2013 Volume 63 Issue 3 Pages 275-283
2013 Volume 63 Issue 3 Pages 275-283
Genetic transformation was successfully established producing both transformed adventitious shoots and calli in Japanese pear (Pyrus pyrifolia Nakai) by using cotyledons as explants. Cotyledons of five cultivars were co-cultivated with Agrobacterium tumefaciens strain LBA4404 carrying the pBIN19-sgfp, which contained a green fluorescent protein gene and the neomycin phosphotransferase gene. In order to increase transformation efficiency, sonication and ethylenedioxybis (ethylamine)-N,N,N′,N′-tetraacetic acid (EGTA) treatments were applied, which could produce physical wounds across the tissue and prevent plant defense reaction, respectively. Green fluorescent protein (GFP) fluorescence was evaluated two weeks and five months after Agrobacterium inoculation as measures of transient and stable transformations, respectively. As a result, sonication significantly increased both transient and stable expression of GFP fluorescence, whereas EGTA treatment did not show a positive effect on either. Out of 18 regenerated plantlets obtained, one plant regenerated from ‘Agenosho Shinanashi’ showed stable GFP fluorescence. This plant was confirmed as a transformant by PCR and genomic Southern blotting. Three other transformed regenerated shoots by myb gene showed red color, which were derived from ‘Imamuraaki’ by the same transformation method. Transformation system in this study was shown to be reproducible since plural transformants were obtained.
Pear (Pyrus spp.) is one of the most important deciduous fruit crops worldwide. Pear has been cultivated in Asia and Europe for at least two thousand years and is presently grown commercially in all of the temperate regions of the world, in more than 50 countries (Bell 1990, Bell et al. 1996). The genus Pyrus contains 22 widely recognized primary species, all indigenous to temperate Asia, Europe, or the mountainous areas of North Africa. The Japanese pear (Pyrus pyrifolia Nakai) is one of the most important fruit trees in Japan, China and Korea, while European pear (P. communis L.) is the main pear species of commerce in Europe, North America, South America, Africa and Australia (Bell et al. 1996).
Controlled hybridization and selection have been performed over the last 200 years (Bell 1990) and have been major contributors to pear breeding. Improvements in disease resistances and other characteristics have been achieved by selection from either indigenous or cultivated Japanese pear germplasms. In traditional breeding programs, recurrent backcrossing has sometimes been tried to introgress genes for desirable traits from wild or related species into cultivars, however, the long juvenile phase of Japanese pear is a discouraging to recurrently introgress important traits by cross-breeding. None of the major commercial Japanese pear cultivars are resistant to scab disease caused by Venturia nashicola (Bell et al. 1996, Ishii et al. 1992), but no scab symptoms were observed on the indigenous Japanese pear cultivar ‘Kinchaku’ (Terakami et al. 2006), the Chinese pear (P. bretschneideri Rehd.) ‘Hongli’ and ‘Mili’ and the European pear ‘Flemish Beauty’ and ‘La France’ (Ishii et al. 1992). Although it will be necessary to pyramid these resistance genes from different origins in order to introduce durable scab resistance, this approach may be impossible because of requiring recurrent hybridizations several times. Other attractive trait useful for stable fruit cultivation is parthenocarpy, which produces seedless fruits without fertilization, especially in self-incompatible species such as pears. Some European pears showed high parthenocarpic ability, whereas no or little parthenocarpy was observed for Asian pears (Nishitani et al. 2012b). However, it will take long time for achievement of parthenocarpy in Japanese pear introgressed from European pear. Alternatively, it is expected that genetic transformation can be utilized to introduce desirable genes and generate unique breeding materials.
Recently, some important genes have been identified related to fruit ripening and dormancy in Japanese pear. Nishitani et al. (2010) designed a custom oligoarray based on 9,812 independent ESTs from different tissues in Japanese pear and used it for comprehensive investigation of gene expression before and during ripening. Sixteen genes were identified that showed a remarkable expression increase of more than 100-fold during ripening, i.e., from 105 to 147 days after full bloom. Nishitani et al. (2012a) analyzed the transcriptome of Japanese pear ‘Kosui’ leaf buds during the dormancy transitional phases using a 10K cDNA microarray. Comparison of selected genes between ‘Kosui’ and the less-dormant Taiwanese pear ‘Hengshanli’ (TP-85–119) identified two novel transcription factors (NAC and PRR) whose expression varied concomitantly with the dormancy phase changes. The lack of genetic transformation system has hampered to identify the function of candidate genes related to important characteristics. In fundamental studies, genetic transformation can also be used for identification and validation of functional genes in Japanese pear.
To the present, genetic transformation system in Japanese pear is not established maybe due to low efficiencies of regeneration and Agrobacterium infection. Regeneration from cotyledons and in vitro leaves of European pear have been reported (Hennayake et al. 2003, Leblay et al. 1991). Leblay et al. (1991) reported a successful regeneration from in vitro leaves of European pear and the regeneration ratios were 76.7% in ‘Passe Crassane’, 70.8% in ‘Comice’, 62.1% in ‘Williams’ and 96.7% in ‘Conference’. According to Hennayake et al. (2003), regeneration ratios of in vitro leaves were 73% in ‘Conference’, 43% in ‘Comice’, 17% in ‘La France’ and 45% in ‘Max Red Bartlett’. Some studies on tissue cultures were reported using in vitro leaves of Japanese pear (Hennayake et al. 2003, Lane et al. 1998), however, regeneration remains unstable for this pear species. Lane et al. (1998) reported regeneration from leaves of Japanese pear, whose values were 21.9% in ‘Kosui’, 16.2% in ‘Nijisseiki’, 8.8% in ‘Hosui’ and 8.7% in ‘Chojuro’. According to Hennayake et al. (2003), regeneration frequencies were 57% in ‘Hosui’, 7% in ‘Kosui’ and 4% in ‘Shinsei’ from in vitro leaves. In many fruit tree species, although cotyledons are not genetically identical to their mother cultivars, juvenile tissues and organs such as cotyledons and immature ovules are better suited for regeneration than are older tissues (Pérez-Clemente et al. 2004). In our previous study, we developed an efficient method for adventitious shoot generation from cotyledons of Japanese pear and a high number of adventitious shoots per explant (1.3–2.3) and high rates of regeneration of adventitious shoots (60–76%) were obtained from the cotyledons of Japanese pears ‘Imamuraaki’ and ‘Agenosho Shinanashi’ (Nakajima et al. 2012).
