2022 年 47 巻 4 号 p. 197-202
2022 年 47 巻 4 号 p. 197-202
The inhibitory effect of propiconazole on strigolactone (SL) production and Striga hermonthica emergence were evaluated using rice plants. A significant reduction in SL levels was detected in root and root exudates after rice was treated with different doses of propiconazole. Propiconazole induced second tiller bud outgrowth in rice seedlings. In accordance with the results of 4-deoxyorobanchol levels in root and root exudates, propiconazole-treated rice attenuated the degree of infestation of the root parasitic weed, S. hermonthica. Overall, these results suggest that propiconazole has potential as a plant growth regulator for agriculture and a new scaffold for developing potent inhibitors of SL production.
Carotenoid-derived natural products—strigolactones (SLs)—which are produced in plants and exuded from the roots, were first discovered as inducers of seed germination against root parasitic weeds.1) Root parasitic weeds, such as Striga, Orobanche, and Phelipanche spp., severely damage agricultural production, especially in warm and temperate zones.2) Hence, effective ways of control have been sought, but to date, definitive control methods have not been established. Almost a decade ago, SLs were identified as a plant hormone that regulates multiple events of growth and development, including shoot branching.3) Therefore, the biosynthesis inhibitors of SL have potential application for various uses: protection against the infestation of root parasitic weeds and alteration of plant body shape. SLs are derived from all-trans-β-carotene and converted into biosynthetic intermediate carlactone (CL) by sequential steps catalyzed by carotenoid isomerase DWARF27, carotenoid cleavage dioxygenase 7 (CCD7), and CCD8.4) CL is sequentially converted to carlactonoic acid (CLA) by cytochrome P450 [CYP711A1/MORE AXILLARY GROWTH1 (MAX1)] and then to methyl carlactonoate (MeCLA) followed by methylation in Arabidopsis thaliana.5) MeCLA could bind to the SL receptor, suggesting that it functions as an active SL in A. thaliana. In rice, four CYP711A isoforms are functional; one of them—CYP711A2—oxidizes CL to 4-deoxyorobanchol (4DO), a canonical SL with the simplest structure via CLA. CYP711A3 hydroxylates CL to CLA and 4DO to orobanchol.6,7) CLA is converted to various structures of SLs in a species-dependent manner.8–11) The mutants caused by disrupting these genes show similar morphological phenotypes: dwarfism and increased branching.
Triazole, a nitrogen-containing heterocycle compound, has been used as a biosynthesis inhibitor of many plant hormones. For instance, paclobutrazol inhibits gibberellin biosynthesis, reducing unwanted shoot elongation and preventing lodging without lowering crop productivity, therefore having agricultural applications.12) Brassinazoles13,14) and abscinazoles15) inhibit the biosynthesis of brassinosteroids (BRs) and abscisic acid, respectively. They are now widely used chemical tools in biological research. Some triazole compounds were also reported to inhibit SL biosynthesis. It has been reported that TIS108 and its derivative, KK5, had a strong inhibitory effect on SL production.16,17) These compounds completely inhibited the content of 4DO in the exudates from rice roots in the nanomolar concentration range. However, these compounds cannot induce tiller bud outgrowth in rice although they slightly increased the number of shoot branches of A. thaliana.17–19)
Propiconazole (1-[[2-(2,4-dichlorophenyl)-4-propyl-1,3-dioxolan-2-yl]methyl]-1,2,4-triazole; Fig. 1A), a widely used triazole-containing fungicide, inhibits the 14-alpha demethylation reaction in the ergosterol biosynthetic pathway of fungi.20) In addition to fungicidal action, propiconazole blocks brassinosteroid biosynthesis in plants.14,21) Furthermore, Ito et al. (2013) previously found that propiconazole treatment reduced the content of endogenous 4DO in exudates from rice.22) In addition, we preliminarily observed longer second tillers in propiconazole-treated rice seedlings. Because propiconazole is a registered fungicide, propiconazole is a promising SL biosynthesis inhibitor for use in practical agriculture. In this study, we have presented the effects of propiconazole on increased numbers of tillers in rice and infection of a root parasitic weed, Striga hermonthica.
