2023 年 48 巻 4 号 p. 156-167
2023 年 48 巻 4 号 p. 156-167
The excessive use of chemical pesticides in agricultural fields for controlling plant pathogenic microorganisms harms human health, the environment, and other beneficial microorganisms in the soil and plants. To address this challenge, it is essential to isolate and discover bioactive compounds from biological resources that could inhibit plant pathogenic microorganisms. In this study, the culture filtrate of the edible mushroom Pleurotus ostreatus was subjected to bioassay-guided isolation, and two phthalide derivatives—4,6-dimethoxyphthalide (1) and 5,7-dimethoxyphthalide (2)—were identified, along with an oxindole compound—3-hydroxy-3-methyloxindole (3). The inhibitory activities of the three compounds were evaluated against four fungal and five bacterial pathogens. Remarkably, 1 and 2 exhibited the lowest IC50 values against the conidial germination and germ tube elongation of the rice blast fungus Pyricularia oryzae. However, their effectiveness against bacterial pathogens was relatively low. The (S) and (R)-enantiomers of 3-hydroxy-3-methyloxindole showed different activities against plant fungal pathogens and bacterial plant pathogens.
Plant diseases caused by pathogenic microorganisms are one of the biggest challenges in the agricultural sectors, leading to substantial economic losses. To combat these diseases, farmers and agricultural producers rely on chemical pesticides owing to the difficulties of eliminating plant pathogenic microorganisms. While commercially available pesticides provide convenience and effectiveness, their continuous and excessive use has several disadvantages. These include pollution of the natural environment, which can negatively impact beneficial microorganisms associated with plants,1) and increasing consumer concerns about pesticide residue in agricultural products. Furthermore, the excessive use of chemical pesticides results in the development of resistance of pathogens to the pesticides. Thus, it is crucial to develop bio-ecofriendly solutions that effectively address the burden caused by pathogenic microorganisms while minimizing harm to beneficial organisms and the environment. A better approach to control and inhibit plant pathogenic microorganisms without compromising other beneficial organisms in the environment is the use of bioactive compounds from natural resources as they provide the advantages of having unique mechanisms and a wide range of biological activities.2)
Mushrooms represent one of the untapped natural bioresources with immense potential. Scientific studies have proven mushrooms as reservoirs of diverse bioactive metabolites, such as saponins, alkaloids, anthraquinones, anthrones, phenols, coumarins, fatty acids, and steroids.3–5) These metabolites possess different biological activities, such as hypoglycemic, antibacterial, antioxidant, and teratogenic.6,7) Additionally, mushroom metabolites exhibit plant growth promoting activities8) and inhibitory activities on tyrosinase.9) While mushrooms are tapped for nutritional benefits and therapeutic applications, they are also promising in sustainable agriculture as a source of secondary metabolites with inhibitory activities against plant pathogens. For instance, the compound grifolin isolated from the mushroom Albatrellus dispansus exhibited antifungal activity against nine plant pathogenic fungi.10) Similarly, the volatile compound isovelleral, isolated from three mushroom species Russula aff. anthracina, R. chloroides, and R. senecis had significant antifungal activity against Alternaria brassicicola, a pathogenic fungus responsible for a dark leaf spot in cabbage.11) Additionally, rufuslactone, a sesquiterpenoid, isolated from the mushroom Lactarius rufus, showed antifungal activity against Alternaria alternata, Alternaria brassicae, Botrytis cinerea, and Fusarium graminearum.12) Moreover, volatile compounds detected in edible mushrooms have antimicrobial activities against plant pathogenic bacteria and fungi.13,14) Given these studies, mushrooms represent excellent candidates for screening structurally diverse bioactive secondary metabolites that can be used for agricultural applications. Among the overall mushroom taxa recorded worldwide, approximately 20% (∼3,284 species) are considered edible.15) These figures call for further research into edible mushrooms as potential sources of biologically active compounds for agricultural applications, specifically for inhibiting the growth of plant pathogenic microorganisms. Their edibility further guarantees its safe use although safety testing of individual compounds is necessary. Thus, apart from their usual purpose as a source of food and medicine, edible mushrooms hold significant promise for agricultural advancements.
P. ostreatus, commonly known as oyster mushroom, is renowned for its oyster-like fruiting body.16) It is mainly found on living trees and decaying trunks,17) displaying characteristics of a wood-destroying saprophyte.18) Among edible mushrooms, P. ostreatus is the second most cultivated mushroom worldwide19,20) and the second most commercially available mushroom in many Asian countries.21) Its relatively short cultivation period compared to other edible mushrooms makes it ideal for cultivation.22) For several years, numerous studies have highlighted the diverse applications and benefits of P. ostreatus. Biotechnologically, it has been recognized as an efficient biosorbent for heavy metals in water.23) Nutritionally, it contains proteins, amino acids, minerals, vitamins,24) and dietary fiber.25) Medicinally, P. ostreatus has demonstrated antiviral properties,26) is an antioxidant,27) possesses antidiabetic potential,28) and exhibits hypolipidemic activities.29) These exceptional attributes made P. ostreatus an ideal mushroom for exploring compounds with promising biological activities.
Thus, herein, the culture filtrate extract of the edible mushroom P. ostreatus was subjected to bioassay-guided isolation and identified bioactive compounds that can inhibit plant pathogenic microorganisms. The findings of this study could add valuable insights into the potential of edible mushrooms as viable sources of biologically active compounds for agricultural application.
The electrospray ionization (ESI) mass spectra (MS) were recorded using a Quattro Micro API mass spectrometer (Waters, Milford, MA, USA) connected with an Acquity UPLC (Waters). The 1H NMR (600 MHz), 13C NMR (150 MHz), and 2D spectra were recorded using a JEOL JNM-ECZ600 (Tokyo, Japan). Preparative high-performance liquid chromatography (HPLC) was performed using a 10A HPLC system (Shimadzu, Kyoto, Japan). The specific rotation was measured using a SEPA-500 polarimeter (HORIBA, Kyoto, Japan).