Although Agrobacterium-mediated genetic transformation has been pursued for several cultivars of European pear (Gao et al. 2002, Kaneyoshi et al. 2001, Matsuda et al. 2005, Mourgues et al. 1996), the transformation rate is still low (about 1% to 5%), with a few exceptions (Mourgues et al. 1996, Sun et al. 2011). Transformation rates were noted as 2.7% for cotyledons of ‘La France’ and 5.2% for cotyledons of ‘Bartlett’ (Gao et al. 2002), and transformation efficiency was 3.2% for ‘Silver bell’ using in vitro leaf sections and 4.8% for ‘La France’ using axillary shoot meristems (Matsuda et al. 2005). Mourgues et al. (1996) reported that up to 42% of inoculated leaves produced transformed buds or bud clusters using wounded leaves of ‘Conference’, whereas 0.5% and 1.3% of inoculated leaves were transformed for ‘Doyenne du Comice’ and ‘Passe Crassane’, respectively. Until now, there have been no reports of genetic transformation of Japanese pear so far, even transgenic callus has not been reported. Franklin et al. (2008) described that cultured cells of Hypericum perforatum L. immediately turned darkening with Agrobacterium co-cultivation, which is a plant species recalcitrant to Agrobacterium-mediated transformation. In our preliminary experiment, since in vitro leaves of Japanese pear turned brownish after Agrobacterium co-cultivation, it is considered that recalcitrance to Agrobacterium infection may be the major reason for the difficulty of genetic transformation in Japanese pear. It is necessary to develop an efficient method for Agrobacterium infection on this species.
Hypericum perforatum L. showed an intense oxidative burst (a typical plant defense reaction), when its cells were infected with Agrobacterium (Franklin et al. 2008). Calcium ions are thought to be a signal of oxidative burst. Ethylenedioxybis (ethylamine)-N,N,N′,N′-tetraacetic acid (EGTA), a chelator of calcium, reduced the oxidative burst in potato tuber discs during the hypersensitive response to fungal infection or elicitor (Miura et al. 1995). Thus, the removal of calcium ions from the co-culture medium, the addition of EGTA or both could reduce the oxidative burst, possibly increasing the efficiency of Agrobacterium-mediated transformation. In addition, Santarém et al. (1998) reported that sonication of soybean explants during infection of Agrobacterium improved the efficiency of infection by introducing large numbers of micro-wounds into the target plant tissues. Therefore, treatment by sonication, alone or in combination with chelation of calcium ions by EGTA treatment, might increase the efficiency of transformation.
In the present study, we used the cotyledons of five Japanese pear cultivars for Agrobacterium-mediated transformation. We examined the effects of sonication and EGTA treatment by observation of transient and stable green fluorescence protein (GFP) fluorescence encoded by the green fluorescent protein gene.
As explants we used cotyledons from mature seeds of four Japanese pear cultivars, ‘Agenosho Shinanashi’, ‘Kosui’, ‘Natsushizuku’ and ‘Hakuteiryu’ and of ‘Ninomiya’, a hybrid cultivar of Japanese pear and European pear. All plant materials were maintained at the NARO Institute of Fruit Tree Science (Ibaraki, Japan).
Mature seeds were excised from open-pollinated fruits and then sterilized for 20 min in sodium hypochlorite solution (1% available chlorine) containing a few drops of Tween 20. The two layers of seed coat were peeled off and two-thirds of the cotyledons, without the hypocotyls, were excised. The cotyledons were bisected along the mid-rib. The pieces were soaked in the 1/10 MS basal medium (Murashige and Skoog 1962) excluding CaCl2·2H2O and containing 63 g L−1 sucrose, 36 g L−1 glucose and 10 mM 2-(N-morpholino) ethanesulfonic acid (MES), pH 5.8 (hereafter, C medium), until Agrobacterium infection.
Genetic transformationAgrobacterium tumefaciens strain LBA4404 harboring the binary plasmid pBIN19-sgfp was used for genetic transformation, which contained a green fluorescent protein gene (Fig. 1). pBIN19-sgfp contains the Nos promoter-NPTII (neomycin phosphotransferase gene)-Nos terminator cassette and the 35S promoter-sgfp-Nos terminator cassette (Ghorbel et al. 1999, Fig. 1). The sgfp gene was engineered to increase GFP expression by replacement of the serine at position 65 with a threonine (Chiu et al. 1996). Overnight-cultured Agrobacterium tumefaciens was collected by centrifugation and resuspended in 1/10 MS basal salts and vitamins excluding CaCl2·2H2O, 63 g L−1 sucrose, 36 g L−1 glucose, 10 mM MES, 0.01% pluronic F68 and 100 μM acetosyringone (pH 5.8) according to the method of Matsuda et al. (2005) for transformation of European pear, at a cell density of 1 × 108 cfu mL−1. The explants were removed from the C medium and soaked in the Agrobacterium suspension for 15 min. For the sonication treatment, cotyledons were placed in 50-ml tube containing 5 ml of the Agrobacterium suspension. The tube was gently suspended at the corner of a bath sonicator (Iuchi ultrasonic cleaning instrument US-2, 38-KHz frequency, Japan), which was run for 20 s. After sonication, the cotyledons were soaked in the Agrobacterium suspension for 15 min.
