2023 年 48 巻 3 号 p. 111-115
2023 年 48 巻 3 号 p. 111-115
Synthesis of (+)-costic acid, isolated from Dittrichia viscosa (L.) W. Greuter as a natural acaricidal sesquiterpenoid, was achieved in 16 steps from (R)-carvone with an overall yield of 4.8%, involving the radical cyclization of selenoester to construct a decalone framework as the key step. Other structurally related natural products, (+)-costal, (+)-costol, and (+)-β-selinene, were also synthesized. The acaricidal activities of these four natural products and some synthetic intermediates were also evaluated against Varroa destructor. Among them, (+)-costal especially exhibited potent acaricidal activity.
Colonies of Apis mellifera (European honey bees), which are used as pollinators in agriculture, have been collapsing globally, threatening food production, ecosystems, and environmental conservation.1) Varroa destructor, an ectoparasitic mite of honey bees,2) is considered one of the main causes and now one of the most serious threats to the health and survival of honey bees.3,4) Treatments with synthetic chemicals and natural compounds are the most common control methods for V. destructor. Synthetic compounds such as coumaphos, amitraz, and fluvalinate show potent acaricidal activity and have been developed as effective acaricides.5) However, their long-term use has led to the emergence of many mites that have acquired drug resistance against synthetic compounds.6) Oxalic acid, formic acid, and thymol have been used as effective natural organic compounds to control V. destructor in practice.7–9) Although natural products with acaricidal activity are not as potent as the synthetic acaricides mentioned above, they have the advantage that drug-resistant mites are less likely to emerge.10) On the other hand, larval growth of A. mellifera is detrimentally affected by oxalic acid,11) and recent studies have hypothesized that V. destructor is symbiotic with bacteria that break down oxalic acid.12) Thymol does not adversely affect the growth of adult or larval honey bees, but its residues in honey may affect the taste of honey during and up to three months after its treatment.13)
Thus, there remains room for improvement in agents that control V. destructor, especially in terms of ecological stability, and many chemists have been attracted to new natural products with acaricidal activity. In 2017, Katerinopoulos et al. reported that (+)-costic acid [(+)-1] (also called β-costic acid : Fig. 1), isolated from Dittrichia viscosa (L.) W. Greuter, was found to exhibit potent acaricidal activity against V. destructor.14) The natural product (+)-1 is a sesquiterpenoid with a eudesmane skeleton and has been isolated from other species in the past (for details, see Ref. 14). Their group also synthesized analogues of (+)-1 that incorporate the structure of oxalic acid as a motif and reported that they exhibited more potent miticidal activity than natural product (+)-1.15) We were interested in the acaricidal activity of (+)-costal [(+)-2],16) (+)-costol [(+)-3],17) and (+)-β-selinene [(+)-4],18) which are also natural products with various degrees for the oxidation state of the carboxylic acid moiety of (+)-1. Surprisingly, however, an asymmetric synthesis of (+)-1, (+)-2, or (+)-3 has not yet been reported.19) Therefore, we decided to develop a route for asymmetric syntheses of these sesquiterpenoids and to elucidate the correlation between their acaricidal activity and the degree of oxidation state at the C12-position of (+)-1.
IR spectra were recorded by a Jasco FT/IR-4200 spectrometer using an ATR (ZnSe) attachment. 1H (400 MHz) and 13C NMR (100 MHz) data were recorded on Jeol JNM-ECS400. Mass spectra were obtained with AB SCIEX TripleTOF 5600. Kanto Chemical silica gel 60 (63–200 µm) was used for column chromatography unless otherwise stated. Analytical thin-layer chromatography was performed using Merck silica gel 60 F254 plates (0.25 mm thick). All air- or moisture-sensitive reactions were conducted under an argon atmosphere unless otherwise stated. Amitraz standard was purchased from Fujifilm Wako pure chemical Co. (Osaka Japan).