Propiconazole was purchased from FUJIFILM Wako Pure Chemical (Osaka, Japan). GR24, synthesized as described previously,23) produced four stereoisomers and was separated with silica gel column chromatography. In this study, GR24 with the same relative stereochemistry as (±)-strigol was used.
2. Plant materials and growth conditionsRice (Oryza sativa) cultivars Nipponbare and IAC-165 were used in this study. The seeds were sterilized with a 2.5% (v/v) sodium hypochlorite solution containing 0.01% (v/v) Tween 20 for 30 min and incubated in sterile water at 25°C in the dark for 2 days. The germinated seeds were transferred into a glass bottle containing hydroponic culture medium24) solidified with 0.6% (v/v) agar, and they were grown at 25°C under LED light (100 µmol m−2 s−1) with long-day conditions (16 hr light/8 hr dark cycle).
For measuring 4DO in rice roots and root exudates and seed germination assay of S. hermonthica, 7-day-old seedlings of cv. Nipponbare were transferred to 12 mL of hydroponic culture media in a brown glass vial and grown under the same conditions for 6 days. Seedlings were then transferred to 12 mL of fresh hydroponic culture medium with or without propiconazole in a new brown glass vial. Propiconazole was diluted 1 : 1000 from an acetone-dissolved stock solution (acetone was used as a control). Twenty-four hours after application, roots and hydroponic culture media were collected. For measuring plant height and the length of the second tiller, 7-day-old seedlings were grown under the hydroponic conditions described above for 10 days.
Seeds of S. hermonthica were collected in Sudan and shipped to Japan, where they were maintained in an incubator at 30°C in the dark before use.
3. Measuring 4DOFor analyzing 4DO in root exudates, the hydroponic culture medium (9 mL) was subjected to extraction twice with ethyl acetate containing 200 pg of deuterium-labeled 5-deoxystrigol (d6-5DS) used as internal standard.25) The ethyl acetate layer was dried under reduced pressure and dissolved in 1 mL of 15% (v/v) ethyl acetate in hexane. The extracts were subjected to Sep-Pak silica 1 mL cartridges (Waters, Milford, MA), washed with 3 mL of the same solvent, and eluted with 3 mL of 35% (v/v) ethyl acetate in hexane. SL-containing fractions were dried in vacuo. To analyze 4DO in roots, the roots were homogenized in acetone containing 200 pg of d6-5DS. The resulting acetone extracts were filtrated, concentrated in vacuo, and then dissolved in 10% (v/v) acetone. The extracts were subjected to Oasis HLB 3 mL cartridges (Waters), washed with 6 mL of water, eluted with 6 mL of acetone, concentrated in vacuo, and dissolved in 1 mL of 15% (v/v) ethyl acetate in hexane. The extracts were subjected to Sep-Pak silica 1 mL cartridges (Waters) in the same way as extraction from root exudates, and elution fractions were then dried in vacuo. The dried SL-containing fractions were dissolved in acetonitrile and subjected to liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) analysis. Analysis was performed using a quadruple/time-of-flight tandem mass spectrometer (Triple TOF 5600 system; AB SCIEX) connected to an ultra-high-performance liquid chromatograph (Nexera; Shimadzu) equipped with a reversed-phase column (Acquity UPLC BEH-C18, 2.1×50 mm, 1.7 µm; Waters). LC-MS/MS conditions were the same as those described previously.26)
4. Striga seed germination assayThe medium culturing propiconazole-treated rice (10 mL) was extracted twice with ethyl acetate. The ethyl acetate layer was concentrated in vacuo and dissolved in 0.1 mL of deionized water. Seed germination assay using S. hermonthica was performed as described previously.26) The seeds were sterilized with a 1% (v/v) sodium hypochlorite solution containing 0.01% (v/v) Tween 20 for 5 min and sown on glass fiber paper disks saturated with distilled water. The seeds were sown at a density of around 50 seeds per disk. The paper disks containing the seeds were arranged on a filter paper disk (70 mm diameter) in a petri dish, and 1.6 mL of distilled water was added to the dish. The dishes were conditioned at 30°C in the dark for 7 days. The paper disks with seeds were transferred to a 96-well plate, a 20 µL aliquot of distilled water or extracts was applied, and the seeds were incubated at 30°C in the dark for 2 days. They were then examined for germination under a microscope.