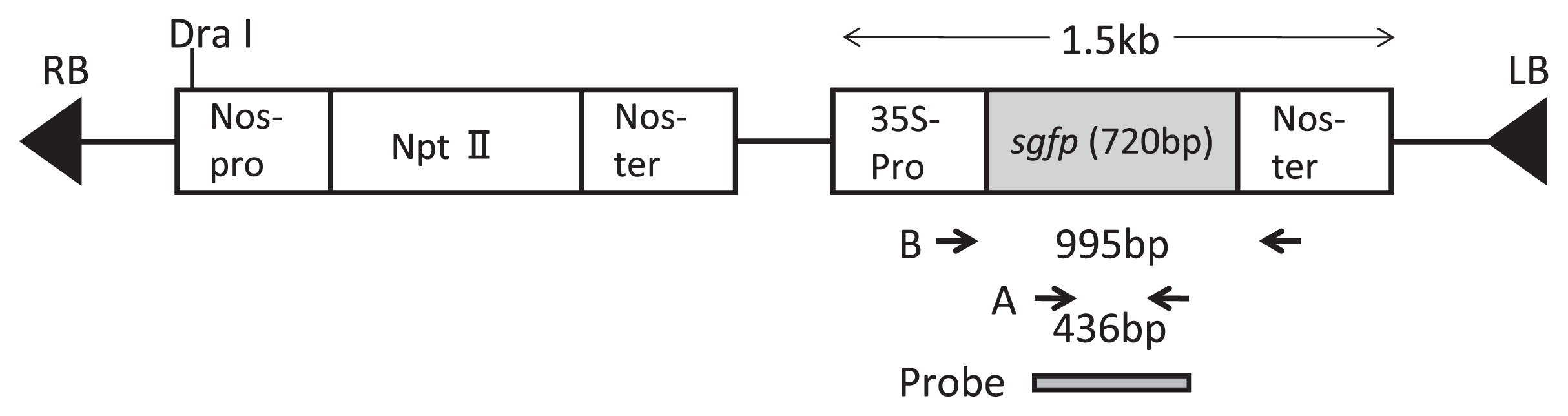
Schematic map of the T-DNA region of the pBIN19-sgfp plasmid, which contains NPTII and sgfp cassettes. The Dra I restriction site used for Southern analysis is shown above the map. Arrow pairs A and B indicate the primer pairs for PCR detection, and the box at the bottom of the diagram indicates the probe for genomic Southern analysis.
Explants were placed on a sterilized filter paper to remove the excess liquid and then placed on co-culture medium at 26°C in the dark for five days. Two kinds of co-culture medium were tested with ‘Kosui’ and ‘Hakuteiryu’, one with and one without 10 mM EGTA. Both media contained 1/10 MS basal salts according to Vega et al. (2008) excluding CaCl2·2H2O and containing 1/10 MS vitamins, 5 μM 1-naphthaleneacetic acid (NAA), 10 μM 6-benzylaminopurine (BA) [or 10 μM N-(1, 2, 3-thiadiazol-5-yl)-N′-phenylurea (thidiazuron) (TDZ)], 10 mM MES, 100 μM acetosyringone, 30 g L−1 sucrose and 0.85% agar (pH 5.8). For the other three genotypes, only the medium containing EGTA was used.
In our preliminary experiments, 5 mg L−1 of kanamycin caused shoot tips whitening in shoot tip culture, suggesting that the concentration was enough for selection in media. Therefore, explants were transferred to selection medium (MS medium containing 5 μM NAA, 10 μM BA (or 10 μM TDZ), 5 mg L−1 kanamycin, 200 mg L−1 cefotaxime, 30 g L−1 sucrose and 0.85% agar) for selection of transformants and elimination of Agrobacterium. The cultures were incubated at 26°C in the dark and subcultured once a month. Regenerated shoots were transferred to MS medium containing 0.1 μM NAA, 2.5 μM BA, 30 g L−1 sucrose, 0.85% agar, 5 mg L−1 kanamycin and 200 mg L−1 cefotaxime (pH 5.8) and grown under a 16/8-h (day/night) photoperiod.
In order to identify whether Agrobacterium tumefaciens exists within shoot tips and calli after elimination by antibiotics, two different tests were conducted, i.e., cultures of shoot tips and calli on LB medium, and PCR detection by Agrobacterium specific primers (VCF: 5′-ATCATTTGT AGCGACT-3′, VCR: 5′-AGCTCAAACCTGCTTC-3′) (Sawada et al. 1995).
Observation of GFP fluorescenceGFP expression of the cotyledons was scored at two weeks after Agrobacterium infection and again at five months after infection. GFP fluorescence was visualized by using an MZ FLIII stereo fluorescence microscope (Leica, Germany) with a 480/40-nm excitation filter and a 510-nm barrier filter.
DNA analysisPCR analysis and genomic Southern hybridization were conducted to detect the sgfp gene in callus and regenerated plants. Genomic DNA was extracted from calli and from in vitro leaves of regenerated plants by using a DNeasy Plant Mini Kit (Qiagen, Germany) according to the supplier’s instruction. Leaves of untransformed plants were collected from orchard trees and genomic DNA was extracted using a Genomic-tip 20/G (Qiagen, Germany) (Yamamoto et al. 2006).
A primer pair (5′-AGCTGACCCTGAAGTTCATC-3′ and 5′-GTGTTCTGCTGGTAGTGGTC-3′) corresponding to sgfp coding regions was used for PCR amplification to confirm the integration of the sgfp gene into regenerated plants (A in Fig. 1). The expected size of the amplified product was 436 bp (region A in Fig. 1). Another primer pair (5′-GATGTGATATCTCCACTGACGTAAG-3′ and 5′-GTA TAATTGCGGGACTCTAAT-3′ corresponding to the 35S promoter region and the Nos terminator region, respectively), was used to amplify 995-bp fragment of the 35S-sgfp-Nos chimeric gene (region B in Fig. 1). PCR amplification was performed in a total volume of 20 μL containing 10 ng genomic DNA, 0.5 μM each primer and 10 μL of GoTaq Colorless Master Mix, which contains GoTaq DNA polymerase (Promega, USA). For amplification of the 35S-sgfp-Nos chimeric gene fragment (B in Fig. 1), the solutions was heated to 95°C for 5 min; followed by 30 cycles of 1 min at 94°C, 1 min at 55°C and 1.5 min at 72°C; then 7 min at 72°C. For the sgfp gene internal fragment (A in Fig. 1), the conditions were 94°C for 5 min; followed by 35 cycles of 1 min at 94°C, 1 min at 60°C and 2 min at 72°C; then 7 min at 72°C. Both amplifications were performed with the GeneAmp PCR system 9700 (Applied Biosystems, USA).