2. ChemicalsPhthalide was purchased from Wako Pure Chemical (Osaka, Japan), while 4-methoxyisobenzofuran-1(3H)-one and 7-methoxyisobenzofuran-1(3H)-one were purchased from AmBeed, Inc. (Illinois, USA). Additionally, 5-methoxyisobenzofuran-1(3H)-one and 6-methoxyisobenzofuran-1(3H)-one were purchased from Accela ChemBio Inc. (California, USA) and Angene Chemicals (London, England), respectively. 4,6-Dimethoxyisobenzofuran-1(3H)-one was obtained from BLD Pharmatech Ltd. (Shanghai, China), while a sufficient amount of 5,7-dimethoxyisobenzofuran-1(3H)-one used for the assays was obtained from a colleague in the same laboratory who is also working on P. ostreatus. 3-Hydroxy-3-methyl-2,3-dihydro-1H-indol-2-one (S and R mixture) was purchased from Nacalai Tesque (Kyoto, Japan).
3. Mushroom strainThe strain of the edible mushroom P. ostreatus (TUFC 100534) was obtained from the Fungus/Mushroom Resource and Research Center (FMRC), Tottori University, Japan. It was inoculated and cultured on potato dextrose agar (PDA) (Difco™) slant and incubated at 25°C until further use.
4. Plant pathogenic fungi and bacteriaColletotrichum gloeosporioides MAFF 243178 (causative of anthracnose disease), Fusarium verticillioides MAFF 240087 (a major pathogen of cereals and corn causing ear and seedling rots), and Pyricularia oryzae MAFF 101512 (causative of rice blast) were obtained from the National Agriculture and Food Research Organization (NARO) Genebank (www.gene.affrc.go.jp/index_en.php). Strain of Fusarium oxysporum f. sp. cepae isolate 61 (causative of Fusarium basal rot in onion) was a fungal culture stock obtained from the Faculty of Agriculture, Tottori University. All fungal strains were sub-cultured on PDA slants and maintained at 25°C for further use.
Clavibacter michiganensis ssp. michiganensis NK-1 (causes bacterial canker disease in tomato) and Pectobacterium carotovorum ssp. carotovorum strain 39 (causes bacterial soft-rot disease in a wide variety of plant species) were isolate stocks from Field Science Center, Tottori University. Burkholderia glumae MAFF 106541 (causes bacterial panicle blight in rice), Xanthomonas oryzae pv. oryzae MAFF 210548 (causes bacterial leaf blight in rice), and Ralstonia solanacearum MAFF 107633 (causative agent of bacterial wilt in many crops) were obtained from the NARO Genebank. Bacterial isolates were sub-cultured in the following liquid culture media: Luria Bertani (LB) medium (10 g tryptone, 5 g yeast extract, 10 g NaCl, and 1 L distilled water) for B. glumae; yeast-peptone-glucose (YPG) medium (5 g yeast extract, 10 g peptone, 5 g glucose, and 1 L distilled water) for Cl. michiganensis ssp. michiganensis and Pe. carotovorum ssp. carotovorum; casamino acid-peptone-glucose (CPG) medium (1 g casamino acid, 10 g peptone, 5 g glucose, and 1 L distilled water) for R. solanacearum; semi-synthetic potato dextrose (SSPD) medium (300 g potato, 2 g Na2HPO4·12H2O, 0.5 g Ca(NO3)2·4H2O, 5 g peptone, 15 g sucrose, and 1 L distilled water) for X. oryzae pv. oryzae. The bacterial cultures were incubated for 12 to 16 hr at 25°C, and used to prepare glycerol stocks, which were preserved at −80°C until further use.
5. Determination of antifungal activityCo. gloeosporioides was first inoculated on a PDA plate, while F. oxysporum f. sp. cepae, and F. verticillioides were inoculated on a V8 juice media plate [200 mL V8 juice (Del Monte Quality Vegetable Juice, Nagano, Japan), 3 g CaCO3, 15 g agar at 1.5%, and 1 L distilled water]. The plates were incubated at 25°C for 7 to 10 days until active sporulation was observed. Py. oryzae was inoculated on oatmeal agar plate [50 g oatmeal (Melvit Secret Whole Rolled Oats, Warszawa, Poland), 20 g agar, 1 L distilled water] and incubated at 20°C for 14 days under a black light lamp (FL15BLB, Hitachi, Tokyo, Japan). Afterward, the aerial hyphae were removed by flooding the plate with distilled water containing 0.01% glucose and gently rubbing the surface with a spatula. The plate was further incubated for another three days at 25°C. To collect conidia, the surface of the fungal colony was flooded with distilled water containing 0.25% Tween 20 and gently rubbed using a spatula. The conidia were then filtered through four layers of cheesecloth. The conidial suspension was centrifuged twice at 1,200 rpm for 3 min to remove debris, and it was subsequently resuspended in distilled water containing 0.25% Tween 20. The conidial suspension was adjusted to approximately 5×105 conidia/mL using a cell counting chamber and immediately used for the antifungal activity experiment.
The bioassay to assess the inhibition of conidial germination followed the protocol described by Morimoto et al. (2018)30) and Kariya et al. (2020),31) with slight modifications. The test compound, dissolved in DMSO, was added to the conidial suspension to achieve the following final concentrations: 10, 30, 100, 300, and 1000 ppm. Three drops (25 µL) of the mixture were placed individually on a microscope glass slide coated with collodion. The slides were then placed in a container lined with tissue paper moistened with distilled water. Conidial suspension with 1% DMSO served as a control because the final concentration of DMSO in the test solutions were fixed at 1%. All the containers were incubated at 25°C in the dark for 24 hr. After incubation, the germinated and ungerminated conidia were counted under a compound microscope (Olympus BX43, Tokyo, Japan). Photographs of the conidia were captured, and the length of germ tube elongation was measured using the ImageJ software (https://imagej.nih.gov/ij/index.html).