2. Synthesis of (+)-costic acid [(+)-1], (+)-costal [(+)-2], (+)-costol [(+)-3], and (+)-b-selinele [(+)-4]The natural compounds, (+)-1, (+)-2, (+)-3 and (+)-4, were synthesized from (R)-carvone in 12–16 steps via the radical cyclization of selenoester as shown in Scheme 2 and 3. Details of the experimental procedures are described in the Supporting Information.
3. Bioassay: Vapor-diffusion methodThis bioassay was carried out in accordance with some references.14,15)
The experiment was conducted in a completely randomized design under laboratory conditions in five replications. With the help of a magnifying glass, the isolated varroa was immediately placed in groups of five at the bottom of 30 mL glass vials. The compounds studied were used to make acetone solutions, with a concentration of 10 mg/mL, which were placed on filter paper fitted to the caps. Measurements were made using 60 µL doses, chosen as the optimal dose for costic acid activity. In some vials, 60 µL of acetone was applied as control (five replicates). Mites were placed on the bottom of the vials to avoid direct contact with the filter paper. Acetone was removed from the vials, to which H2O (20 µL) was added to maintain the necessary moisture levels. The vials were then sealed with varroa and left in a room 20°C. Mortality of mites was recorded under magnifying glass at 2 hr time intervals and assessed to detect subtle limb movement. Mites that were completely motionless (i.e., lack of appendage movement), while probing were considered dead.
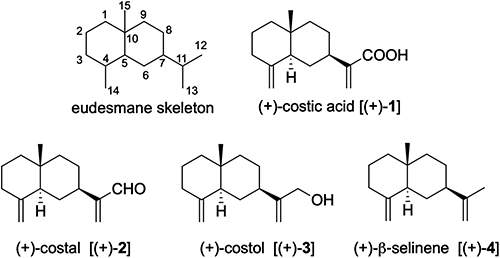
Scheme 1 outlines the retrosynthetic analysis of (+)-costic acid [(+)-1]. The natural products (+)-1, (+)-2, and (+)-3 could be synthesized from an eudesmane-type hydrocarbon, (+)-β-selinene [(+)-4], via stepwise oxidation at C12. The (+)-4 would be obtainable from selenoester 5 by radical cyclization,20) followed by Wittig olefination of the corresponding bicyclic ketone. The key intermediate 5 would be accessible from ester 6 by homologation and appropriate functional group transformation. The cyclohexenepropionate 6 would be transformed from a corresponding cyclohexanone 7 by a Bamford–Stevens reaction.21) The compound 7 would be synthesized from the known dihydrocarvone 8 by a diastereoselective Michael addition with methyl acrylate.

Synthetic studies of (+)-costic acid [(+)-1] commenced with the construction of the stereocenter at C10 (Scheme 2). A conjugate double bond of (R)-carvone [(R)-9]] was reduced by its treatment with zinc in the presence of KOH22) to afford dihydro carvone (8). An organocatalytic asymmetric Michael addition successfully affected the diastereoselective construction of the quaternary chiral center at C10 using thiourea organocatalyst A,23,24) furnishing ketoester 7 in a satisfactory yield (79%, 97.7% d.e.). Silica gel chromatography readily separated the undesired epimer of 7 at C10 (10-epi-7). Treatment of ketoester 7 with tosylhydrazine followed by exposure of the corresponding hydrazone progressed the Bamford–Stevens reaction, installing a double bond at C4 to afford 6. Subsequently, the side chain of 6 was homologated via a three-step transformation as follows. The ester moiety of 6 was reduced to the corresponding primary alcohol by LiAlH4, and its subsequent mesylation, followed by nucleophilic substitution with sodium cyanide, afforded 10 in excellent overall yield from 6. Exposure of 10 to aqueous NaOH affected the hydrolysis of the nitrile moiety to give acid, which, upon treatment with a mixture of tri-n-butylphosphine and diphenyl diselenide, was transformed into the corresponding selenoester 5 in excellent yield.