For Southern hybridization, 5 μg of each genomic DNA sample was digested with Dra I (Nippon Gene, Japan). The digested DNA was separated by electrophoresis in 0.8% agarose gel at 25 V 15 h and blotted onto a nylon membrane (Hybond-N, Amersham, UK). The filter was probed with a DIG-labeled coding region of the sgfp gene (DIG-PCR labeling kit, Roche, Germany) and the same primer pair as used for PCR analysis of the sgfp coding regions (A in Fig. 1). Pre-hybridization (1 h) and hybridization (overnight) were carried out in a high-SDS hybridization buffer containing 50% formamide, 5× SSC (750 mM NaCl, 75 mM trisodium citrate, pH 7.0), 2% blocking solution (Roche, Germany), 0.1% lauroylsarcosine and 7% SDS at 42°C. After hybridization, the membrane was washed with 2× SSC and 0.1% SDS at room temperature for 15 min and then twice with 1× SSC and 0.1% SDS at 65°C for 15 min (Ban et al. 2007). The detection was performed by using DIG-CSPD system (Roche, Germany) according to the manufacturer’s instructions and the membrane was exposed to X-ray film (Fuji Photo Film, Japan).
Statistical analysisArcsine-transformed frequencies of cotyledons showing GFP fluorescence were statistically analyzed by two-factor ANOVA for all five cultivars and the two treatments tested with them (EGTA alone and EGTA plus sonication). Data were similarly analyzed by three-way ANOVA for ‘Kosui’ and ‘Hakuteiryu’ and the four treatments tested with them (sonication alone, EGTA alone, EGTA plus sonication and no treatment).
Genetic transformation of cotyledon explants by myb geneGenetic transformation system was re-examined by using different combination of cultivar ‘Imamuraaki’ and a myb gene, with sonication treatment. In our previous report, Japanese pear ‘Imamuraaki’ showed high rates of regeneration of adventitious shoots, which was similar values for ‘Agenosho Shinanashi’ (Nakajima et al. 2012). Open-pollinated fruits were harvested from trees of ‘Imamuraaki’ at 134 to 155 days after full bloom. Cotyledons were excised and inoculated for tissue cultures by the same methods above-described using four co-culture media, i.e., 1/10 MS, 1/10 MS-Ca, 1/10 MS + EGTA and 1/10 MS-Ca + EGTA.
Agrobacterium tumefaciens strain EHA 105 harboring the binary plasmid pBI121/VlmybA1–2 was used for genetic transformation. VlmybA1–2 is a myb-related gene isolated from mature-berry cDNA library of ‘Kyoho’ grape (Kobayashi et al. 2002, Koshita et al. 2008). Genetic transformation was conducted by the same methods above-described. Red color caused by myb gene expression in the cotyledons was scored by visual inspection as well as by observation using a stereo microscope (Nikon SMZ800, Japan) at five months after Agrobacterium tumefaciens infection.
A total of 497 cotyledons from the five cultivars were infected by Agrobacterium with or without sonication treatment; 168 cotyledons of ‘Hakuteiryu’, 133 of ‘Kosui’, 82 of ‘Agenosho Shinanashi’, 75 of ‘Natsushizuku’ and 39 of ‘Ninomiya’. By two weeks after Agrobacterium infection, the cotyledons were two to three times their original size and they had begun to produce callus. Spots of GFP fluorescence were observed on the surface of 24% to 85% of the cotyledons, depending on cultivar and treatments (Table 1). One month after infection, adventitious shoot–like organs (leaf–like organs without an apical meristem) began to emerge from the cotyledons. Two months after infection, many adventitious shoot–like organs were present and a few of them showed GFP fluorescence (Fig. 2A). Several of these organs had started to develop into adventitious shoots and the number of regenerated adventitious shoots gradually increased. At five months after infection, calli were observed on the surfaces of almost all of the cotyledons and about one-third to two-thirds of the calli showed GFP fluorescence. Few adventitious roots were observed. Finally, 18 of shoots were recovered from the cotyledons. No shoots or roots seem to have regenerated from calli. By this time, some cotyledons and calli had become brownish.