5. Striga shoot emergence testWhen germinated S. hermonthica succeeds in parasitizing a host root, it begins to grow a shoot, which eventually emerges on the soil surface. The number of S. hermonthica buds appearing on the soil surface was considered to be an indicator of parasitism, and the effect of propiconazole on surface bud appearance was examined. Seeds of S. hermonthica (3 mg) were sown in plastic pots (70 mm i.d. and 84 mm in height; AS ONE, Osaka, Japan) containing 200 g of sterilized soil [Bonsol No.2 (Sumitomo Chemical, Osaka, Japan) and river sand=1 : 1, v/v] and 60 mL of deionized water and conditioned in the dark at 30°C for 7 days. Five-day-old seedlings of rice cultivar IAC-165, which were cultivated in solidified culture medium,24) were transplanted into pots containing conditioned S. hermonthica (one seedling per pot) and grown in a growth chamber (NK System, Tokyo, Japan) at 30°C under LED lights (500 µmol m−2 s−1) with long-day conditions (16 hr light/8 hr dark cycle). Propiconazole was diluted 1 : 1,000 from an acetone-dissolved stock solution (acetone was used as the control) in distilled water, and it was applied to the soil (50 mL per pot) once a week for 7 weeks. The S. hermonthica plants that emerged in each pot were counted.
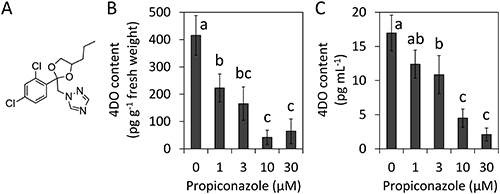
A previous study showed that 10 µM propiconazole decreased the level of 4DO in the root exudates of rice to one-eleventh of that with mock treatment.22) A petunia SL exporter, mutant pdr1, has lowered the SL level in the root exudates but did not reduce the endogenous level of SL.27) Hence, we thought that the reduced 4DO level in the root exudates from rice treated with propiconazole could be induced by inhibiting the export of 4DO. To determine whether propiconazole inhibits SL biosynthesis, we quantified the endogenous level of SL in roots by LC-MS/MS. Figure 1B shows that propiconazole reduced the endogenous level of 4DO in a dose–dependent manner up to 10 µM. We also confirmed that propiconazole reduced the level of 4DO in root exudates the same as endogenous 4DO, and dose dependency of propiconazole was observed in the reduction of 4DO content in the tested concentration ranges (Fig. 1C). These results indicated that propiconazole inhibited SL production in plants.