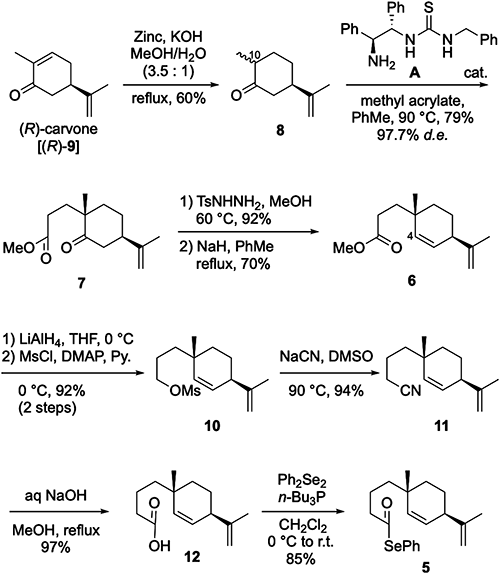
For the synthesis of (+)-costic acid [(+)-1] (Scheme 3), suitable conditions for the key transformation–radical cyclization–for selenoester 5 were realized through studies of reaction conditions using tri-n-butyltin hydride (n-Bu3SnH) in the presence of 2,2′-azibis(isobutyronitrile) (AIBN) in various solvents. The most suitable reaction conditions, viz., adding a solution of n-Bu3SnH and AIBN in benzene under reflux, promoted the desired radical cyclization, which furnished cis-fused decalone 13 accompanied by undesired aldehydes 14 and 15. cis-Decalone 13 was isomerized to trans-decalone 16 by exposure of 13 to a solution of NaOMe in methanol, and the subsequent Wittig olefination of 16 completed the synthesis of (+)-β-selinene [(+)-4] {[α]30D +61 (c 0.31, hexane), lit.25} [α]23D +49 (c 0.23, hexane)}. The 1H and 13C NMR data of (+)-4 were in good accordance with those of the natural product.26,27)
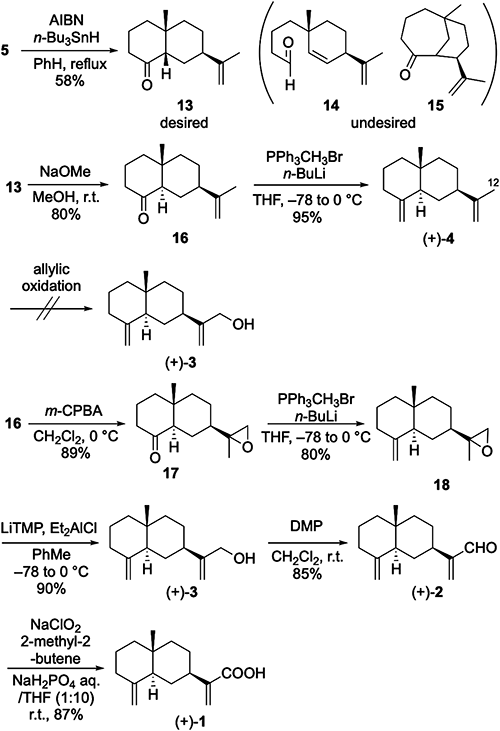
Having established the synthetic route to (+)-4, we turned our attention to its elaboration into three derivatives, (+)-1, (+)-2, and (+)-3, by the subsequent oxidation at C12 of (+)-4. However, direct oxidation using SeO2 or CrO3 resulted in a low yield of the desired oxidized product due to concomitant nonselective allylic oxidation. Therefore, we decided to improve the synthetic plan and consider the synthesis of 1–3 from trans-decalone 16. Treatment of 16 with m-CPBA brought about epoxidation to give 17 as a mixture of diastereomers at C11 in an excellent yield (diastereomer ratio=1 : 1). The subsequent Wittig olefination of 17 proceeded smoothly to afford 18 in 80% yield. Exposure of 18 to lithium tetramethylpiperidide (LiTMP), freshly prepared from 2,2,6,6-tetramethylpiperidine (2,2,6,6-TMP) and n-BuLi, in the presence of Et2AlCl28) gave rise to a ring-opening reaction of the epoxide group, furnishing (+)-costol [(+)-3 {[α]25D +34 (c 0.54, CHCl3), lit.26) [α]20D +31.5 (c 1.00, CHCl3)}] in an excellent yield of 90%. Oxidation of (+)-3 with DMP uneventfully afforded (+)-costal [(+)-2 {[α]25D +30 (c 0.53, CHCl3), lit.26) [α]20D +29.5 (c 1.00, CHCl3)}]. Finally, further oxidation of the aldehyde group of (+)-2 by a Pinnick oxidation completed the enantioselective synthesis of (+)-costic acid [(+)-1 {[α]25D +27 (c 0.51, CHCl3), lit.29} [α]30D +28 (c 0.665, CHCl3)}].