| Cultivar name | Treatment | No. of cotyledons inoculated | No. of cotyledons with transient GFP expression | Frequency of cotyledons with transient GFP expression (%) | |
|---|---|---|---|---|---|
| EGTA | Sonication | ||||
| Hakuteiryu | − | − | 40 | 16 | 40 |
| + | − | 38 | 12 | 32 | |
| − | + | 46 | 29 | 63 | |
| + | + | 44 | 34 | 77 | |
| (subtotal) | 168 | 91 | 54 | ||
| Kosui | − | − | 25 | 6 | 24 |
| + | − | 39 | 10 | 26 | |
| − | + | 29 | 19 | 66 | |
| + | + | 40 | 16 | 40 | |
| (subtotal) | 133 | 51 | 38 | ||
| Agenosho Shinanashi | − | − | nt* | nt | nt |
| + | − | 42 | 25 | 60 | |
| − | + | nt | nt | nt | |
| + | + | 40 | 34 | 85 | |
| (subtotal) | 82 | 59 | 72 | ||
| Natsushizuku | − | − | nt | nt | nt |
| + | − | 38 | 19 | 50 | |
| − | + | nt | nt | nt | |
| + | + | 37 | 29 | 78 | |
| (subtotal) | 75 | 48 | 64 | ||
| Ninomiya | − | − | nt | nt | nt |
| + | − | 18 | 11 | 61 | |
| − | + | nt | nt | nt | |
| + | + | 21 | 14 | 67 | |
| (subtotal) | 39 | 25 | 64 | ||
| Total | 497 | 274 | 55 | ||
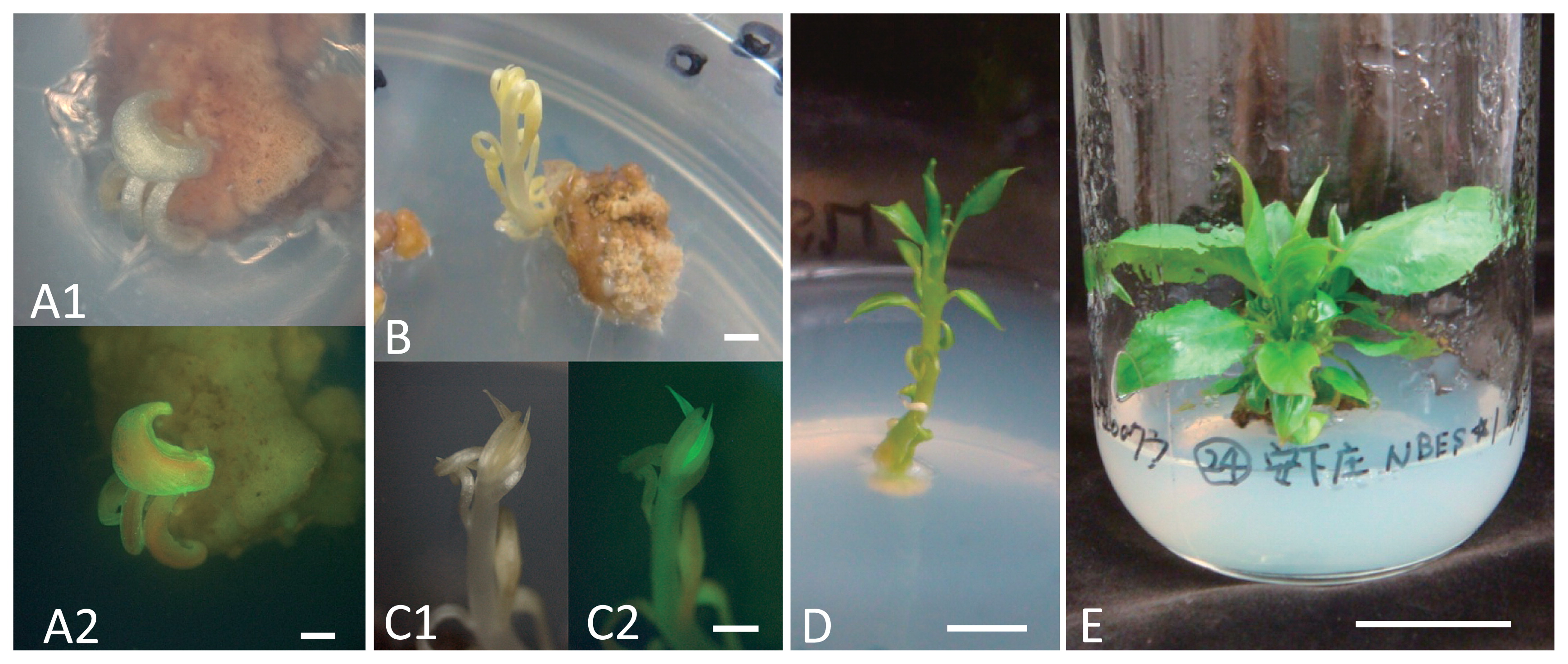
Regeneration of a shoot expressing GFP fluorescence and recovery of a transgenic plant from cotyledon explants of Japanese pear ‘Agenosho Shinanashi’. A1, A2: GFP-expressing adventitious shoot–like organ at 2.5 months after inoculation (A1: under white incandescent light, A2: under 480 nm-excited blue light), B: GFP-expressing adventitious shoot at 4.5 months after inoculation, C1, C2: Magnified view of B (C1: under white incandescent light, C2: under 480 nm blue light), D: GFP-expressing adventitious shoot incubated under the 16-h light/8-h dark (5.5 months after inoculation), E: GFP-expressing adventitious shoot. Scale bar on each picture represents 1 mm (A), 3 mm (B, C), 5 mm (D) and 2 cm (E).
Two weeks after inoculation, 91 out of 168 cotyledons of ‘Hakuteiryu’ (54%) showed GFP fluorescence, with the highest frequency (77%) in the EGTA plus sonication treatment (Table 1). Fifty-one out of 133 cotyledons of ‘Kosui’ (38%) showed GFP fluorescence, with the highest frequency (66%) in the sonication alone treatment. The average frequencies of cotyledons with transient GFP fluorescence among both cultivars were 32% with no treatment, 65% with sonication alone, 29% with EGTA alone and 59% with EGTA plus sonication. In a three-way ANOVA with factors of cultivar, EGTA treatment and sonication treatment, none of the three interactions were significant, so the sums of squares of those interactions were pooled with the error of sum of squares to increase the testing power. The ANOVA revealed that only the sonication treatment was significant at the 5% level.
Two weeks after inoculation, 59 out of 82 cotyledons (72%) of ‘Agenosho Shinanashi’ showed GFP fluorescence, with the highest frequency (85%) in the EGTA plus sonication treatment. Forty-eight out of 75 cotyledons (64%) of ‘Natsushizuku’ showed GFP fluorescence, with the highest frequency (78%) in the EGTA plus sonication treatment. Fourteen out of 21 cotyledons (67%) of ‘Ninomiya’ showed GFP fluorescence when treated with EGTA plus sonication and 61% did so when treated with EGTA alone.
Across the five cultivars, the average frequencies of cotyledons showing GFP fluorescence were 46% for EGTA alone and 69% for EGTA plus sonication (Table 1). Statistical analysis by two-factor ANOVA revealed that EGTA plus sonication treatment produced significantly more cotyledons with GFP fluorescence cotyledons than EGTA treatment alone at the 5% level and that there was no significant difference among cultivars.