We next examined whether propiconazole induced SL-deficient phenotypes in rice plants. The number of tillers significantly increases in SL-deficient mutants of rice with dwarfism.28–30) Young seedlings of SL-deficient rice mutants show elongation of the first and second tiller buds, whereas those of wild types remain dormant. Propiconazole induced the outgrowth of second tiller buds, and the length of the second tiller was dependent on concentration up to 10 µM in 17-day-old rice seedlings (Fig. 2A), whereas propiconazole suppressed plant height to the same degree at all tested concentrations (Fig. 2B). In SL-deficient mutants, these phenotypes are restored by the application of SLs to the medium.24) To determine whether propiconazole-induced phenotypes are due to SL deficiency in plants, GR24, a synthetic analog of SL, was co-treated with 10 µM propiconazole. When rice was co-treated with 1 µM GR24, the length of the second tiller was partially suppressed (Fig. 2C), whereas plant height was not rescued by this treatment (Fig. 2D). These results indicate that propiconazole induces the outgrowth of the second tiller bud by inhibiting SL biosynthesis; however, propiconazole-induced dwarfism is a side effect. In fact, it has been reported that propiconazole blocked BR biosynthesis and induced dwarf phenotypes in maize, A. thaliana, and cress seedlings.14,15) The phenotypes of these plants are similar to those of BR-deficient mutants, and the phenotypes can be restored by BR treatment. These reports explain why the application of propiconazole-induced dwarfism could not be recovered by SL treatment. Further testing is required to assess whether propiconazole-induced bud outgrowth and dwarfism are restored by co-treatment with SL and BR.

In addition to suppressing shoot branches, SL stimulates the seed germination of root parasitic weeds such as Striga and Orobanche spp. Therefore, propiconazole might be utilized to control root parasitic weeds since the exudation of SL from roots was reduced in propiconazole-treated rice seedlings. To evaluate the utility as a regulator of germination of root parasitic weeds, we performed a seed germination assay using S. hermonthica. The culture medium of rice cv. Nipponbare treated with 10 µM propiconazole attenuated germination-stimulating activity against S. hermonthica as compared with that of mock-treated rice (Fig. 3A). The addition of 1 µM GR24 to the culture medium of 10 µM propiconazole-treated rice restored the rate of seed germination. These results indicate that the reduction of germination activity of the culture medium of propiconazole-treated rice should be attributed to the reduction of SL levels in the culture medium and not propiconazole’s phytotoxicity to S. hermonthica seeds.

Next, we performed a pot test to confirm the effect of propiconazole on the shoot emergence of S. hermonthica plants in the soil. To more clearly evaluate the inhibitory effect of propiconazole on S. hermonthica infection of the host, we used S. hermonthica-susceptible rice cultivar IAC-165 in this assay. Rice plants were grown in pots containing soil with S. hermonthica seeds, and 3 or 10 µM propiconazole was applied to the soil. Propiconazole showed a significant reduction of the number of emerged S. hermonthica seedlings as compared with those with mock treatment, and at 10 µM, no seedlings of S. hermonthica were detected (Figs. 3B, C). Plant height was shorter of rice mixed with S. hermonthica than of rice not mixed, with or without propiconazole treatment; however, the number of tillers was significantly higher only on rice treated with propiconazole (Figs. 3D, E). As the concentration of propiconazole decreased, the effect on rice height also decreased, but there was no difference in the number of tillers. Since S. hermonthica did not emerge in pots treated with 10 µM propiconazole, it can be assumed that the change in rice morphology at this concentration was simply induced by propiconazole. These results indicate that propiconazole induces dwarfing and excessive tillering. Mock-treated plants showed shorter plant height than that of seedlings grown in soil without S. hermonthica seeds because the parasite suppressed the growth of the host plant by draining nutrients from the host plant (Fig. 3D). Although propiconazole-treated rice plants showed morphological alteration such as dwarfism owing to the side effect of this chemical, as shown in Fig. 2B (Fig. 3D), the stunting of propiconazole-treated rice plants was milder than that in mock-treated rice plants. Thus, these results indicate that propiconazole prevents damage caused by root parasitic weeds. Previous studies have shown that the inhibition of SL biosynthesis alleviated damage from the infestation of root parasitic weeds. Carotenoid biosynthesis inhibitors reduced SL levels and the number of S. hermonthica attached to rice plants.31) Additionally, TIS108 subjected to tomato plants attenuated the degree of infection of O. minor.32) To date, several approaches for controlling root parasitic weeds have been developed,33,34) but further effective approaches are needed, due to their own weak point. Overall, it is suggested that the inhibition of SL biosynthesis is one effective option for attenuating a big agricultural problem.
Although we have not examined the target site of this chemical, information on the fungistatic action of propiconazole might present the putative target in the SL biosynthesis pathway. Propiconazole contains a triazole skeleton that functions as an inhibitor of cytochrome P450s. In fact, propiconazole inhibits the activity of CYP51A1—lanosterol 14-alpha demethylase—in phytopathogenic fungi.19,20) It is plausible to assume that propiconazole also blocks the SL biosynthesis steps catalyzed by cytochrome P450. In rice, two paralogs of P450 CYP711A were identified as genes encoding CL oxidases. CYP711A2 catalyzes sequential steps from CL to 4DO via CLA, and CYP711A3 converts CL to CLA and 4DO to orobanchol.6,7) However, TIS108 targets these CYP711As but does not induce a phenotype of excessive tillers in rice.19) If the propiconazole-induced increase in tillers is due to the inhibition of SL biosynthesis, then this chemical may inhibit the biosynthesis enzymes involved in CL biosynthesis, upstream of the catalytic step of CYP711As. Kawada et al. demonstrated that the triazole-containing P450 inhibitors bromuconazole and diniconazole reduced 4DO levels in rice exudates to half the levels in control rice, but they did not inhibit CYP711A2 enzyme activity. This suggests that they may inhibit D27, CCD7, or CCD8 enzyme activity.35) Following the speculation of Kawada et al.,35) triazoles may chelate non-heme iron in the active sites of D27 and CCDs,36) thereby inhibiting their enzyme activity. Therefore, it is reasonable to suppose that D27 and the CCDs are also targets of this chemical. Enzyme inhibition analysis will provide decisive information on propiconazole’s target site(s).
Hydroxamic acid derivatives inhibited the activity of various CCDs, including A. thaliana CCD7, and increased the number of shoot branches of Arabidopsis plants at 100 µM.37) When tomato plants were treated with 50 µM of one of these compounds, the level of solanacol—a SL—in the root exudates was reduced by around 50%.38) On the other hand, no more than 10 µM propiconazole reduces 4DO level and induces the outgrowth of tiller buds in rice seedlings (Figs. 1B, 2A). Therefore, propiconazole might be an inhibitor more potent than hydroxamic acid derivatives. Further studies are required to compare the effectiveness of these two types of inhibitors under the same assay conditions. Meanwhile, propiconazole inhibited the production of 4DO at higher concentration than either TIS108 or KK5. These two types of inhibitors completely inhibit the 4DO level in the nanomolar concentration range, although it has not been shown whether they can induce tiller outgrowth of rice plants.16,17) Although TIS13 was a SL inhibitor with the induction of dwarfism, an obvious side effect, to treated plants,26) chemical modification achieved the development of TIS108, a potent SL inhibitor without the induction of obvious growth retardation.16) Therefore, the rigorous chemical modification of propiconazole will lead to the development of a specific and potent inhibitor of SL biosynthesis.
Here, we collectively showed that propiconazole was a potent regulator of plant growth by inhibiting the biosynthesis of SLs, which function as regulators of morphological alterations and the accumulating germination of root parasitic weeds. Therefore, we propose that propiconazole can be a plant growth regulator for agriculture and a new scaffold for developing potent inhibitors of SL biosynthesis.
The authors thank Professor Abdelgabbar Eltayeb Babiker (Sudan University of Science and Technology, Sudan) for kindly providing the S. hermonthica seeds. This work was supported in part by grants from the Core Research for Evolutional Science and Technology (CREST) Program of the Japan Science and Technology Agency (JST), the Science and Technology Research Partnership for Sustainable Development (SATREPS) Program of JST, the Program for the Promotion of Basic and Applied Researches for Innovation in Bio-oriented Industry (BRAIN), and a JSPS Grant-in-Aid for Scientific Research (grant number 18H05266).