We assessed the acaricidal activities of natural products [(+)-1, (+)-2, (+)-3, and (+)-4] and two synthetic intermediates (16 and 18) against V. destructor under vapor-diffusion conditions. This bioassay was conducted following a previous report by Katerinopoulos et al.14,15) We found that all the compounds except (+)-3 exhibited acaricidal activities (Table 1). Notably, the compound (+)-2 was more potent than the lead natural product (+)-1, and acted as one of the most potent acaricidal agents among these samples. Focusing on the substructures common to (+)-1 and (+)-2, the α,β-unsaturated carbonyl structure may be important for the acaricidal activity. In addition, it is also worth mentioning that (+)-2 in particular begins to act faster than (+)-1. The higher activity of (+)-2 over (+)-1 may be due to the stronger electron-withdrawing of the aldehyde carbonyl group and/or its higher volatility. More interestingly, (+)-4 and epoxide 18 also exhibited quite potent acaricidal activity. The mechanism of action of (+)-4 and 18 may be different from those of (+)-1 and (+)-2, since the properties of the functional groups on (+)-4 and 18 are different from those of (+)-1 and (+)-2. However, the target and mechanism of acaricidal agents synthesized by us have not yet been clarified. Therefore, further studies are required to determine structure–activity relationships and elucidate the mechanism and possible targets.
| Compounds | Mortality (±S.E.) | ||
|---|---|---|---|
| 8 hr | 16 hr | 24 hr | |
| (+)-costic acid [(+)-1] | 16±4% | 48±5% | 80±6% |
| (+)-costal [(+)-2] | 48±5% | 100% | 100% |
| (+)-costol [(+)-3] | 0% | 12±5% | 28±8% |
| (+)-β-selinene [(+)-4] | 0% | 48±5% | 88±5% |
| ketone 16 | 0% | 56±4% | 92±5% |
| epoxide 18 | 28±5% | 100% | 100% |
| blank | 0% | 4±3% | 20±7% |
Synthesis of (+)-costic acid [(+)-1] was achieved in 16 steps from (R)-carvone [(R)-9] in 4.8% overall yield, which involves the radical cyclization of selenoester 5 as the key step. (+)-Costal [(+)-2], (+)-costol [(+)-3], and (+)-β-selinene [(+)-4] were also synthesized. Acaricidal activities of these four natural products and some synthetic intermediates (16 and 18) were evaluated against V. destructor. Among them, (+)-2 and epoxide 18 exhibited potent acaricidal activities. Synthetic efforts toward other members of this class of natural products and their synthetic analogues, including diastereomers of (+)-1, are being planned for more detailed structure–activity relationship studies on their acaricidal activity. These studies will be carried out and reported in due course.
We thank Dr. Atsushi Sakai and Mr. Ryutaro Natsusaka (Nippon Pesticide Co., Ltd.) for their useful advice on the bioassay. We are also grateful to Dr. Kazuo Furihata and Dr. Hiroyuki Watanabe (The University of Tokyo) for 1D and 2D NMR measurements. This work was financially supported by JSPS KAKENHI (No. 22K05449) and JST FOREST program (No. JMPJFR2203).
The online version of this article contains supplementary material, which is available at https://www.jstage.jst.go.jp/browse/jpestics/.