Stable GFP fluorescence in cotyledonsThe numbers of cotyledons showing GFP fluorescence were evaluated five months after inoculation to estimate the frequency of stable genetic transformation (Table 2). Forty-three out of 147 cotyledons of ‘Hakuteiryu’, showed stable GFP fluorescence with the highest frequency (40%) in the sonication alone treatment. Forty-three out of 118 cotyledons of ‘Kosui’ did so, with the highest frequency (48%) again in the sonication treatment. The average frequencies of cotyledons with stable GFP fluorescence in both cultivars were 18% with no treatment, 34% with EGTA alone, 44% with sonication alone and 32% with EGTA plus sonication. Three-way ANOVA showed significant differences for sonication treatment and sonication × EGTA treatment at the 5% level, but no significant differences for cultivar, EGTA treatment, cultivar × EGTA, or cultivar × sonication.
| Cultivar name | Treatment | No. of cotyledons inoculated | No. of cotyledons with stable GFP expression | Frequency of cotyledons with stable GFP expression (%) | No. of cotyledons with regenerated shoots | No. of shoots with GFP fluorescence | |
|---|---|---|---|---|---|---|---|
| EGTA | Sonication | ||||||
| Hakuteiryu | − | − | 31 | 4 | 13 | 4 | 0 |
| + | − | 33 | 10 | 30 | 0 | 0 | |
| − | + | 42 | 17 | 40 | 4 | 0 | |
| + | + | 41 | 12 | 29 | 2 | 0 | |
| (subtotal) | 147 | 43 | 29 | 10 | 0 | ||
| Kosui | − | − | 22 | 5 | 23 | 1 | 0 |
| + | − | 35 | 13 | 37 | 0 | 0 | |
| − | + | 27 | 13 | 48 | 1 | 0 | |
| + | + | 34 | 12 | 35 | 0 | 0 | |
| (subtotal) | 118 | 43 | 36 | 2 | 0 | ||
| Agenosho Shinanashi | − | − | nt* | nt | nt | nt | nt |
| + | − | 32 | 2 | 6 | 3 | 0 | |
| − | + | nt | nt | nt | nt | nt | |
| + | + | 36 | 17 | 47 | 2 | 1 | |
| (subtotal) | 68 | 19 | 28 | 5 | 1 | ||
| Natsushizuku | − | − | nt | nt | nt | nt | nt |
| + | − | 38 | 14 | 37 | 1 | 0 | |
| − | + | nt | nt | nt | nt | nt | |
| + | + | 37 | 25 | 68 | 0 | 0 | |
| (subtotal) | 75 | 39 | 52 | 1 | 0 | ||
| Ninomiya | − | − | nt | nt | nt | nt | nt |
| + | − | 17 | 4 | 24 | 0 | 0 | |
| − | + | nt | nt | nt | nt | nt | |
| + | + | 21 | 13 | 62 | 0 | 0 | |
| (subtotal) | 38 | 17 | 45 | 0 | 0 | ||
|
| |||||||
| Total | 446 | 161 | 36 | 18 | 1 | ||
Nineteen out of 68 cotyledons of ‘Agenosho Shinanashi’ (28%) showed GFP fluorescence: 47% with EGTA plus sonication and of 6% with EGTA alone (Table 2). Thirty-nine out of 75 cotyledons of ‘Natsushizuku’ (52%) showed GFP fluorescence: 68% with EGTA plus sonication and 37% with EGTA alone. Seventeen out of 38 cotyledons of ‘Ninomiya’ (45%) showed GFP fluorescence: 62% with EGTA plus sonication and 24% with EGTA alone.
Across the five cultivars, the average frequencies of cotyledons with stable GFP were 48% with EGTA plus sonication and 27% with EGTA alone. Although EGTA plus sonication gave higher frequency of GFP fluorescence than EGTA treatment alone, two-factor ANOVA showed no significant differences between treatments or among cultivars.
Shoot regeneration and transformationFive months after inoculation, shoots were regenerated from ‘Hakuteiryu’ (10 shoots), ‘Kosui’ (2), ‘Agenosho Shinanashi’ (5) and ‘Natsushizuku’ (1), but none were obtained from ‘Ninomiya’ (Table 2). One shoot showing GFP fluorescence was obtained from ‘Agenosho Shinanashi’ (Fig. 2B, C1, C2, D); the other 17 shoots had no GFP fluorescence. Unrooted shoots (Fig. 2E) could be propagated by shoot-tip grafting.
DNA analysisPCR analysis was performed on GFP florescent calli and regenerated shoot to confirm the integration of T-DNA into the pear genome. Some GFP-fluorescent calli and one GFP fluorescent plantlet generated amplified products of the expected 0.4-kb in size corresponding to an internal region of the sgfp gene (Fig. 3) and of the expected 1-kb size when amplified with primers corresponding to the 35S promoter and Nos terminator regions (data not shown). Agrobacterium tumefaciens was not observed for cultures of GFP florescent calli and regenerated shoot on LB medium and Agrobacterium specific amplified band was not detected for calli and regenerant by PCR analysis, which showed that Agrobacterium tumefaciens was eliminated by antibiotics during selection process (data not shown). Therefore, we concluded that some calli and one regenerated plantlet were successfully transformed, and that the sgfp gene had stably integrated into the genome.