6. Determination of antibacterial activityInitially, 250 µL of glycerol stocks of five plant pathogenic bacteria were inoculated in a 50 mL conical tube containing 5 mL of respective liquid culture medium. LB medium was used for B. glumae, YPG medium for Cl. michiganensis ssp. michiganensis and Pe. carotovorum ssp. carotovorum, CPG medium for R. solanacearum, and SSPD medium for X. oryzae pv. oryzae. The tubes were then incubated at 25°C with shaking at 100 rpm for 12 to 16 hr. To prepare bacterial suspensions, 1 mL of the culture was transferred to a new 50 mL conical tube containing 9 mL of liquid culture medium. The absorbance (OD600) of bacterial suspensions was measured using a UV-Vis spectrophotometer (Infinite M200 PRO, Grödig, Austria). The OD600 was adjusted to a range of 0.02 to 0.04 for B. glumae and X. oryzae pv. oryzae, and 0.06 to 0.08 for Cl. michiganensis ssp. michiganensis, Pe. carotovorum ssp. carotovorum, and R. solanacearum by adding culture medium as needed. The absorbance of the liquid culture medium was also measured and recorded as blank.
The antibacterial activity was determined using the growth inhibition assay following the protocol described by Morimoto et al. (2018)30) with slight modifications. The test compound, dissolved in DMSO, was added to the bacterial suspension to obtain the following final concentrations: 10, 30, 100, 300, and 1000 ppm. The mixture was then incubated at 25°C with shaking at 100 rpm. The duration of incubation was determined based on the growth curve of each of the bacteria, which was optimized before the actual assay (Supplemental Fig. S7). After incubation, the absorbance (OD600) was measured using a UV-Vis spectrophotometer during the exponential growth phase of each bacterium. A bacterial suspension containing 1% DMSO was used as a control because the final concentration of DMSO in the test solutions were fixed at 1%. The inhibition rate was computed following the formula:
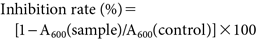 |
Where A600(sample) is the increase in absorbance of the bacterial suspension treated with the compound after incubation, whereas A600(control) is the absorbance of the bacterial suspension with 1% DMSO.
7. Bioassay-guided isolation of two phthalide derivatives and an oxindole compound from the culture filtrate of P. ostreatusThe isolation of two phthalide derivatives and an oxindole compound was guided with the antifungal bioassay against the four plant pathogenic fungi. P. ostreatus was first grown in a malt extract medium plate (50 g malt grain, 30 g glucose, 3 g peptone, 15 g agar, and 1 L tap water) for 14 days at 25°C. After incubation, three mycelial blocks each measuring ∼6 mm in size, were inoculated into a 500-mL Erlenmeyer flask containing 200 mL of malt extract liquid medium (50 g malt grain, 30 g glucose, 3 g peptone, and 1 L tap water). A total of 3 L equally divided into 15 flasks were prepared and incubated at 25°C for 46 days or until the surface of the medium was fully covered with the growth of P. ostreatus mycelia. The contents of the 15 flasks were combined and filtered through layers of Whatman filter paper no. 1 and cheesecloth. The resulting culture filtrate was extracted thrice with 1 L of ethyl acetate (EtOAc) and the EtOAc layer obtained was then dehydrated over Na2SO4 overnight and evaporated to dryness.
The culture filtrate extract (350 mg) was subjected to silica gel column chromatography (17.5 g silica gel, Daisogel IR-60-63/210; Daiso, Osaka, Japan) using 350 mL of acetone and hexane mixtures as eluting solvent (0–100% acetone, 20% increment, v/v). The eluted fractions were evaporated to dryness and assayed for their ability to inhibit conidial germination. The strongest inhibitory activity was observed in the fractions eluted with 20% and 40% acetone. The 20% (81 mg) and 40% acetone (169.8 mg) fractions were individually applied to a silica gel column, with 4.05 and 8.5 g silica gel for 20% and 40% acetone fractions, respectively. Elution was performed using 40 and 85 mL EtOAc and hexane mixtures (0–100% EtOAc, 20% increment, v/v) for the 20% and 40% acetone fractions, respectively. Each eluted fraction was concentrated to dryness and subjected to inhibition of conidial germination assay against the four plant pathogenic fungi.
In the chromatographic fraction from the 20% acetone fraction, the fraction demonstrating the highest activity (EtOAc 20%) was subjected to preparative HPLC under the following conditions: Column: Cosmosil 5C18 AR-II 10 mm ID×250 mm; temperature: 40°C; flow rate: 3 mL/min; solvent: 50% acetonitrile; detection: 210 nm). The fraction corresponding to the peak obtained from the HPLC analysis was then evaporated to dryness, yielding 3.5 mg and regarded as compound 1.
Compound 1 (3.5 mg). Positive ESI-MS: m/z 195.1 [M+H]+, m/z 236.1 [M+H+CH3CN]+. 1H NMR (600 MHz, CDCl3): δH 6.92 (d, J=1.8 Hz); 6.67 (d, J=1.8 Hz, H); 5.19 (s, 2H); 3.86 (s, 3H); 3.85 (s, 3H). 13C NMR (150 MHz, CDCl3): δC 171.4 (C1); 162.6 (C6); 154.9 (C4); 128.4 (C8); 128 (C9); 104.9 (C5); 98.6 (C7); 68.2 (C3); 56.0 (C10); 55.7 (C11).
In the chromatographic fraction from the 40% acetone fraction, the fraction exhibiting the highest activity (60% EtOAc) (64 mg) was further fractionated using ODS column chromatography (3.2 g; Cosmosil 75C18-OPN, Nacalai Tesque, Kyoto, Japan) using 60 mL of methanol (MeOH) and water mixtures as eluting solvent (20–100% MeOH, 20% increment, v/v). The inhibitory activity was detected at the 20% MeOH fraction. The two major peaks were detected and isolated through preparative HPLC using the following conditions: Column: Cosmosil 5C18 AR-II 10ID×250 mm; temperature: 40°C; flow rate: 3 mL/min; solvent: 20% acetonitrile; detection: 210 nm). The peak at a retention time of 14.7 min yielded 4.0 mg and was assigned as compound 2, while the peak at a retention time of 11.5 min yielded 1.6 mg and was regarded as compound 3. Compound 2 was also isolated in our laboratory by a colleague working on P. ostreatus. The three isolated compounds were further tested for their antimicrobial potential against plant pathogenic microorganisms.
Compound 2 (4 mg). Positive ESI-MS: m/z 195.2 [M+H]+. 1H NMR (600 MHz, CDCl3): δH 6.47 (s, H); 6.42 (s, H); 5.16 (s, 2H); 3.94 (s, 3H); 3.88 (s, 3H). 13C NMR (150 MHz, CDCl3): δC 169.1 (C1); 166.9 (C5); 159.8 (C7); 151.8 (C9); 106.6 (C8); 98.9 (C6); 97.7 (C4); 68.7 (C3); 56.1 (C10); 56.0 (C11).
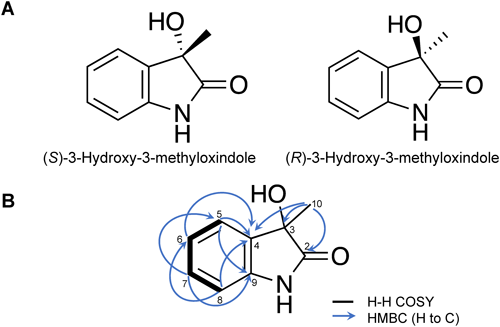
Compound 3 (1.6 mg) (S and R, 84 : 16 enantiomeric ratio). Positive ESI-MS: m/z 146.0 [M–OH]+. Negative ESI-MS: m/z 162.1 [M–H]−. [α]D20=−39.4° (c 0.0008 g/mL, CHCl3). 1H NMR (600 MHz, CDCl3): δH 7.39 (d, J=7.8 Hz); 7.26 (t, J=7.8 Hz); 7.08 (t, J=7.8 Hz); 6.87 (d, J=7.8 Hz), 1.6 (s, 3H, overlapped with H2O); 13C NMR (150 MHz, CDCl3): δC 180.0 (C2); 139.9 (C9); 131.6 (C4); 129.9 (C7); 124.0 (C5); 123.4 (C6); 110.1 (C8); 73.8 (C3); 24.9 (C10).
8. Optical resolution of 3-hydroxy-3-methyloxindoleA commercially available 3-hydroxy-3-methyloxindole was subjected to chiral HPLC. The chiral HPLC conditions were as follows: column: CHIRALPAK IA 4.6 mm ID×150 mm (Daicel Corporation, Tokyo, Japan); temperature: 40°C; flow rate: 0.8 mL/min; solvent: 20% MeOH; detection: 210 nm. The peak corresponding to (S)-3-hydroxy-3-methyloxindole was detected at retention time of 8.18 min, while (R)-3-hydroxy-3-methyloxindole was detected at retention time of 10.7 min. Their absolute configurations were determined by measuring optical rotation.
(S)-3-Hydroxy-3-methyloxindole, [α]D20=−58° (c=0.00175 g/mL, CHCl3); (R)-3-Hydroxy-3-methyloxindole [α]D20=+58° (c=0.00170 g/mL, CHCl3).
The isolation of antifungal compounds from the culture filtrate of P. ostreatus against plant pathogenic microorganisms, was guided with inhibition of conidial germination assay against plant pathogenic fungi. The fractions with the strongest activity against any of the four plant pathogenic fungi were further subjected to fractionation and isolation. The bioassay-guided fractionation resulted in the isolation and identification of three compounds.
Compound 1 was purified through two rounds of silica gel column chromatography and preparative HPLC. ESI-MS showed positive ions at m/z 195.1 [M+H]+ and m/z 236.1 [M+H+CH3CN]+. Combined with NMR spectra, these findings suggested the molecular formula of 1 as C10H10O4 with a molecular weight (MW) of 194, indicating hydrogen deficiency index of 6. The NMR spectra revealed a 1,2,3,5-tetrasubstituted benzene ring corresponding to six carbon signals (δC 98.6, 104.9, 128.0, 128.4, 154.9, and 162.6 ppm) and two doublet proton signals (δH 6.67 and 6.92 ppm, J=1.8 Hz). A carbon signal at δC 171.4 ppm corresponds to a carboxyl group. The methylene group, corresponding to the carbon signal at δC 68.2 ppm and the proton signal at δH 5.19 ppm, is linked to the oxygen atom of a carboxyl group and the benzene ring on the basis of the chemical shifts. Thus, we assumed a phthalide skeleton. Furthermore, proton signals at δH 3.85 and 3.86 ppm, along with carbon signals at δC 56.0 and 55.7 ppm, indicated the presence of two methoxy groups. The small coupling constant (J=1.8 Hz) of proton signals on the benzene ring indicated their location at meta positions on the benzene ring (Fig. 1, Supplemental Fig. S8). Based on these findings, we assumed 1 is either 4,6-dimethoxyphthalide or 5,7-dimethoxyphthalide. To confirm the identity, the obtained NMR data were compared with the data from these compounds in published literature, identifying 1 as 4,6-dimethoxyphthalide.32)

Compound 2 was purified through two rounds of silica gel column chromatography, ODS column chromatography, and preparative HPLC. ESI-MS detected a positive ion at m/z 195.1 [M+H]+, suggesting the molecular formula of 2 as C10H10O4 indicating a hydrogen deficiency index of 6. Similar to 1, a phthalide skeleton was confirmed. The 1H NMR signals at δH 3.94 and 3.88 ppm, along with carbon signals at δC 56.0 and 56.1 ppm, indicated the presence of two methoxy groups (Fig. 1, Supplemental Fig. S9). Compound 2 was identified to be 5,7-dimethoxy phthalide by comparing the NMR data with those in literature.33)
Furthermore, compound 3 was isolated from the same fraction as 2 but eluted at an earlier retention time in HPLC. Its molecular weight was suggested to be 163 as deduced from the positive and negative ions detected in ESI-MS: m/z 146.0 [M–OH]+ and m/z 162.1 [M–H]−, respectively. The molecular formula C9H9NO2 was suggested by the molecular weight and 1H and 13C NMR signals. The hydrogen deficiency index was calculated to be 6. The NMR spectra showed four aromatic proton signals at δH 6.87, 7.08, 7.26, and 7.39 ppm. Their coupling pattern indicated ortho-disubstitution of the benzene ring. A carbon signal at 180.0 ppm is an indicative of a carbonyl group, while a singlet at δH 1.6 ppm corresponded to a methyl group. In addition, a quaternary carbon at 73.8 ppm was detected. In the HMBC spectrum (Fig. 2B), cross-linkages of peaks were observed between the methyl group at δH 1.6 ppm and carbon signals at 73.8 ppm (C-3), 180.0 ppm (C-2), and 131.6 ppm (C-4). This determined the position of a methyl group at C-3. The chemical shift of the C-3 carbon indicated that an oxygen atom is also connected to C-3 carbon. Based on this information, the chemical structure of 3 was determined as an oxindole bearing a hydroxy group and a methyl group, both attached at the C-3 position (Fig. 1, Supplemental Fig. S10). The NMR data were identical to the data of 3-hydroxy-3-methyloxindole found in the literature.34) Then, 3 was subjected to chiral HPLC, which revealed the presence of two peaks corresponding to (S) and (R)-enantiomers (84 : 16 enantiomeric ratio based on peak area) (Fig. 2A, Supplemental Fig. S11). Due to the small amount of 3 isolated in this study, commercially available 3-hydroxy-3-methyloxindole was subjected to preparative HPLC to isolate the two enantiomers. Peaks corresponding to (S) and (R)-enantiomers were detected at 8.18 and 10.7 min, respectively. These two enantiomers were subjected to antifungal and antibacterial assays.
Compound 1 (4,6-dimethoxyphthalide) showed the strongest activity against the conidial germination of Py. oryzae with an IC50 value of 6.1 ppm (31.4 µM), followed by F. verticillioides with an IC50 of 89.5 (461.3 µM) ppm, F. oxysporum f. sp. cepae with an IC50 of 263.9 ppm (1,360.3 µM) and Co. gloeosporioides with the highest IC50 of 407.5 ppm (2,100.5 µM) (Table 1). These results were consistent with the IC50 values calculated for inhibitory activity against germ tube elongation, where 4,6-dimethoxyphthalide (1) displayed the strongest inhibitory activity against Py. oryzae with an IC50 value of 256.2 ppm (1,320.6 µM), followed by F. verticillioides (IC50=265.9 ppm) (1,370.6 µM) and F. oxysporum f. sp. cepae (IC50=676.8 ppm) (3,488.7 µM) (Table 1, Supplemental Fig. S1). However, the germ tube elongation of Co. gloeosporioides was not inhibited by >50%, even at 1000 ppm (32% inhibition rate) (Supplemental Fig. S2). Moreover, 5,7-dimethoxyphthalide (2) showed the strongest activity on conidial germination and germ tube elongation against Py. oryzae, with IC50 values of 28.5 ppm (146.9 µM) and 521.1 ppm (2,686.1 µM), respectively (Table 1). However, the germ tube elongation of Co. gloeosporioides and F. oxysporum f. sp. cepae was not inhibited by >50%, even at 1000 ppm (Supplemental Fig. S2). Based on the calculated IC50 values, all four plant pathogenic fungi showed relatively high sensitivity to both 4,6-dimethoxyphthalide (1) and 5,7-dimethoxyphthalide (2) in conidial germination than germ tube elongation.
| Compound | Plant pathogenic fungi | Conidial germination | Germ tube elongation |
|---|---|---|---|
| IC50 (ppm)* | IC50 (ppm)* | ||
| 4,6-Dimethoxyphthalide (1) | Colletotrichum gloeosporioides | 407.5±103.3 | >1000 (32%) |
| Fusarium oxysporum f. sp. cepae | 263.9±56.3 | 676.8±194.2 | |
| Fusarium verticillioides | 89.5±33.0 | 265.9±20.9 | |
| Pyricularia oryzae | 6.1±0.5 | 256.2±132.8 | |
| 5,7-Dimethoxyphthalide (2) | Colletotrichum gloeosporioides | 355.2±57.8 | >1000 (42%) |
| Fusarium oxysporum f. sp. cepae | 242.2±69.8 | >1000 (46%) | |
| Fusarium verticillioides | 44.7±37.6 | 756.9±125.8 | |
| Pyricularia oryzae | 28.5±4.7 | 521.1±44.8 | |
| (S)-3-Hydroxy-3-methyloxindole (3) | Colletotrichum gloeosporioides | 300.3±121.3 | 273.3±15.1 |
| Fusarium oxysporum f. sp. cepae | 93.4±60.2 | 566.2±61.7 | |
| Fusarium verticillioides | 17.3±4.6 | >1000 (49%) | |
| Pyricularia oryzae | 44.8±15.4 | 81±8.2 | |
| (R)-3-Hydroxy-3-methyloxindole (3) | Colletotrichum gloeosporioides | 21.5±5.3 | >1000 (48%) |
| Fusarium oxysporum f. sp. cepae | 745.8±50.1 | 835.7±86.6 | |
| Fusarium verticillioides | 20.4±3.1 | 83±4.1 | |
| Pyricularia oryzae | 73.7±9 | 860.1±72.3 |
*Values are means±S.D. (n=3). Values in parentheses are inhibition rates at 1000 ppm.
For 3-hydroxy-3-methyloxindole (3), the (S)-enantiomer showed the strongest inhibitory activity against the conidial germination of F. verticillioides with an IC50 value of 17.3 ppm (106.1 µM) among tested fungi, and its activity was slightly stronger than the (R)-enantiomer with an IC50 value of 20.4 ppm (125.2 µM) (Table 1). However, regarding germ tube elongation, the (R)-enantiomer (IC50=83 ppm) (509.2 µM) showed greater effectiveness than the (S)-enantiomer (49% inhibition rate at 1000 ppm) against the same fungus (Table 1, Supplemental Fig. S2). Interestingly, the results differed for Co. gloeosporioides, where the (S)-enantiomer showed relatively stronger inhibitory activity on germ tube elongation (IC50=273.3 ppm) (1,676.7 µM) than the (R)-enantiomer (48% inhibition rate at 1000 ppm) (Table 1, Supplemental Fig. S2). Conversely, against conidial germination, the (R)-enantiomer (IC50=21.5 ppm) (131.9 µM) exhibited more than ten times higher effectiveness than the (S)-enantiomer (IC50=300.3 ppm) (1,842.3 µM) (Table 1). Overall, the general data of IC50 values for both enantiomers suggested their effectiveness in inhibiting the conidial germination rather than the germ tube elongation of the tested fungi.
3. Antibacterial activityThe inhibition rate of each compound was determined based on the absorbance (OD600) of the bacterial suspension mixed with the compound during the logarithmic phase of each of the test bacteria. Compound 1 (4,6-dimethoxyphthalide) showed inhibitory activity against the growth of X. oryzae pv. oryzae and R. solanacearum with IC50 values of 440.7 ppm (2,271.6 µM) and 441.9 ppm (2,277.8 µM), respectively. However, B. glumae, Cl. michiganensis ssp. michiganensis, and Pe. carotovorum ssp. carotovorum were not inhibited by >50%, even at 1,000 ppm (Table 2). Compound 2 (5,7-dimethoxyphthalide) inhibited the growth of three bacterial pathogens with inhibition rates of >50% (Supplemental Fig. S3). It inhibited the growth of B. glumae (IC50=276.8 ppm) (1,426.8 µM), Pe. carotovorum ssp. carotovorum (IC50=278.8 ppm) (1,437.1 µM) and R. solanacearum (IC50=375.3 ppm) (1,934.5 µM) (Table 2).
| Compound | Plant pathogenic bacteria | IC50 (ppm)* |
|---|---|---|
| 4,6-Dimethoxyphthalide (1) | Burkholderia glumae | >1000 (45%) |
| Clavibacter michiganensis ssp. michiganensis | >1000 (13%) | |
| Pectobacterium carotovorum ssp. carotovorum | >1000 (34%) | |
| Ralstonia solanacearum | 441.9±360.5 | |
| Xanthomonas oryzae pv. oryzae | 440.7±44.2 | |
| 5,7-Dimethoxyphthalide (2) | Burkholderia glumae | 276.8±10.6 |
| Clavibacter michiganensis ssp. michiganensis | >1000 (45%) | |
| Pectobacterium carotovorum ssp. carotovorum | 278.8±20.2 | |
| Ralstonia solanacearum | 375.3±173.8 | |
| Xanthomonas oryzae pv. oryzae | >1000 (39%) | |
| (S)-3-Hydroxy-3-methyloxindole (3) | Burkholderia glumae | >1000 (21%) |
| Clavibacter michiganensis ssp. michiganensis | 523±5 | |
| Pectobacterium carotovorum | >1000 (19%) | |
| Ralstonia solanacearum | >1000 (47%) | |
| Xanthomonas oryzae pv. oryzae | >1000 (9%) | |
| (R)-3-Hydroxy-3-methyloxindole (3) | Burkholderia glumae | >1000 (27%) |
| Clavibacter michiganensis ssp. michiganensis | 836.9±18.3 | |
| Pectobacterium carotovorum | >1000 (15%) | |
| Ralstonia solanacearum | 502.8±29.3 | |
| Xanthomonas oryzae pv. oryzae | >1000 (17%) |
*Values are means±S.D. (n=3). Values in parentheses are inhibition rates at 1000 ppm.
Furthermore, both (S) and (R)-enantiomers of 3-hydroxy-3-methyloxindole could not inhibit the growth of gram-negative plant bacterial pathogens by >50%, while they were observed to inhibit the growth of the gram-positive bacterium, Cl. michiganensis ssp. michiganensis by >50% (Table 2, Supplemental Fig. S3).
4. Structure–activity relationship of substituted phthalidesThe structure–activity relationship of phthalides was evaluated specifically focusing on the importance of methoxy substitutions at the benzene ring. Compounds 1 (4,6-dimethoxyphthalide) and 2 (5,7-dimethoxyphthalide), containing two methoxy substituents attached at the aromatic ring, showed strong inhibitory activities against the conidial germination and germ tube elongation of Py. oryzae and F. verticillioides. To further confirm the importance of the number of methoxy substitutions at the benzene ring, closely related phthalide compounds with one methoxy substituent were tested. In terms of inhibitory activity on conidial germination, F. verticillioides showed the highest sensitivity among the tested fungi. In particular, phthalide and 4-methoxyphthalide showed the smallest IC50 values, 77.7 ppm (579.9 µM) and 41.3 ppm (251.8 µM), respectively. Relatively small IC50 values were also recorded for 4-methoxyphthalide against F. oxysporum f. sp. cepae (IC50=83.6 ppm) (509.8 µM) and for 6-methoxyphthalide against Fusarium verticillioides (IC50=79.8 ppm) (486.6 µM). All compounds did not exhibit strong inhibitory activity on Py. oryzae although 4,6-dimethoxyphthalide (1) and 5,7-dimethoxyphthalide (2) showed small IC50 values for the fungus (Table 3, Supplemental Fig. S4 and S5).
| Compound | Plant pathogenic fungi | Conidial germination | Germ tube elongation |
|---|---|---|---|
| IC50 (ppm)* | IC50 (ppm)* | ||
| Phthalide | Colletotrichum gloeosporioides | 601.9±99.7 | 824.3±31.9 |
| Fusarium oxysporum f. sp. cepae | >1000 (38%) | >1000 (37%) | |
| Fusarium verticillioides | 77.7±19.3 | >1000 (45%) | |
| Pyricularia oryzae | >1000 (8%) | >1000 (29%) | |
| 4-Methoxyphthalide | Colletotrichum gloeosporioides | 252.6±24 | >1000 (48%) |
| Fusarium oxysporum f. sp. cepae | >1000 (14%) | 83.6±7.4 | |
| Fusarium verticillioides | 41.3±15.8 | 479.9±226 | |
| Pyricularia oryzae | 663.7±23.7 | 529.3±46.5 | |
| 5-Methoxyphthalide | Colletotrichum gloeosporioides | 924.8±114.8 | 523.4±27.4 |
| Fusarium oxysporum f. sp. cepae | >1000 (23%) | 279.3±24.9 | |
| Fusarium verticillioides | 157.0±82.6 | 424.7±221.9 | |
| Pyricularia oryzae | >1000 (34%) | 518.7±50.7 | |
| 6-Methoxyphthalide | Colletotrichum gloeosporioides | 307.0±168.8 | 902.7±40.9 |
| Fusarium oxysporum f. sp. cepae | >1000 (21%) | >1000 (18%) | |
| Fusarium verticillioides | 487.8±214.5 | 79.8±40.8 | |
| Pyricularia oryzae | 512.1±66 | 177.1±13.6 | |
| 7-Methoxyphthalide | Colletotrichum gloeosporioides | 683.7±280 | 402.5±62.9 |
| Fusarium oxysporum f. sp. cepae | >1000 (25%) | 974.3±48.3 | |
| Fusarium verticillioides | 353.3±168.3 | 582.5±174.4 | |
| Pyricularia oryzae | 867.0±15 | 157.1±16.8 |
*Values are means±S.D. (n=3). Values in parentheses are inhibition rates at 1000 ppm.
Varying results were also observed for plant pathogenic bacteria. Phthalide (IC50=23.6 ppm) (176.1 µM), 4-methoxyphthalide (IC50=23.7 ppm) (144.5 µM) and 6-methoxyphthalide (IC50=24.8 ppm) (151.2 µM) demonstrated relatively strong inhibitory activity against the growth of X. oryzae pv. oryzae compared to 5-methoxyphthalide and 7-methoxyphthalide. This trend parallels the results observed for 4,6-dimethoxyphthalide (1) that possesses methoxy groups at 4 and 6 positions. Compound 4,6-dimethoxyphthalide (1) showed a lower IC50 value than 5,7-dimethoxyphthalide (2). The mono-methoxy phthalides and the unsubstituted phthalide showed higher inhibitory activity against four gram-negative bacteria, B. glumae, Pe. carotovorum ssp. carotovorum, R. solanacearum, and X. oryzae pv. oryzae, compared to the gram-positive bacterium, Cl. michiganensis ssp. michiganensis (Table 4, Supplemental Fig. S6). These results indicated the selectivity of mono-methoxy phthalides with methoxy substituents at the benzene ring in inhibiting fungal and bacterial pathogens.
| Compound | Plant pathogenic bacteria | IC50 (ppm)* |
|---|---|---|
| Phthalide | Burkholderia glumae | 327.0±0 |
| Clavibacter michiganensis ssp. michiganensis | >1000 (35%) | |
| Pectobacterium carotovorum ssp. carotovorum | 681.2±34.4 | |
| Ralstonia solanacearum | 560.9±24.4 | |
| Xanthomonas oryzae pv. oryzae | 23.6±0.6 | |
| 4-Methoxyphthalide | Burkholderia glumae | 118.5±6.4 |
| Clavibacter michiganensis ssp. michiganensis | >1000 (10%) | |
| Pectobacterium carotovorum ssp. carotovorum | 163.8±6.6 | |
| Ralstonia solanacearum | 337.4±94 | |
| Xanthomonas oryzae pv. oryzae | 23.7±0.1 | |
| 5-Methoxyphthalide | Burkholderia glumae | 212.0±4.1 |
| Clavibacter michiganensis ssp. michiganensis | 921.8±36.6 | |
| Pectobacterium carotovorum | 80.5±2.7 | |
| Ralstonia solanacearum | 295.6±33.7 | |
| Xanthomonas oryzae pv. oryzae | 470.5±7.8 | |
| 6-Methoxyphthalide | Burkholderia glumae | 469.7±9.2 |
| Clavibacter michiganensis ssp. michiganensis | >1000 (22%) | |
| Pectobacterium carotovorum | 26.5±0.1 | |
| Ralstonia solanacearum | 442.5±56.3 | |
| Xanthomonas oryzae pv. oryzae | 24.8±0.3 | |
| 7-Methoxyphthalide | Burkholderia glumae | 454.8±2.9 |
| Clavibacter michiganensis ssp. michiganensis | >1000 (27%) | |
| Pectobacterium carotovorum | 466.8±38.5 | |
| Ralstonia solanacearum | 449.6±72.6 | |
| Xanthomonas oryzae pv. oryzae | 236.1±4.3 |
*Values are means±S.D. (n=3). Values in parentheses are inhibition rates at 1000 ppm.
The bioassay-guided approach resulted in the isolation of two phthalide derivatives and an oxindole compound from the culture filtrate of P. ostreatus. Among them, two phthalide derivatives, 4,6-dimethoxyphthalide (1) and 5,7-dimethoxyphthalide (2), had been previously isolated from the same mushroom, P. ostreatus35) and other Pleurotus species such as Pleurotus cornucopiae.36) Previous studies have also reported the isolation of 4,6-dimethoxyphthalide from the mushroom Lignomyces vetlinianus32) and Coprinus comatus.37) On the other hand, 5,7-dimethoxyphthalide has been isolated from the mushroom Sparassis crispa38) and the fungus Scytalidium album.33) Despite relevant studies on the isolation of these phthalide compounds, their biological activities, particularly their potential applications in agriculture, have yet to be fully explored.
The present study showed the strong inhibitory activities of 4,6-dimethoxyphthalide (IC50=6.1 ppm) and 5,7-dimethoxyphthalide (IC50=28.5 ppm) against the conidial germination of Py. oryzae, the causative agent of rice blast disease. Additionally, these two phthalide derivatives have shown promising activity against F. verticillioides, a fungal pathogen responsible for ear rot in corn. The high selectivity of phthalides toward Py. oryzae and F. verticillioides indicates their potential for developing new selective fungicides as alarming fungicide resistance are reported in these and related fungal pathogens.39,40) Notably, both phthalide derivatives showed inhibitory activity against the germ tube elongation of all the tested fungal pathogens. However, all the tested fungi displayed greater sensitivity to both compounds on conidial germination than germ tube elongation, as evidenced by lower IC50 values for the inhibition of the conidial germination. This distinction indicates differences in the mechanism by which conidial germination and germ tube elongation are inhibited. Further investigation into the mode of action of these two phthalide derivatives could lead to identifying potential targets in the germination process, thus enhancing our understanding of their fungicidal properties.
Phthalides are widely distributed in plants and fungi41) and have been reported to offer significant biological activities, including antifungal,42,43) antibacterial,44) anti-inflammatory,45) anticancer,46) and cytotoxic activity.47) Phthalide derivatives, such as 5-(3′-carboxyl-3′-methyl-2E-allyloxy)-3-methoxy-4-methylphthalide, 5-(3′,3′-dimethylallyloxy)-3-methoxy-4-methylphthalide, 5-(3′,3′-dimethylallyloxy)-3-methoxy-4-methylphthalide, zinniol anhydride, and porriolide isolated from the fungus Pestalotiopsis photiniae, have shown antifungal activities against F. graminearum, B. cinerea, and Phytoptora nicotianae.42) Similarly, two phthalide derivatives, namely 7-hydroxy-4,6-dimethyl-3H-isobenzofuran-1-one and 6-formyl-4-methyl-7-methoxy-3H-isobenzofuran-1-one, isolated from the fungus Hypoxylon anthochroum, possessed inhibitory activities against F. oxysporum, A. alternata, Pythium aphanidermatum, and Phytophthora capsici.48)
Despite these studies, there are few relevant reports on the mode of action of phthalides as fungicides and bactericides. A study regarding the phthalide compound, 3-butylidene phthalide reported that its mode of action is the inhibition of melanin biosynthesis in the fungus Macrophomina phaseolina.49) Plant pathogenic fungi protect themselves against ultraviolet light, toxins, and other environmental factors through the production of melanin.50) Additionally, bacteria can produce several types of melanin important for their pathogenesis, survival in different types of environments, and interactions with other organisms.51) Recently, 3-butylidene phthalide was reported to inhibit the biosynthesis of ergosterol, resulting in damage to the fungal cell membrane.43) Moreover, the phytotoxicity of some phthalide compounds were also reported in the literature. The 3-butylidene phthalide exhibited phytotoxicity against the plant Lemna minor but non-toxic at concentration that inhibited the fungus M. phaseolina.49) Additionally, 5,7-dihydroxy-3(R)-methylphthalide isolated from the endophytic fungus Xylaria brevipes was also found to be phytotoxic.52) The effectiveness of 4,6-dimethoxyphthalide and 5,7-dimethoxyphthalide against fungal pathogens serves as baseline information for future studies aiming to elucidate the possible mode of action of these phthalide compounds.
The simple structure of two dimethoxyphthalides prompted us to examine the structure–activity relationship of phthalides with mono-methoxy substitutions on the aromatic ring. Based on the IC50 values, mono-methoxy phthalides exhibited lower activity compared to dimethoxyphthalides, specifically against two fungal pathogens, F. oxysporum f. sp. cepae and Py. oryzae, indicating the importance of having at least two methoxy groups on the benzene ring for optimal activity. Phthalide compounds with one or two methoxy substitutions at the aromatic ring showed selectivity in inhibiting plant pathogenic microorganisms, with varying inhibitory activities across different microorganisms. Previous studies primarily focused on the C-3 position of the lactone ring when exploring the structure–activity relationship of phthalides.53,54) However, the effect of methoxy substitutions at the aromatic ring of phthalide on fungi and bacteria has not yet been reported in the literature. The present study highlights the relevance of phthalide with methoxy substitutions at the aromatic ring and their antifungal and antibacterial activities. Further examining the activity of phthalides with a larger number of methoxy groups attached at the aromatic ring could be interesting in understanding the role of these methoxy groups. In addition, introducing other substituents to the benzene ring may provide the potential for strong antifungal activities.
The isolation of 3-hydroxy-3-methyloxindole (3) from P. ostreatus is one of the most interesting findings in this study. This compound was first isolated from human urine55) and in mice urine dosed with 3-methylindole, which was believed to be the possible metabolic product of 3-methylindole.56) This compound has also been reported as a product of the conversion of indole-3-acetic acid by plant enzymes57) and a possible catabolism product of beta-indolylacetic acid.58) However, these studies did not determine the stereochemistry of this compound. Previously, Chen et al. (2015) reported (S)-3-hydroxy-3-methyloxindole isolated from an endophytic fungus Hypoxylon sp. found in the plant Ilex formosana.34) This is the first and only isolation of this compound from a biological source other than mammals. The authors also reported the potent inhibitory activity of 3-hydroxy-3-methyloxindole on lipopolysaccharide-induced nitric oxide (NO) and interleukin-6 production in RAW 264.7 murine macrophages.
Enantiomerically oppositive compounds can be isolated from one species as either a racemic or scalemic mixture,59) and these enantiomers may possess different biological activities. For instance, the polyphenolic bissesquiterpene, gossypol isolated as mixture of (S)-gossypol and (R)-gossypol from cottonseed60) showed that (R)-gossypol had higher activity than (S)-gossypol as antifertility, antioxidant and anticancer.61) To the best of our knowledge, this is the first isolation of a mixed (S) and (R)-enantiomers (86 : 14 enantiomeric ratio) or a scalemic mixture of 3-hydroxy-3-methyloxindole from macrofungi, specifically the edible mushroom P. ostreatus, and second from a biological resource. Because of the rare isolation of this oxindole compound from natural resources, studies on its biological activities are limited. The present study tested the potential of the two enantiomers of 3-hydroxy-3-methyloxindole for their inhibitory effects on plant pathogenic microorganisms. Both (S) and (R)-enantiomers showed inhibitory activity against the conidial germination and germ tube elongation of the four plant pathogenic fungi. However, the two enantiomers showed different dose–response curves depending on the fungi, suggesting that despite their structural similarity, they possess different effects on fungal pathogens. Moreover, they were found to be less effective in inhibiting the growth of bacterial pathogens.
The present study reported a newly isolated oxindole compound from P. ostreatus and two known phthalide derivatives. Their inhibitory activities against plant pathogenic microorganisms may indicate their potential as alternatives to synthetic chemicals for agricultural applications. However, to fully establish their effects on plant pathogenic microorganisms, further in vivo studies are recommended.
Pleurotus ostreatus (TUFC100534) was provided by the Fungus/Mushroom Resource and Research Center, Tottori University with support in part by National BioResource Project (NBRP), AMED, Japan. E. M. C. is indebted to the Department of Science and Technology-Science Education Institute (Foreign Graduate Scholarship Program) of the Republic of the Philippines for the scholarship grant.
E. M. C., T. E. E. D. C and A. I. conceived and designed the research; E. M. C. and A. I. performed experiments; E. M. C., K. U., and A. I. analyzed data; E. M. C., and A. I. interpreted the results of the experiments; E. M. C. and A. I. prepared the figures; E. M. C., K. U., K. O., K. K., T. E. E. D. C and A. I. drafted, edited, and revised the manuscript; all authors approved the final version of the manuscript.
The online version of this article contains supplementary materials (Fig. S1–S11) which are available at https://www.jstage.jst.go.jp/browse/jpestics/.