2018 Volume 68 Issue 2 Pages 227-232
2018 Volume 68 Issue 2 Pages 227-232
Apomixis, or asexual seed formation, is of great value for plant breeding and seed production, and is desirable in modern agriculture, but natural apomixis occurs in cassava at very low frequency. In present study, apomixis was induced by the treatments of female flower buds with 1%, 1.5% and 2% (v/v) dimethyl sulfoxide (DMSO) and the results showed that 1.5% DMSO treatment was most effective for the induction of apomictic seed formation in cassava cultivar SC5 with the highest percentages of fruit set and true apomictic seeds. The germinated seedlings resembled their parents and displayed no morphological characteristics of cassava polyploid. Flow cytometry and chromosome counting showed that these plants were uniform diploids. Analysis of 34 DMSO-induced cassava progenies by the expressed sequence tag-simple sequence repeat (EST-SSR) and sequence-related amplified polymorphism (SRAP) markers showed that three true apomictic seeds were obtained from the group of SC5 treated with 1.5% DMSO.
Apomixis is a form of asexual reproduction through seeds that leads to the clonal progeny genetically identical to the mother plant (Koltunow and Grossniklaus 2003). Apomixis includes three steps: embryo sac formation bypass of meiosis (apomeiosis), development of an embryo independent of fertilization (parthenogenesis) and formation of endosperm. Apomixis can be classified into two categories: gametophyte apomixis (the embryo develops from a diploid embryo sac) which further subdivided into diplospory (the embryo sac is produced from the unreduced megaspore or megaspore mother cell) and apospory (the embryo sac arises from somatic cells of the nucellus avoiding both meiosis and fertilization) depending the origin of the unreduced embryo sac, and sporophytic apomixis or adventitious embryony (the embryo directly develops from diploid somatic cells adjacent to the sexual embryo sac within the ovule) (Hand and Koltunow 2014, Ramulu et al. 1999).
Due to its huge advantages such as the fixation of a given genotype or heterosis via the clonal production of seeds, transformation of current plant breeding paradigm by apomixis breeding and dramatic reduction in time and costs for breeding and seed production, the introduction of apomixis into sexual crops has been considered as a revolutionary technology and will have a huge impact on agriculture (Rodriguez-Leal and Vielle-Calzada 2012, van Dijk et al. 2016). In addition, for vegetative crops, apomictic seed is an alternative to conventional vegetative propagule which is disease-free and high productive (eradicating of viruses accumulated over successive rounds of vegetative propagation and thus higher-yielded), easily stored and transported, and convenient for the exchange of germplasm between countries (Spillane et al. 2004).
Despite the occurrence of apomixis in more than 400 species belonging to over 40 families of angiosperms, it is found scarce in agriculturally important crops (Bicknell and Koltunow 2004, Carman 1997). Harnessing its full potential would depend on the successful introduction of the apomixis trait to a crop variety via an introgression-based approach or genetic engineering method (Spillane et al. 2001). However, the introgression of apomixis through back-crossing program has been largely unsuccessful and the engineering of conditional apomixis through biotechnology has been limited by the lack of the understanding of molecular mechanisms that trigger apomixis (Barcaccia and Albertini 2013, Spillane et al. 2001, van Dijk et al. 2016).
Cassava (Manihot esculenta Crantz), a woody shrub of the Euphorbiaceae with 36 chromosomes in somatic cells (2n = 36), is a major staple food for over 800 million people in the tropics and subtropics (Nassar 2000). Because its roots are rich in carbohydrates and leaves are rich in proteins, cassava can feed both humans and animals and also a potential energy crop for its starchy tuberous roots (Ceballos et al. 2010, Cock 1982, Jennings and Iglesias 2001). Cassava is propagated vegetatively using stem cuttings and this asexual propagation favors the accumulation of viruses and bacteria leading to the decline of production and the degeneration of variety (Nassar et al. 2007). Apomixis is perceived as an effective way of maintaining cultivar superiority and avoiding contamination by pathogens (Freitas and Nassar 2013). However, apomixis occurs in cassava at very low frequency and attempts to introduce apomixis genes from wild Manihot species into cultivated cassava through artificial hybridization are frequently encountered with the resulting hybrid sterility and the association of apomixis with aneuploidy or polyploidy (Freitas and Nassar 2013).
A major effort in our laboratory is devoted to the utilization of 2n gametes and apomixis in cassava breeding programs and previously we reported the induction of 2n female gametes with colchicine treatment and production of tetraploids through sexual hybridization in cassava (Lai et al. 2015). The aim of the present work was to induce apomixis in cassava by dimethyl sulfoxide (DMSO) and identify apomictic plants with both cytogenetic and molecular marker methods.
Three cassava cultivars, South China No. 5 (SC5) (Lin et al. 2001), No. 8 (SC8) (Ye et al. 2006) and No. 10 (SC10) (Ye et al. 2001), provided by Tropical Crops Genetic Resources Institute, Chinese Academy of Tropical Agriculture, were planted at the base of teaching and research (Danzhou Campus), Tropical Agriculture and Forestry Institute, Hainan University in March 2016. All three cultivars were grown in the field (19°30′N and 109°28′E; 146.8 m above sea level; with a mean daily temperature of 23.1°C and annual rainfall of 1823 mm in 2016) in red loam soil with a spacing of 1.5 × 2.0 mm. The apomixis induction was conducted from September to November in 2016 and fruit harvest was carried out from January to February in 2017. Field management practices consisted of no application of agrochemicals and fertilizers.
Apomixis inductionThe relationship between morphology of inflorescences (racemes or panicles) at various phonological stages, and gynoecia and megaspore development was determined following the method described by Lai et al. (2015). The cassava female cyathia at the developmental stage 1 (raceme of 1.8–2.5 cm in length with ramification initiated and corresponding to the archesporial cell differentiation stage of female gametophyte development) were selected for apomixis induction. These female flower buds were applied with 1%, 1.5% and 2% (v/v) DMSO-soaked cotton plugs between 9:00 and 10:00 am. After treatments of 24 h, cotton plugs were removed and the buds were rinsed with water at least 3 times. Untreated buds were defined as the control group (CK). Female cyathia were bagged before anthesis to prevent contamination. Three days after cyathium opening, fruit development was observed after removing the bags. Ripe fruits were counted to determine percent fruit set and dried in the shade 100 days after cyathium blooming. Differences in percent fruit set among the different treatments were compared using ANOVA. The seeds were removed from the dehisced fruits and sowed in sand bed for germination.
Determination of DNA content and chromosome numberDNA content determination and chromosome counting for the leaves of seedlings was performed according to the protocol of Lai et al. (2015). Leaf tissue samples of 1 cm2 were chopped with a razor blade and ground up in a Petri dish containing 1 ml of cold LB01 buffer for nuclei release. The obtained suspension was filtered and stained with DAPI (4,6-diamidino-2-phenylindole) solution. After 2 min of incubation, the tested samples were analyzed for relative fluorescence intensity with a flow cytometer (BD FACSCalibur, USA). The results were analyzed with CellQuest Pro software. For chromosome determination, young leaves were collected between 8:30–10:00 am and pretreated with a mixture of 0.1% colchicine and 0.002 M 8-hydroxy quinoline for 3 h at room temperature. Afterwards, the samples were fixed in a solution (ethanol:chloroform:acetic acid = 6:3:1) for 24 h at 4°C and hydrolyzed in 1 M HCl for 8 min at 60°C. The hydrolyzed materials were squashed and chromosome number was counted under an Olympus BX51 microscope (Olympus America Inc., NY, USA).
Molecular analysisIn order to exclude the seedlings developed from the potential doubled gametes, assessment of heterozygosity and homozygosity of seedlings was made with the expressed sequence tag - simple sequence repeat (EST-SSR) markers (Brown et al. 1996, Varshney et al. 2005). EST-SSR primer pairs used in this study were kindly provided by Wang’s lab, Tropical Crops Genetic Resources Institute, Chinese Academy of Tropical Agriculture. These EST-SSR markers were previously developed for cassava genetic linkage map construction and their polymorphism, size, and location on the cassava linkage map have been previously validated (We et al. 2008). Total genomic DNA was extracted from fresh young leaves with modified cetyl trimethyl ammonium bromide (CTAB) method and selection of EST-SSR primers was carried out with parent DNA according to Wei et al. (2008). A total of 159 EST-SSR primer pairs were assayed on SC5 and SC10 and 6 combinations (Table 1) were selected for their simultaneous appearance of two bands in the same heterozygous locus. Subsequently, PCR amplification was conducted in 10 μL reaction mixtures containing 5 μL 2 × PCR mix, 3 μL dd H2O, 1 μL forward/reverse and 1 μL DNA template using following condition: initial denaturation at 94°C for 10 min, 35 cycles of 94°C for 1 min, 52–58°C for 1 min and 72°C for 1 min, for denaturing, annealing and extension respectively, followed by extension for 10 min at 72°C. The amplified PCR products from the parent and progenies were resolved by TBE agarose gel electrophoresis.
| Name | Annealing Tm | Forward primer | Reverse primer |
|---|---|---|---|
| CESR-0410 | 57 | CAGCTCAGTACTCTCTCTCTCTC | GCTTATCAGAATCAACAATCC |
| CESR-0604 | 54 | AAAGAGGCTGGAGGAGGT | TCAACAGTGATCACAAGGAA |
| CESR-0624 | 54 | AAGCCTTAATTTGTCTTCCC | ACAGACAGAAACCACCACTC |
| CESR-0702 | 52 | ATATTTATGCTCGCTTCCTG | GTACCAGACACATGAATCCC |
| CESR-0745 | 54 | CACCTTCAAGCTCACAAA | CACGGTAGAAAGACCATAGC |
| CESR-0831 | 56 | CTTACACACCACCTTCAAGC | AGCACGGTAGAAAGACCATA |
For further confirmation of true apomictic plants, sequence-related amplified polymorphism (SRAP) procedure was performed according to Xia et al. (2008). Of these 64 primer combinations tested for polymorphism and reproducibility, 33 primer pairs (18 for SC5 and 15 for SC10) were selected for their consistent amplifications, and clear and polymorphic banding patterns (Table 2). After selection of SRAP primers, PCR amplification was carried out in 20 μL reaction mixtures that contain 10 μL 2 × PCR mix, 6 μL dd H2O, 1 μL forward/reverse primer and 2 μL DNA template. The reaction conditions include initial denaturation at 94°C for 5 min, the first five cycles of 94°C for 1 min, 35°C for 1 min and 72°C for 1 min, another 35 cycles with the annealing temperature raised to 50°C and last extension for 5 min at 72°C. The amplicons from the parent and progenies were separated by denaturing acrylamide gels and detected by autoradiography.
| Varieties | No. | Primer combinations |
|---|---|---|
| SC5 | 1 | me1-em2 |
| 2 | me1-em5 | |
| 3 | me1-em7 | |
| 4 | me2-em4 | |
| 5 | me2-em7 | |
| 6 | me2-em8 | |
| 7 | me3-em5 | |
| 8 | me3-em6 | |
| 9 | me4-em2 | |
| 10 | me4-em3 | |
| 11 | me5-em4 | |
| 12 | me5-em5 | |
| 13 | Me5-em6 | |
| 14 | Me6-em5 | |
| 15 | me7-em2 | |
| 16 | Me7-em4 | |
| 17 | me8-em4 | |
| 18 | me8-em6 | |
| SC10 | 1 | me1-em5 |
| 2 | me2-em3 | |
| 3 | me2-em4 | |
| 4 | me2-em8 | |
| 5 | me3-em1 | |
| 6 | me3-em5 | |
| 7 | me3-em7 | |
| 8 | me4-em3 | |
| 9 | me5-em5 | |
| 10 | me5-em6 | |
| 11 | me6-em5 | |
| 12 | me7-em2 | |
| 13 | me7-em4 | |
| 13 | me8-em4 | |
| 15 | me8-em6 |
Percentage of fruit set was evaluated for both DMSO-induced and non-induced (CK) female cyathia. The DMSO-induced female cyathia presented an average percent fruit set of 0.79% and the non-induced presented a rate of 0 (Table 3). The average percentage of fruit was 0.48% for 1.0%, 1.74% for 1.5% and 0.43% for 2.0% DMSO treatment, respectively. The plants applied with 1.5% DMSO had a significantly higher percentage of set than that of the plants treated with 1.0% or 2.0% DMSO. Cultivars responded differently to DMSO treatments. SC5 was the best among the three cultivars tested, producing a total of 44 fruits and a percent fruit set of 1.04% which was significantly higher than that of SC10 (0.52%) with SC8 forming no fruit.
| DMSO concentration (%) | SC5 | SC10 | SC8 | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of flowers treated | No. of fruit set | Percentage of fruit set (%) | No. of flowers treated | No. of fruit set | Percentage of fruit set (%) | No. of flowers treated | No. of fruit set | Percentage of fruit set (%) | No. of flowers treated | No. of fruit set | Percentage of fruit set (%) | |
| 1.0 | 1787 | 10 | 0.28 | 1167 | 5 | 0.86 | 167 | 0 | 0 | 3121 | 15 | 0.48ABb |
| 1.5 | 1108 | 27 | 2.44 | 510 | 5 | 0.98 | 218 | 0 | 0 | 1836 | 32 | 1.74Aa |
| 2.0 | 1333 | 7 | 0.53 | 831 | 3 | 0.36 | 135 | 0 | 0 | 2299 | 10 | 0.43Bb |
| Total | 4228 | 44 | 1.04Aa | 2508 | 13 | 0.52Ab | 520 | 0 | 0Ab | 7256 | 57 | 0.79 |
| CK | 300 | 0 | 0 | 200 | 0 | 0 | 80 | 0 | 0 | 580 | 0 | 0 |
Different capital letters indicate significant difference at 0.01 level, different small letters indicate significant difference at 0.05 level.