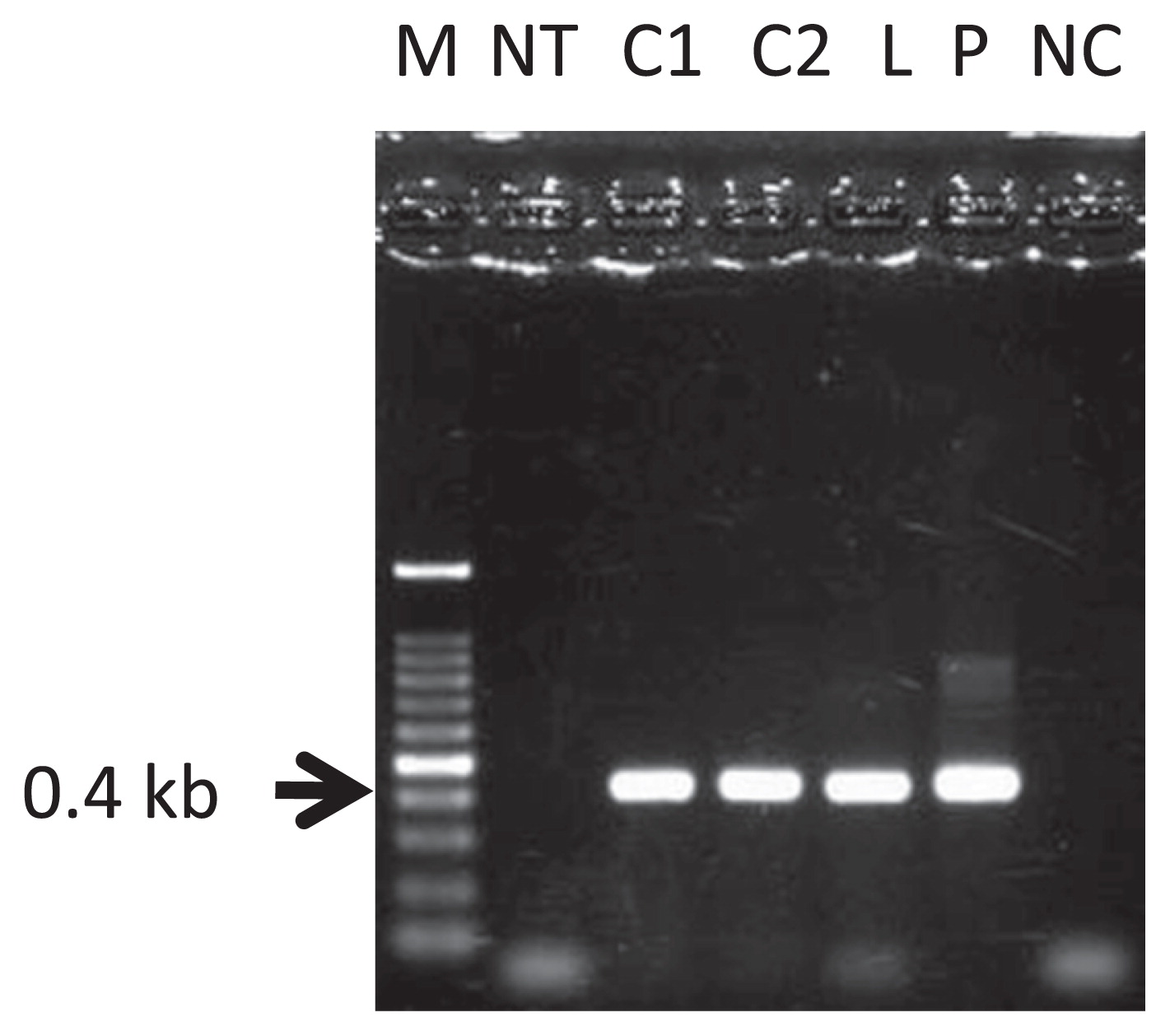
PCR analysis of internal region of the sgfp gene of GFP-fluorescent calli and shoot. Primers used correspond to “A” in Fig. 1. NT, untransformed plant; C1and C2, calli with GFP fluorescence; L, leaf from GFP-fluorescent shoot; P, plasmid; NC, no DNA (negative control); M, 100-bp ladder.
Genomic Southern hybridization was conducted with the one regenerated plantlet showing GFP fluorescence. When the DNA was cut with Dra I and probed with the sgfp gene (probe shown in Fig. 1), three bands of 10.5, 8.4 and 5.4 kb were detected (Fig. 4). Since only one Dra I restriction site exists within the 3.6 kb T-DNA (Fig. 1) and three hybridized bands detected with longer than 5.4 kb, this result indicates that three or more copies of the sgfp gene were stably integrated into the pear genome.
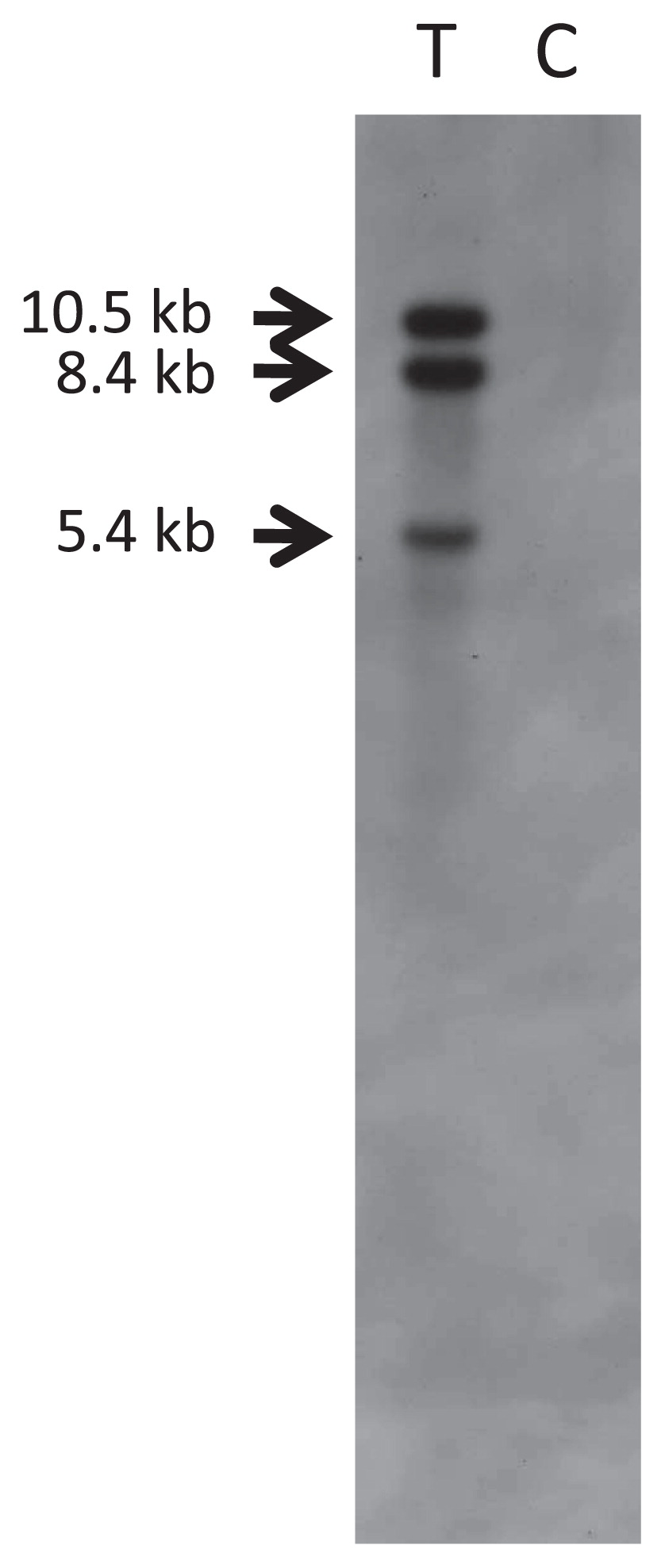
Southern hybridization analysis of the sgfp gene. The probe was a DIG-labeled segment of a 0.4-kb intragenic region of sgfp (probe shown in Fig. 1). T, GFP-fluorescent ‘Agenosho Shinanashi’ plant; C, untransformed plant.
