to obtain the crystal of the complex of D14 and a hydrolysis product, hydroxy D-ring (D-OH). Next, we solved the crystal structure of the D14–D-OH complex and identified amino acid residues interacting with D-OH in the binding pocket rice D14.59) Expecting that compounds that bind to the binding pocket of D14 by similar mechanism to D-OH may function as chemical regulators of D14, a pharmacofore model was constructed based on the information of the D14–D-OH interaction, followed by in silico virtual screening.60) As a result, XM-47 was identified as a candidate D14 inhibitor. XM-47 inhibited the SL-dependent interaction between D14 and D53 in a dose-dependent manner. Because XM-47 was predicted to be easily degraded in the medium or in cells and converted to 2-methoxy-1-naphthaldehyde (2-MN), the D14 inhibitory effect of 2-MN was investigated, and it was found that 2-MN inhibited the D14-D53 interaction more strongly than XM-47. In addition, 2-MN inhibited the SL-induced suppression of rice tiller outgrowth and the SL-suppressed high-temperature germination of Arabidopsis by SL. However, 2-MN did not show a strong inhibitory effect on the SL-induced seed germination of S. hermonthica.
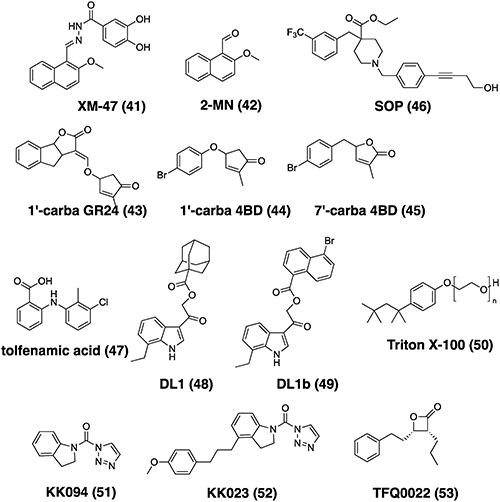
In addition, based on the idea that SL analogs that are not hydrolyzable by D14 could be D14 inhibitors, we designed carba-SL in which the butenolide ring of the D-ring was converted to the cyclopentenone ring or the oxygen atom in the enol ether bond was replaced with the methylene group, 1′-carba GR24, 1′-carba 4BD, or 7′-carba 4BD.61) These blocked the interaction between D14 and D53 by inhibiting D14 hydrolytic activity, as well as inhibiting the SL-induced suppression of rice tiller outgrowth. In addition, carba-SLs were found to suppress the SL response in S. hermonthica seed germination.
In Arabidopsis, SLs are known to positively regulate photomorphogenesis and suppress hypocotyl elongation in the light.62,63) Holbrook-Smith et al. identified soporidine (SOP) as a compound that restores the suppression of hypocotyl elongation by SL.64) SOP inhibited the suppression of hypocotyl elongation by SL by inhibiting the karrikin receptor AtHTL/AtKAI2. SOP also inhibited the SL-induced seed germination of S. hermonthica, inhibiting the SL receptors ShHTLs/ShKAI2ds.
Similarly, Hamiaux et al. identified tolfenamic acid as a compound that inhibits the destabilization of the petunia SL receptor DAD2.65) Tolfenamic acid inhibited the hydrolytic activity of DAD2 and Arabidopsis D14, resulting in an increase in the branches of petunia and Arabidopsis.
Tsuchiya’s group developed Yoshimulactone G (YLG), which emits fluorescence when it is received and degraded by SL receptors, which has greatly contributed to the elucidation of the SL receptor mechanism.66) This group discovered SL receptor-inhibitors in an in vitro chemical screening system using YLG, identifying DL1 as a compound that inhibits YLG hydrolysis by Arabidopsis D14, as well as finding that DL1 increases the branching of Arabidopsis.67) In addition, by performing structural optimization of DL1, DL1b was found to increase the branching of Arabidopsis and rice at a lower concentration.68)
Al-Babilli et al. found Triton X-100 acts as a selective inhibitor of ShHTL/ShKAI2d.69) They had used Triton X-100 as a detergent during ShHTL7 purification. However, as a result of X-ray crystallography analysis, they found that it fitted into the SL binding pocket of ShHTL7, which blocked the binding of ShHTL7 and SL and inhibited the seed germination of S. hermonthica.
D14 and HTL/KAI2 are serine hydrolases whose catalytic center is a serine residue. Serine hydrolases form a large family in various organisms. Several chemicals that inhibit serine hydrolases have been identified. Among them, β-propiolactone derivatives and triazoleurea derivatives inhibit enzyme activity by covalently binding to the serine residue at the catalytic center of the serine hydrolase.70) Adibekian et al. developed triazole urea derivatives to obtain compounds that act on a specific serine hydrolases.71) Inspired by this report, we decided to search among triazole derivatives for compounds that selectively inhibit the SL receptor. Using X-ray crystallography, KK094 was found to inhibit the hydrolytic activity of D14,72) showing an inhibition of the interaction with SL and hydrolysis activity by forming a covalent bond with the catalytic center serine residue of rice D14, as well as increasing tiller outgrowth in rice.
While KK094 showed strong inhibitory activity against rice D14, it showed low seed germination inhibitory activity against root parasitic weeds.72,73) Al-Babilli et al., who previously found that Triton X-100 could be an SL receptor inhibitor, synthesized compound KK023 with a chimeric structure consisting of a triazole urea structure and Triton X-100 in collaboration with our group. As a result, they found that KK023 inhibits ShHTL/KAI2d catalytic activity and the SL-induced seed germination of S. hermonthica.74) These results suggest that further structural development of the triazole urea derivative could lead to the development of various SL receptor-specific inhibitors.
Another example of SL receptor covalent bond inhibitors was reported by Xiang et al.75) They found that TFQ0022, which was designed based on β-propiolactone, covalently bound to the serine residue of Arabidopsis D14, inhibited SL signaling, and restored the suppression of hypocotyl elongation by SL in Arabidopsis seedlings.75)
As mentioned above, SL receptor inhibitors have been developed using various strategies. Therefore, it is now possible to obtain receptor inhibitors based on a large number of options. From a practical point of view, the promotion of branching is directly linked to an increase in crop yield, and therefore shows potential for use as a plant growth regulator in agriculture. Okazaki et al. found that KK094 promotes the formation of adventitious shoots of the medicinal plant ipecac.76) This suggests that KK094 may be a plant growth regulator applicable to various plant species. However, no studies have shown that SL receptor inhibitors promote branching and increase yields at the field level. In addition, although a number of reports have shown that SL-receptor inhibitors inhibit the seed germination of root parasitic weeds, whether it is possible to grow crops in root parasitic weed seed-contaminated soil at the field level using these SL-receptor inhibitors remains poorly understood. We hope that research on the improvement of the chemical structure and treatment methods for receptor inhibitors will proceed with a view to such agricultural applicability in the future.
Parasitic plants develop a unique multicellular organ called the haustorium, which is essential for parasitism and forms upon the detection of haustorium-inducing factors (HIFs) derived from the host plant.77) This organ penetrates the host stem or root and connects to its vasculature, allowing the exchange of materials, such as water, nutrients, proteins, nucleotides, pathogens, and retrotransposons, between the host and the parasite. As this organ requires small-molecule stimulation for its formation, it is considered a good target for chemical control. The process of haustoria formation can be divided into two steps, namely prehaustorium formation and haustorium maturation, which occur before and after host attachment, respectively. Cell wall-related quinones and phenolics have long been known as host-derived HIFs to induce haustoria in many Orobanchaceae species. Here, the structures of HIFs are shown in Fig. 7.
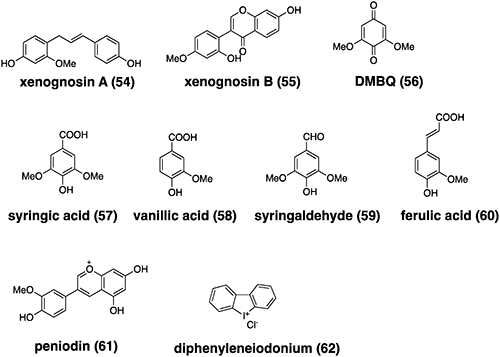
Two flavonoids, xenognosin A and B, were isolated from gum tragacanth, an exudate of Astragalus spp., as HIFs for Agalinis.6,79) A quinone 2,6-dimethoxy-1,4-benzoquinone (DMBQ) was isolated from the root extracts of sorghum, a natural host for Striga, and found to be an HIF for S. asiatica and Agalinis.80) Phenolic acids (including syringic acid, vanillic acid, and ferulic acid), aldehydes (including syringaldehyde), and flavonoids (including peonidin), have also been reported to induce haustoria in Triphysaria versicolor, P. japonicum, and S. hermonthica as compounds with structures similar to DMBQ.81,82) The plant hormones cytokinins, structurally different from phenolic compounds, also trigger prehaustorium formation in Orobanchaceae.77) Castilleja tenuiflora was reported to react with vanillic acid, catechin, and hydrogen peroxide (H2O2),83) although H2O2 alone did not induce haustoria in S. hermonthica.84)
Within this context, the application of a series of ROS inhibitors and scavengers revealed that reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase inhibitors, especially diphenyleneiodonium (DPI), could efficiently reduce haustorium formation in S. hermonthica.84) This is the first report on a haustorium formation inhibitor. However, based on the function of HIFs, further inhibitors of haustorium formation could be developed.
In summary, further research into the common and specific signaling pathways of different types of HIFs should reveal how parasitic plants sense the presence of a host and begin their parasitic lifestyles. Specific and potent HIF inhibitors designed and developed based on this research could be used as chemical regulators to reduce damage caused to crops by root-parasitic weeds.
As we have discussed, there are advantages and disadvantages to controlling root parasitic weeds with compounds compared to other methods, such as weed resistant crops and cover crops. Although this review did not address the application of herbicides, this method has been the subject of much effort and has high potential for practical application. In the future, control methods integrating various methods, not just one method, will become available.
The authors are gratefuly thank to Ms. Suto for the illustration. This work was supported in part by a grant from the Core Research for Evolutional Science and Technology (CREST) Program of Japan Science and Technology Agency (JST) to T.A. and a JSPS Grant-in-Aid for Scientific Research (grant No. 18H05266) to T.A.