2023 年 48 巻 1 号 p. 22-27
2023 年 48 巻 1 号 p. 22-27
Developed by Mitsui Chemicals Agro, Inc. (Tokyo, Japan), quinofumelin is a novel fungicide with a distinct chemical structure including 3-(isoquinolin-1-yl) quinoline, demonstrating fungicidal activity against a variety of fungi, including rice blast and gray mold. We screened our compound library to identify curative compounds for rice blast and evaluated the effect of fungicide-resistant strains of gray mold. Our research demonstrated that quinofumelin has curative effects against rice blast and is not cross-resistant to existing fungicides. Accordingly, the use of quinofumelin can be considered a novel approach for disease control in agricultural production. In this report, the discovery of quinofumelin from the initial compound is described in detail.

Pyricularia oryzae, colloquially known as rice blast, is a pathogen responsible for the most severe and damaging disease in rice production.1) Accordingly, numerous effective fungicides for blast control have been developed, particularly melanin biosynthetic inhibitors (MBI) and plant defense activators (PDA). MBI, such as tolprocarb, fthalide, tricyclazole, pyroquilon, carpropamid, and diclocymet, inhibit the formation of adherents necessary for infection required for infection. PDA, such as probenazole, isotianil, and tiadinil, induce host resistance against pathogens. The disadvantage of these modes of action, however, is that they have no effect after infection. Consequently, the development of a new curative fungicide for rice blast is essential.2)
Botrytis cinereal, also known as gray mold, is an airborne necrotrophic plant pathogen that attacks numerous fruits and vegetables, drastically reducing crop yields and global crop productivity.3) Although numerous fungicides exist to control gray mold, such as benzimidazoles, dicarboximides, phenylpyrroles, ergosterol biosynthesis inhibitors, succinate dehydrogenase inhibitors, and quinone outside inhibitors, their efficacies are declining under the emergence of fungicide-resistant strains, and therefore novel fungicides must be continually developed for the control of gray mold.4)
To respond to these needs, we screened our compound library to identify curative compounds for rice blast and evaluated the effect of fungicide-resistant strains of gray mold.
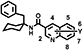
First, we found compound 1, which is active in both diseases in screening. Next, we found that compound 2 with a benzyl group at the first position of the cyclohexane ring has a 10-fold effect on both diseases. The antifungal activity of compound 2 was not enhanced by introducing a substituent into a quinone or benzene ring or by altering the linkage types. Thus, we sought to improve its fungicidal activities by modifying its molecular structure through conformational analysis to confirm an active conformation of compound 2. Based on the results, we synthesized compound 3, which cemented the conformation, but the activity decreased. Upon further examination, compound 4 obtained by converting the cyclohexane ring to a dimethyl group increased its activity, and compound 5, in which the difluoro group was introduced at the 4-position of the isoquinoline ring reached such a high level of activity that it could be expected to be effective even in practical situations (Fig. 1).
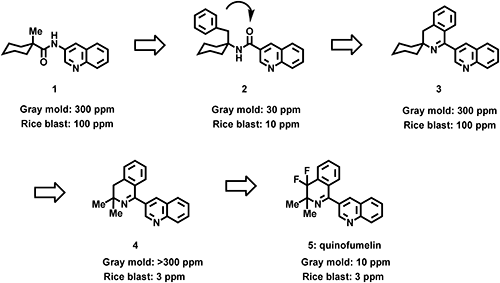
In the present report, we describe the details of the discovery, synthesis, and structure–activity relationships of quinofumelin and its derivatives.
Chemical structures were confirmed by 1H-NMR and 13C-NMR spectroscopy using a Varian Gemini 200, a JEOL JNM-GX270, a JEOL JNM-ECA500, and a Bruker DPX-400 FTNMR system with tetramethylsilane as the internal standard and MS were obtained using a JEOL JMS-D300 and a VG Auto Spec M mass spectrometer. In the case of a solid as a physical property value, the melting point was measured. None of the melting points (MP) were adjusted.
1.1. 1-methyl-N-(quinolin-3-yl)cyclohexane-1-carboxamide (1)Compound 1 of Fig. 1 was synthesized as follows. 2-chloro-1-methylpyridinium iodide (0.19 g, 0.75 mmol), 3-aminoquinoline (72 mg, 0.50 mmol) and triethylamine (0.20 mL, 1.50 mmol) were added to a solution of 1-methylcyclohexanecarboxlic acid (71 mg, 0.50 mmol) in dichloromethane (1 mL) at room temperature and stirred for 3 hr. The mixture was poured into ice water (50 mL), and the resulting mixture was extracted with ethyl acetate (50 mL). The organic layer was dried over anhydrous magnesium sulfate and concentrated in vacuo. The residue was purified using silica gel chromatography (hexane/ethyl acetate=1/1) on a preparative TLC plate to generate compound 1 (30 mg, 22% yield) as colorless crystals. MP: 126°C–129°C. 1H-NMR (200 MHz, CDCl3) δ ppm: 1.32 (3H, s), 1.42–1.63 (8H, m), 2.05–2.12 (2H, m), 7.48–7.66 (2H, m), 7.70 (1H, bs), 7.78 (1H, dd, J=1.8, 8.1 Hz), 8.03 (1H, d, J=8.4 Hz), 8.73 (1H, d, J=2.6 Hz), 8.82 (1H, d, J=2.6 Hz). MS m/z: 268 (M+), 144, 97, and 83.
1.2. N-(1-benzylcyclohexyl)quinoline-3-carboxamide (2): Method ACompound 2 of Fig. 1 could be synthesized in two ways.
Thionyl chloride (3 mL) was added slowly to quinoline-3-carboxylic acid (0.15 g, 0.90 mmol) and the mixture was refluxed for 1 hr. Excess thionyl chloride was removed under reduced pressure and the residue was dissolved in dichloromethane (10 mL). A solution of 1-benzylcyclohexylamine (0.10 g, 0.54 mmol) in pyridine (0.3 mL) was added to the solution at room temperature and stirred for 3 days. The mixture was poured into ice water (50 mL), and the resulting mixture was extracted with ethyl acetate (50 mL). The organic layer was dried over anhydrous magnesium sulfate and concentrated in vacuo. The residue was purified using silica gel chromatography (hexane/ethyl acetate=1/1) on a silica gel column to yield compound 2 (87 mg, 29% yield) as an oil.
1.3. N-(1-benzylcyclohexyl)quinoline-3-carboxamide (2): Method BWe found that Method B, known as the Ritter Reaction, is more efficient than Method A as a method for obtaining compound 2.
Quinoline-3-carbonitrile (1.0 g, 6.7 mmol) and sulfuric acid (3.5 mL) were added to a solution of 1-benzylcyclohexanol (0.84 g, 4.4 mmol) in acetic acid (10 mL), and the mixture was stirred at room temperature for 18 hr. The mixture was then poured into a 50 mL saturated sodium bicarbonate aqueous solution, and the resulting solution was extracted with ethyl acetate (50 mL). The organic layer was dried over anhydrous magnesium sulfate and concentrated in vacuo. The residue was purified using silica gel chromatography (hexane/ethyl acetate=1/1) on a silica gel column to form compound 2 (1.3 g, 85% yield) as an oil. 1H-NMR (200 MHz, CDCl3) δ ppm: 1.40–1.80 (8H, m), 2.04–2.32 (2H, m), 3.23 (2H, s), 5.58 (1H, s), 7.13–7.24 (5H, m), 7.56–7.64 (1H, m), 7.75–7.89 (2H, m), 8.13 (1H, d, J=8.4 Hz), 8.41 (1H, d, J=2.2 Hz), 9.16 (1H, d, J=2.2 Hz). MS m/z: 334 (M+), 301, 287, 253, 172, 156, 128, and 101.
1.4. 1′-quinolin-3-yl-4′H-spiro[cyclohexane-1,3′-isoquinoline] (3)Compound 3 of Fig. 1 was synthesized as follows. Sulfuric acid (0.4 mL) was added at 0°C to a solution of quinoline-3-carbonitrile (0.15 g, 1.0 mmol) in benzene (1 mL), and stirred at room temperature for 10 min. 1-benzylcyclohexanol (0.19 mg, 1.0 mmol) was added to the mixture and stirred at 80°C for 1 hr. With the resulting mixture being extracted with benzene (50 mL), the mixture was poured into ice water (50 mL). The organic layer was dried over anhydrous magnesium sulfate and concentrated in vacuo. The residue was purified using silica gel chromatography (hexane/ethyl acetate=1/1) on a preparative TLC plate to form compound 3 (0.12 g, 37% yield) as an oil. 1H-NMR (270 MHz, CDCl3) δ ppm: 1.51–1.54 (6H, m), 1.74–1.81 (4H, m), 2.85 (2H, s), 7.23–7.28 (3H, m), 7.37–7.42 (1H, m), 7.56 (1H, t, J=8.0 Hz), 7.75 (1H, t, J=8.0 Hz), 7.86 (1H, d, J=8.0 Hz), 8.15 (1H, d, J=8.0 Hz), 8.36 (1H, d, J=2.0 Hz), 9.18 (1H, d, J=2.0 Hz). MS m/z: 326 (M+), 283, 230, 128, and 115.
1.5. 3-(3,3-dimethyl-3,4-dihydroisoquinolin-1-yl)quinoline (4)To synthesize compound 4 of Figs. 1, 2-methyl-1-phenylpropan-2-ol (54 g, 0.36 mol) was added to a solution of quinoline-3-carbonitrile (48 g, 0.30 mol) in sulfuric acid (300 mL) at 0°C and stirred at room temperature for 1 hr. The mixture was then heated to 80°C and then stirred for 1 hr. The mixture was poured into ice water (500 mL) and the resulting mixture was washed with ethyl acetate (500 mL) to remove by-products. The aqueous layer was alkalized by aqueous ammonia and extracted using ethyl acetate (500 mL). The organic layer was dried over anhydrous magnesium sulfate and concentrated in vacuo. To the residue, diethyl ether was added and stirred for 10 min. Insoluble matter was filtered and the filtrate was concentrated in vacuo to generate compound 4 (44 g, 51% yield) as an oil. 1H-NMR (270 MHz, CDCl3) δ ppm: 1.33 (6H, s), 2.86 (2H, s), 7.20–7.27 (3H, m), 7.37–7.40 (1H, m), 7.56 (1H, t, J=8.4 Hz), 7.74 (1H, t, J=8.4 Hz), 7.86 (1H, d, J=8.4 Hz), 8.16 (1H, d, J=8.4 Hz), 8.39 (1H, d, J=2.0 Hz), 9.11 (1H, d, J=2.0 Hz). MS m/z: 286 (M+), 285, 271, 230, 128, and 115.
1.6. 3-(4,4-difluoro-3,3-dimethyl-3,4-dihydroisoquinolin-1-yl)quinoline (5)As a method for obtaining compound 5, a method of introducing fluorine at the 4-position was adopted. A suspension of chromic acid (8.0 g, 28 mmol) in acetic acid (5 mL) was added to a solution of 3-(3,3-dimethyl-3,4-dihydroisoquinolin-1-yl)quinoline (4.2 g, 42 mmol) in acetic acid (10 mL) and refluxed for 6 hr. The mixture was diluted with an aqueous sodium sulfite solution (200 mL), and extracted with ethyl acetate (200 mL). The organic layer was then washed with an aqueous sodium hydrogen carbonate solution (200 mL) and brine (200 mL). The organic layer was dried over anhydrous magnesium sulfate and concentrated in vacuo. The residue was purified using silica gel chromatograpy (hexane/ethyl acetate=1/1) on a silica gel column to generate 3,3-dimethyl-1-(quinolin-3-yl)isoquinolin-4(3H)-one (4.6 g, 55% yield) as colorless crystals. MP: 137°C. 1H-NMR (270 MHz, CDCl3) δ ppm: 1.62 (6H, s), 7.24–7.28 (1H, m), 7.38–7.43 (1H, m), 7.58–7.91 (5H, m), 8.20 (1H, d, J=7.1 Hz), 8.37 (1H, d, J=2.2 Hz), 9.11 (1H, d, J=2.2 Hz). MS m/z: 300 (M+), 285, 271, 257, 244, 231, 216, 189, 149, 128, 107, and 94.
Next, (diethylamino)sulfur trifluoride (6.0 mL, 61.5 mmol) was added to a solution of 3,3-dimethyl-1-(quinolin-3-yl)isoquinolin-4(3H)-one (4.6 g, 15.3 mmol) in toluene (5 mL), and the mixture was stirred at 70°C for 18 hr. The mixture was poured into ice water (200 mL) and extracted with ethyl acetate (200 mL). The organic layer was dried over anhydrous magnesium sulfate and concentrated in vacuo. The residue was purified using silica gel chromatography (hexane/ethyl acetate=1/1) on a silica gel column to generate compound 5 (0.42 g, 9% yield) as colorless crystals. MP: 105°C–109°C. 13C-NMR (100 MHz, CDCl3) δ ppm: 21.4, 60.3, 120.1, 124.1, 127.1, 127.2, 127.6, 127.7, 128.3, 129.3, 130.4, 130.7, 130.9, 131.5, 132.0, 136.2, 148.4, 150.1, and 160.9. 1H-NMR (500 MHz, CDCl3) δ ppm: 1.46 (6H, s), 7.34 (1H, d, J=7.7 Hz), 7.55 (1H, d, J=7.7 Hz), 7.61 (1H, ddd, J=1.1, 7.1, 8.2 Hz), 7.67 (1H, td, J=1.1, 7.7 Hz), 7.80 (1H, ddd, J=1.6, 7.1, 8.2 Hz), 7.87–7.90 (2H, m), 8.18 (1H, d, J=8.2 Hz), 8.40 (1H, d, J=2.2 Hz), 9.14 (1H, d, J=2.2 Hz). MS m/z: 322 (M+), 307, 287, 266, and 230.
1.7. Other derivativesThe other derivatives were synthesized using similar methods, and commercially available reagents and solvents were used. 1H-NMR spectrum data and MS data of the other derivatives are described in the Supplemental material.
2. Biological assay and photostability test 2.1. Rice blast (R.B.) control test (curative effects)Conidial suspensions of Pyricularia oryzae were sprayed onto potted test plants of Oryza sativa cv. Sachikaze, in the third to fourth leaf stage. The onset of disease was promoted by placing the pots in an inoculation room at 20°C–23°C. Test compounds were dissolved in a mixed solution of dimethyl sulfoxide and methanol (volume ratio: 7/3) to prepare the test solutions. Diluted test solutions were uniformly sprayed onto the pots. Seven days after inoculation, the minimum effective concentration of each compound was evaluated.
2.2. Tomato gray mold (G.M.) control test (preventive effects)Test compounds were dissolved in a mixed solution of dimethyl sulfoxide and methanol (volume ratio: 7/3) to prepare test solutions. Diluted test solutions were uniformly sprayed, in in the second–fourth leaf stage, onto potted tomato test plants (Solanum lycopersicum cv. Ogata Fukuju). To promote the onset of disease, 1 day after treatment, conidial suspensions of Botrytis cinerea were sprayed onto the pots in an inoculation room at 20°C–23°C. After 2 days of inoculation, the minimum effective concentration of each compound was evaluated.
2.3. Photostability testThe photostability of fungicides is an important metric because pesticides must be light-resistant to be effective in practical situations such as in the field. In this study, the following method was used to quantitatively measure photostability. A 25-cm-diameter Petri dish, was coated with a test substance (1.0 mmol) dissolved in acetone (10 mL) to form a thin film, then exposed to a black lamp for 18 hr. The thin film was dissolved in acetone, and the acetone solution was purified using silica gel chromatography (hexane/ethyl acetate=1/1) on a preparative TLC plate to isolate the starting material and the by-products.
3. Computational proceduresThe stable conformations of compound 2 were analyzed with density functional theory calculations. The structure for each conformation was optimized in vacuo using the GAUSSIAN-16 computational package.5) All of the calculations were performed at the B3LYP level using a 6-31+G (d) basis set.6) The zero-point vibrational energies and thermochemical parameters were estimated using frequency calculations. At 298.15 K and 1 atm, the relative Gibbs free energies of two chair conformations (Chair 1 and Chair 2) were calculated for cyclohexane (Fig. S1). The benzyl group was studied in three conformations: trans is defined as a structure in which the phenyl group is trans to the amide bond; gauche minus (g−) and gauche plus (g+) are structures in which the benzene faces the N–H side and the carbonyl side, respectively (Fig. S1). Other rotational bonds were optimized without restriction to achieve the most stable structure in each conformation.
Compound 1 was found to be effective against diseases of both susceptible strains. Next, the effects of resistant gray mold strains to fenhexamid, benzimidazoles, and dicarboximides were confirmed using their resistant fungi. As a result, compound 1 was chosen as the lead compound because it was effective against these resistant strains of gray mold.
As shown in Table 1, among the substituents at the first position of the cyclohexane ring, the benzyl substituent, compound 1g exhibited ten times higher fungicidal activity than compound 1 against both diseases. Other substituent derivatives (1a–1f) were inactive or had low activity.7,8)
|
|||
|---|---|---|---|
| No. | R | R.B. LC50 (ppm) |
G.M. LC50 (ppm) |
| 1 | Me | 100 | 300 |
| 1a | Et | 50 | >300 |
| 1b | n-Pr | >100 | >300 |
| 1c | i-Pr | >100 | 200 |
| 1d | Allyl | 100 | 200 |
| 1e | Ph | >100 | >300 |
| 1f | c-HexCH2 | >100 | >300 |
| 1g | Bn | 10 | 30 |
The effects of the cyclohexane-quinoline linkage on fungicidal activity are shown in Table 2. Similar activities were exhibited by the cyclohexane-1-carboxamide derivative, compound 1g, and the quinoline-3-carboxamide derivative, compound 2. In contrast, the derivatives with the other linkage types (2a–2f) lost their activity.9,10) The rigid conformations of amide bonds compared with those of other linkage types (2a–2f) suggest that the cyclohexane and quinoline rings may be taking a specific placement.
|
|||
|---|---|---|---|
| No. | L1–L2 | R.B. LC50 (ppm) |
G.M. LC50 (ppm) |
| 1g | CO–NH | 10 | 30 |
| 2a | CO–O | >100 | >300 |
| 2b | CH2NH | >100 | >300 |
| 2c | O–CO | >100 | >300 |
| 2d | CH2CO | >100 | >300 |
| 2e | CH2CH2 | >100 | >300 |
| 2f | CH=CH | >100 | >300 |
| 2 | NH–CO | 10 | 30 |
Compound 2 was efficiently synthesized by the Ritter reaction, in which alcohol and 3-cyanoquinoline react in the presence of a strong acid to form an amide compound (Method B) (Scheme 1).11)

Each of the compounds 2a–2f were prepared by a different synthesis method. The synthesis methods are described in the supplementary material.
3. The effect of the phenyl ring substituentsSubstituents were introduced into the phenyl ring using the synthesis method shown in Scheme 1. The evaluation results for compounds in which substituents are introduced into a phenyl ring (2xa–2xi) are presented in Table 3.9) The activity of 3-substituted phenyl compounds (2xb, 2xe, and 2xh) were typically greater than that of compounds substituted at other positions. Although fluorine groups were the most efficient substituent, they did not improve fungicidal activity.
|
|||
|---|---|---|---|
| No. | X | R.B. LC50 (ppm) |
G.M. LC50 (ppm) |
| 2 | H | 10 | 30 |
| 2xa | 2-Me | 30 | 200 |
| 2xb | 3-Me | 30 | 200 |
| 2xc | 4-Me | 100 | 100 |
| 2xd | 2-Cl | >100 | >300 |
| 2xe | 3-Cl | 30 | 300 |
| 2xf | 4-Cl | 30 | 300 |
| 2xg | 2-F | 10 | >300 |
| 2xh | 3-F | 10 | 50 |
| 2xi | 4-F | 10 | 50 |
Substituents were also introduced into the quinoline ring using the synthesis method described above, and the evaluation results of these compounds are shown in Table 4.9) Activities were low, with the exception of the 8-methyl and 8-fluorine groups. However, the activity of the compounds 2ye and 2yf did not exceed that of the unmodified compound 2.
|
|||
|---|---|---|---|
| No. | Y | R.B. LC50 (ppm) |
G.M. LC50 (ppm) |
| 2 | H | 10 | 30 |
| 2ya | 2-Me | >100 | >300 |
| 2yb | 4-Me | 100 | 100 |
| 2yc | 6-Me | 100 | >300 |
| 2yd | 6-F | >100 | >300 |
| 2ye | 8-Me | 10 | 50 |
| 2yf | 8-F | 10 | 50 |
| 2yg | 8-MeO | >100 | >300 |
Conformational analysis was therefore carried out to confirm an active conformation of compound 2 to improve its fungicidal activities by modifying its molecular structure. The benzyl group, which is necessary for high fungicidal activity, was most stabilized in the g− conformation in which the benzene faces the amide bond because of the steric influence of the cyclohexane ring (Table 5). We hypothesized that the gauche conformation, in which the benzyl group faces the amide bond, is beneficial for fungicidal activity, as stable conformations were thought to be taken at the active site. To demonstrate the validity of this hypothesis, and to cement the benzyl group in gauche conformation by constructing a benzyl- and amide-bonded ring structure system, we modified the core molecular structure of compound 2. In accordance with the conformational analysis, the g− conformation of compound 2 overlapped with the cyclic compound 3 in a favorable manner (Fig. S2).
| c-Hexa | Bnb | ΔG (kcal/mol) |
|---|---|---|
| Chair 1 | g− | 0 |
| g+ | 3.65 | |
| trans | 1.08 | |
| Chair 2 | g− | 1.43 |
| g+ | 5.21 | |
| trans | 5.84 |
a) In Chair 1 and Chair 2, benzyl group are in equatorial and axial position with respect to chair form cyclohexane, respectively.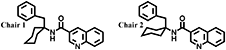
b) Trans is defined as a conformation in which amide is trans to phenyl group. g− and g+ are gauche conformations in which benzenes face the NH side and the carbonyl side, respectively. 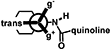
As shown in Table 6, the fungicidal activity of compound 3 is ten times lower than that of compound 2 but its activity is not entirely absent. In contrast, dimethyl derivative 4 showed higher activity against rice blast.12) The cyclohexane ring of compound 2 appears to be important to bring the benzyl group closer to the gauche position. When fixed through cyclization, hydrophobic groups such as the cyclohexane ring were rendered unnecessary, and smaller hydrophobic groups showed higher effects. These results demonstrate that our active conformation hypothesis was accurate.

|
||||
|---|---|---|---|---|
| No. | R1 | R2 | R.B. LC50(ppm) |
G.M. LC50 (ppm) |
| 3 | c-Hex | 100 | 300 | |
| 3a | c-Hep | 50 | 300 | |
| 3b | c-Pen | 3 | 200 | |
| 4 | Me | Me | 3 | >300 |
| 3c | Et | Et | 3 | >300 |
| 3d | Bn | Me | 10 | 30 |
| 3e | CF3CH2 | Me | 3 | 50 |
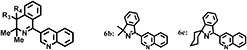
Finally, substituents at positions that had not been studied so far were examined. That is the effect of 4-position substituents of isoquinoline ring of compound 4 was investigated. As shown in Table 7, the presence of substituents at this position improved the fungicidal activity of the compound against gray mold.12,13) However, compounds with rings in R3 and R4 were less effective (4c and 4d). Pesticides must be light-resistant to be effective in practical situations. Unfortunately, the addition of aliphatic carbon to the 4-position of the isoquinoline ring decreased its photostability. In some compounds (4b and 4c), photodegraded products (6b and 6c) were observed wherein the dimethyl group at the 3-position was desorbed. The photodegraded products indicated radical cleavage in the isoquinoline ring and the production of a radical intermediate with a 4-positioned radical14) (Scheme S1). The introduction of fluorine atoms into R3 and R4 is therefore expected to reduce the stability of the radical intermediate and the photodegradability. In fact, difluoro derivative compound 5 possessed remarkably high photostability together with strong efficacy against both plant diseases.
|
||||||
|---|---|---|---|---|---|---|
| No. | R3 | R4 | R.B. LC50(ppm) |
G.M. LC50(ppm) |
Recovery (%) | By-product (%) |
| 4 | H | H | 3 | >300 | 83 | 0 |
| 4a | Me | H | 3 | 200 | 51 | 0 |
| 4b | Me | Me | 3 | 200 | 34 | 6b: 48 |
| 4c | c-Hex | >100 | >300 | 5 | 6c: 37 | |
| 4d | c-Pen | 30 | 100 | 44 | 0 | |
| 5 | F | F | 3 | 10 | 99 | 0 |
This study is the first presentation of novel quinoline derivatives for use against rice blast curatively and gray mold. Initially, we found compound 1 that is active in both diseases in screening. The effects of resistant gray mold strains of compound 1 were confirmed using their resistant fungi. Although, we evaluated substituent derivatives, the antifungal activity of the derivatives was not enhanced by introducing a substituent into a quinone or benzene ring or by altering the linkage types. Conformational analysis was carried out to confirm an active conformation of compound 2. Based on the results, compound 3, which cemented the conformation was prepared, but the activity decreased. Finally, compound 5 in which a difluoro group was introduced at the 4-position of the isoquinoline ring reached a high level of activity. The fungicidal activity against rice blast and gray mold can be increased to a practical level, leading to quinofumelin.
Quinofumelin showed preventive and curative activity against rice blast and gray mold. Furthermore, quinofumelin did not show cross-resistance to existing fungicides. Therefore, quinofumelin appears to be a new disease control solution in agricultural production.
We would like to express our deep gratitude to our colleagues at the Agrochemical Research Center of Mitsui Chemicals Agro Inc. for their professional and technical support in discovering a novel fungicide.
The online version of this article contains supplementary material, which is available at https://www.jstage.jst.go.jp/browse/jpestics/.