A total of 1,014 cotyledons of ‘Imamuraaki’ were used for infection by Agrobacterium tumefaciens with sonication treatment. Cotyledons grew to two to three times to their original size by two weeks after infection and then began to produce callus. Several red spots were observed on the surface of the cotyledons. Several shoot initials began to develop into adventitious shoots, and the number of regenerated adventitious shoots gradually increased. At five months after infection, calli were observed on the surfaces of almost all cotyledons and about 5–11% of them showed red. Few adventitious roots were observed (Table 3). Finally, 58 regenerated shoots were obtained at five months after infection. No shoots or roots seemed to have regenerated from callus. Of these 58, three regenerated shoots were red.
| Co-culture medium | (a) No. of cotyledons inoculated | (b) No. of cotyledons showing red calli | (b/a × 100) Frequency (%) of cotyledons showing red calli | No. of regenerated adventitious shoot | No. of adventitious shoots showing red color |
|---|---|---|---|---|---|
| 1/10MS | 267 | 27 | 10.1 | 21 | 1 |
| 1/10MS-Ca | 251 | 19 | 7.6 | 12 | 1 |
| 1/10MS + EGTA | 255 | 28 | 11.0 | 14 | 1 |
| 1/10MS-Ca + EGTA | 241 | 12 | 5.0 | 11 | 0 |
| Total | 1014 | 86 | 8.4 | 58 | 3 |
To our knowledge, this report describes the first successful transformation of Japanese pear. In this study, sonication treatment significantly increased the frequencies of both transient and stable transformation of sgfp into cotyledons of Japanese pear. These results were in good accordance with the previous reports in soybean (Santarém et al. 1998), black locust (Zaragozá et al. 2004) and chickpea (Pathak and Hamzah 2008), which also showed increases in transformation frequencies when tissues were sonicated. Although plant cells have hard and thick cell walls, sonication can produce a large number of small and uniform wounds across the tissue, which allow Agrobacterium to more easily access to the target plant cells. Sonication allows the Agrobacterium to travel deeper and more completely throughout the tissue than normal co-cultivation, thus enhancing the bacterial colonization and infection of the tissue (Oliveira et al. 2009).
We obtained many calli and one shoot with GFP fluorescence. The GFP fluorescent shoot was regenerated not from GFP-fluorescent calli but directly from cotyledon. Furthermore, the GFP-fluorescent calli in this study did not generate shoots. According to Lane et al. (1998), shoot regeneration from in vitro leaves in Japanese pear was not preceded by visible callus growth. Out of 18 regenerated shoots obtained in this study, 17 did not show GFP fluorescence and were thus regarded as escapees from kanamycin selection. A higher concentration of kanamycin might decrease the number of escapees. The one transformed shoot was regenerated directly from a transformed part of the cotyledon, so a high rate of adventitious shoot regeneration will be necessary to establish an efficient transformation system. Three other transformed adventitious shoots were obtained using the same methods in Japanese pear ‘Imamuraaki’, which has high regeneration ability similar to ‘Agenosho Shinanashi’ (Nakajima et al. 2012). Although cotyledons of different cultivar ‘Imamuraaki’ were used for genetic transformation by Agrobacterium tumefaciens strain EHA 105 harboring myb gene, three transformants were obtained from 58 regenerated shoots. Since plural transformants were obtained, our transformation system seemed to be reproducible.
In this study, the obtained transformants were not true-to-type of mother cultivars, but hybrids between mother cultivar and unknown pollen parents. As the transformants were hybrids, it is difficult to improve a pinpointed phenotypic trait of original cultivar. However, transformation system using cotyledons can introduce desirable genes and generate unique breeding materials in Japanese pear, which will lead to breed new cultivars after one or more generation crossed with prominent cultivars. It is considered that the mother source seemed to have some influence on regeneration efficiency; for example, the cotyledons from ‘Imamuraaki’ and ‘Agenosho Shinanashi’ showed high shoot production. On the other hand, pollen parents might have some influence on regeneration and transformation. It will be interesting to identify the parent combination producing high shoot regeneration and genetic transformation.
Mase et al. (2007) reported that overexpression of Arabidopsis ENHANCER OF SHOOT REGENERATION 1 (ESR1) (also known as DORNRÖNSCHEN; DRN), which encodes a member of the ETHYLENE-RESPONSIVE FACTOR (ERF) family, enhanced shoot regeneration from root explants of Arabidopsis. Thus, the use of shoot regeneration enhancing genes may improve regeneration ability and the efficiency of Agrobacterium-mediated transformation. An intense oxidative burst was detected during Agrobacterium infection of Hypericum perforatum L., which may explain the recalcitrance of this species to Agrobacterium-mediated transformation (Franklin et al. 2008). EGTA and calcium-ion-channel blockers reduced the generation of active oxygen substances induced by elicitor-treatment in a dose-dependent manner in potato tuber discs (Miura et al. 1995). In the present study, EGTA had no significant effects on either transient or stable transformation of Japanese pear cotyledons; this difference might be due to the different of plant species used in the two studies.
To the best of our knowledge, this is the first report of the induction of genetic transformed calli and regeneration of a transformed plantlet of Japanese pear. This result is an important step toward functional analysis of the genes of interest in Japanese pear and accelerating improvement of Japanese pear cultivars by genetic transformation.
We thank Drs. H. Iketani, A. Ito and T. Imai for valuable discussion and suggestions, and Drs. S. Komazaki and M. Yamada for statistical analysis. We also thank Mss. M. Kimura, T. Akashi and M. Kimura for their technical help. This work was supported in part by a Grant-in-Aid for Challenging Exploratory Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan.