2023 年 48 巻 2 号 p. 61-64
2023 年 48 巻 2 号 p. 61-64
Cyclopropene derivatives have been used as extremely reactive units in organic chemistry owing to their high ring-strain energy. They have become popular reagents both for bioorthogonal chemistry and for chemical biology because of their small size and ability to be genetically encoded. In this context, we conducted an exploratory study to identify the biologically active cyclopropenes that affect normal plant growth. We synthesized several cycloprop-2-ene-1-carboxylic acid derivatives and evaluated their effects on the early growth stage of Arabidopsis thaliana. Eventually, we identified the chemicals that affect apical hook development in Arabidopsis thaliana. Their mode of action is different from those of ethylene receptor inhibition and gibberellin biosynthesis inhibition. We expect that some of the chemicals reported here can be new tools in chemical biology to determine useful molecular targets for herbicides or plant growth regulators.
Cyclopropene derivatives have been used as extremely reactive units in organic chemistry1) owing to their high ring-strain energy.2) They have become popular bioorthogonal reagents3) and chemical biological reagents4–7) because of their small size and ability to be genetically encoded. In addition, cyclopropenes are present in some naturally occurring acids. Malvalic acid and sterculic acid are used as causative agents of abnormalities in animals.8) Cycloprop-2-ene carboxylic acid is also a natural compound isolated from Asian toxic mushrooms as a causative agent for fatal rhabdomyolysis, a new type of mushroom poisoning.9) The physicochemical characteristics and biological activities of cyclopropene derivatives strongly suggest the usefulness of cyclopropenes as tools for chemical biological studies, which can dissect physiological and/or genetic events in plants. In this context, we expect that cyclopropenes could be major compounds for herbicides or plant growth regulators. However, there has been no report on the physiological activities of cyclopropenes in plants, except for the gaseous ethylene receptor inhibitor 1-methylcyclopropene (1-MCP).10) Thus, we conducted an exploratory study to identify the biologically active cyclopropenes that affect normal plant growth.
In this study, we used an in planta assay system to identify the chemicals that affect plant phenotypes. Cyclopropenes substituted with a carbonyl group at C1 are generally more stable than those with an alkyl functional group,7) and cyclopropenes with carboxy or alkoxycarbonyl groups are expected to be liquid or solid at ambient temperature. For these reasons, we focused on the cycloprop-2-ene carboxylic acid isolated from mushroom9) and prepared several cycloprop-2-ene-1-carboxylic acid derivatives. We evaluated their effects on the early growth stage of Arabidopsis and identified the chemicals that affect plant phenotypes.
We first synthesized the derivatives of ethyl 2-cyclopropenecarboxylate via the rhodium-catalyzed reaction of alkynes with α-diazo esters9,11) and named them TK compounds (Table 1).
 | |||
|---|---|---|---|
| R1 | R2 | R3 | |
| TK1 | -Et | -H | -CH2-O-Si(Me)2-t-Bu |
| TK2 | -Et | -H | -CH2OH |
| TK3 | -Et | -Me | -Me |
| TK3A | -H | -Me | -Me |
| TK4 | -Et | -H | -n-Pr |
| TK4A | -H | -H | -n-Pr |
| TK5 | -Et | -H | -benzyl |
| TK5A | -H | -H | -benzyl |
| TK6 | -Et | -H | -Me |
| TK6A | -H | -H | -Me |
| TK7 | -Et | -H | -Octyl |
| TK7A | -H | -H | -Octyl |
| TK8 | -Et | -Et | -Et |
| TK8A | -H | -Et | -Et |
| TK9 | -Et | -Me | -CH2Br |
| TK9A | -H | -Me | -CH2Br |
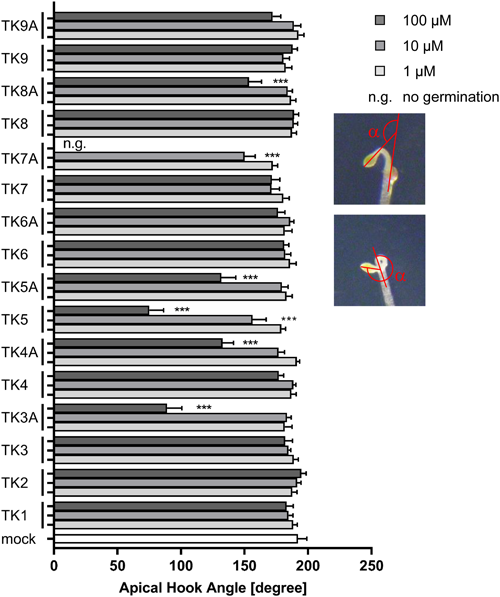
In the first screening, the effects of TKs (TK1–9) that included the ester group were evaluated by comparing the phenotype of TK-treated Arabidopsis grown under light and dark conditions with those of non-treated plants. Under the light condition, none of the compounds induced morphological changes (data not shown). Under dark conditions, none of the compounds induced morphological changes, except TK5, which has a benzyl group in its molecule and reduced the angle of apical hook curvature of Arabidopsis grown under the dark condition, as shown in Fig. 1. In this study, the angle of hook curvature (α) was defined as 180° minus the angle between the tangential of the apical part and the axis of the lower part of the hypocotyl, as previously reported.12) In the case of hook exaggeration, 180° plus that angle is defined as the angle of the hook curvature (see Fig. 1, inset). TK5 significantly inhibited hook development at a concentration of 10 µM. As hook development is an essential morphogenesis for normal plant development,13) we focused on the inhibitory effect of cyclopropene derivatives on hook development in Arabidopsis grown in the dark in subsequent experiments. Thereafter, TK3–TK9 were hydrolyzed in aqueous potassium hydroxide solution/methanol to obtain the corresponding carboxylic acids TK3A–TK9A, which were then subjected to the same biological assay as described above. Interestingly, TK3A, TK4A, TK5A, TK7A, and TK8A showed inhibitory activity on hook development. TK5A, a TK5 derivative with a benzyl group in its molecule, significantly inhibited hook development and showed the highest activity at 10 µM among the acids. The reason for the high activity of TK5A is not clear, but considering that TK3A, TK4A, and TK8A, which have lipophilic substituents, also showed significant activity, we can assume that some degree of lipophilicity should be important. In addition, the benzene ring of TK5A may contribute to the activity increase through, for example, π–π interactions. As reported above, we found that some cyclopropenes could induce morphological changes in Arabidopsis, but the underlying mechanisms are still unclear.
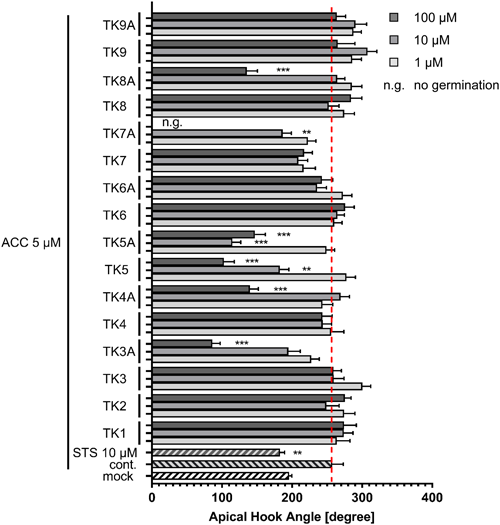
Several plant hormones affect apical hook development in the dark.14) Ethylene increases the angle of hook curvature of Arabidopsis grown in the dark, and in turn, gibberellin (GA) deficiency suppresses hook development in seedlings grown in the dark. To explore the involvement of plant hormones in the inhibition of hook development by TKs, we evaluated the involvement of TKs in ethylene signaling pathway. As shown in Fig. 2, treatment with 1-aminocyclopropane-1-carboxylate (ACC), an ethylene biosynthesis intermediate, increased the angle of hook curvature, and this morphological change was inhibited by the application of sodium thiosulfate (STS), an ethylene receptor inhibitor, as reported previously.15,16) In addition to STS, TKs, which inhibited hook development in WT plants (TK5, TK3A, TK4A, TK5A, TK7A, and K8A) inhibited ethylene-induced hook development, suggesting that these compounds could disturb ethylene signaling pathways and thus induce morphological changes. To further confirm the possibility of TKs’ inhibitory activity on hook development through the inhibition of ethylene function, we tested the effect of TKs on ctr1 (constitutive triple response 1) mutant, in which the ethylene signaling pathway downstream of the ethylene receptor is constitutively activated17) and making it insensitive to ethylene biosynthesis inhibitors and receptor inhibitors. The results demonstrated that ctr1 was insensitive to STS treatment, as shown in Fig. 3A, but was sensitive to treatment with active TKs (TK5, TK3A, TK4A, TK5A, TK7A, and K8A). Based on these results, we concluded that these active TKs could inhibit hook development not by inhibiting ethylene biosynthesis or ethylene receptors; however, there is still a possibility that TKs inhibited the ethylene signaling pathways downstream of CTR1.
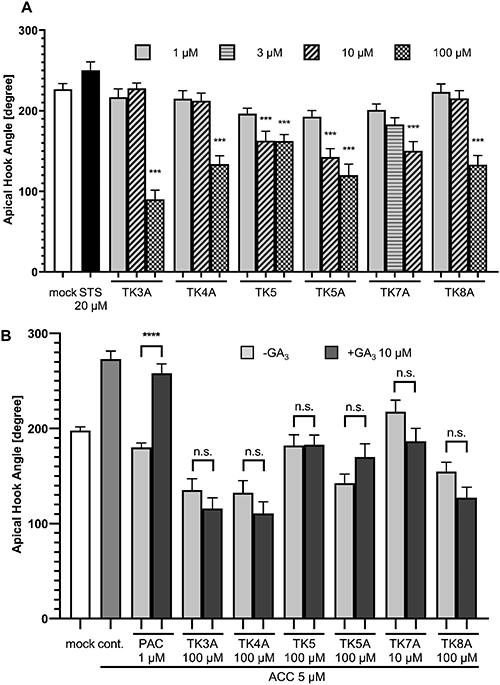
Afterward, we compared the effect of the gibberellin (GA) biosynthesis inhibitor paclobutrazol (PAC)18) with that of TKs on hook development, as shown in Fig. 3B. PAC treatment suppressed the effects of ACC treatment, and this suppression by PAC treatment was reversed by GA treatment. This result demonstrated that GA deficiency should inhibit the effect of ethylene (ACC) on the promotion of hook development and exogenous application of GA could reverse this hook formation due to GA deficiency. TKs treatment also suppressed the hook formation induced by ACC treatment, but in this case the hook formation was not reversed by GA treatment. This result strongly suggests that the promotive activity of TKs in hook development is independent of GA deficiency.
In this study, we reported our findings on the TK compounds that induce morphological changes in Arabidopsis. We also investigated the mechanism of action of TKs and found that ctr1 was insensitive to ethylene receptor inhibitor STS but sensitive to TKs, suggesting that their mode of action is different from that of ethylene receptor inhibitors. These results suggest that it is difficult to find a meaningful association between TK-induced morphology and the plant hormones ethylene and GA, although it is possible to think that the ethylene signal downstream could be controlled. Therefore, we expect that TKs can be new chemical biology tools for determining useful molecular targets for herbicides or plant growth regulators by identifying TK-insensitive mutants and their causal genes because they inhibit hook development, which is essential for normal plant growth.
Most of the ester-type TKs (TK3–TK4, TK5, TK7, TK8, and TK9) were synthesized in a step via the reaction of carbene from ethyl diazoacetate with the corresponding alkyne in the presence of rhodium (II) acetate dimer, as shown in the Supplementary materials. TK2 was synthesized from TK1 via the removal of t-butyldimethylsilyl (TBS) group with HF-pyridine treatment. TK6 was synthesized from the ethyl ester of 2-methyl-3-(trimethylsilyl)cycloprop-2-ene-1-carboxylate, which was synthesized by the coupling of α-diazo ester and 1-trimethylsilyl-1-propyne, via the removal of the TMS group with tetrabutylammonium fluoride (TBAF) in tetrahydrofuran (THF). All the synthesized 2-cyclopropene carboxylic acid derivatives had liquid or solid properties, as expected. Synthetic scheme and procedures are in Supplementary materials.
2. Apical hook angle assayAnalyses of apical hook angles were performed using etiolated Arabidopsis thaliana seedlings of the Columbia ecotype, as described by Vandenbusscche et al.12) Seeds were surface-sterilized with 70% ethanol for 20 min, placed on sterile filter paper to dry, and plated on half-strength MS media (pH 5.7) containing 1% sucrose and 0.8% agar. Seeds were stratified at 4°C in the dark for 3 day and exposed to fluorescent white light (20 μmol m−2 s−1) at 22°C for 3 hr prior to being grown vertically in the dark for 3 day at 22°C. The seedlings were then transferred to a transparent film, and their images were obtained. The apical hook angle was measured using the ImageJ software. The angle of hook curvature (α) was defined as 180° minus the angle between the tangent of the apical part and the axis of the lower part of the hypocotyl, as shown in Fig. 1.
3. Statistical analysisStatistical analyses were performed using ANOVA followed by Dunnett’s test using GraphPad Prism 9 (GraphPad Software, CA, USA). Statistical significance was defined as p<0.01 or 0.05.
This study was funded by the JSPS Grant-in-Aid for Scientific Research (grant number 18H05266 to T.A.).
The online version of this article contains supplementary material which is available at https://www.jstage.jst.go.jp/browse/jpestics/.