A total of 70 seeds (46 for SC5 and 24 for SC10) were harvested and sowed for germination (Table 4). Thirty four plantlets (23 for SC5 and 11 for SC10) were obtained and showed no obvious morphological difference among the groups obtained with different concentrations of DMSO applied (Fig. 1). In addition, these plants resembled their diploid parents and displayed no morphological variations such as enlarged and round leaves, and thicker veins occurred in the tetraploid seedlings (Lai et al. 2015).
| Cultivars | No. of seeds harvested | No. of geminated seedlings (%) | No. of apomictic plants identified (%) |
|---|---|---|---|
| SC5 | 46 | 23 (50) | 3 (13.04) |
| SC10 | 24 | 11 (45.83) | 0 (0) |
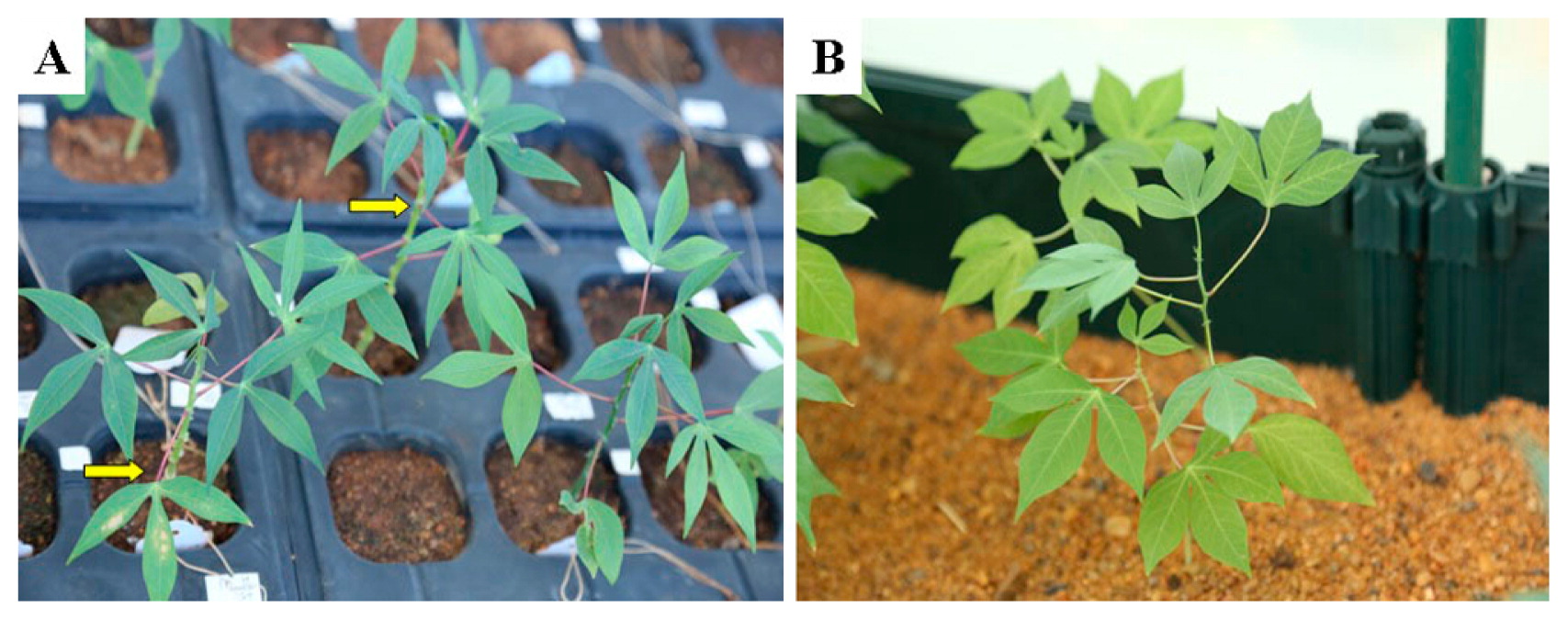
Growth of the cassava seedlings obtained with DMSO application. A: seedlings 20 days after germination (true apomictic plants are indicated by the yellow arrows). B: seedlings transferred 35 days after germination.
Flow cytometric measurement and chromosome observation confirmed that thirty four seedlings obtained with DMSO treatment were uniform diploids (Fig. 2).
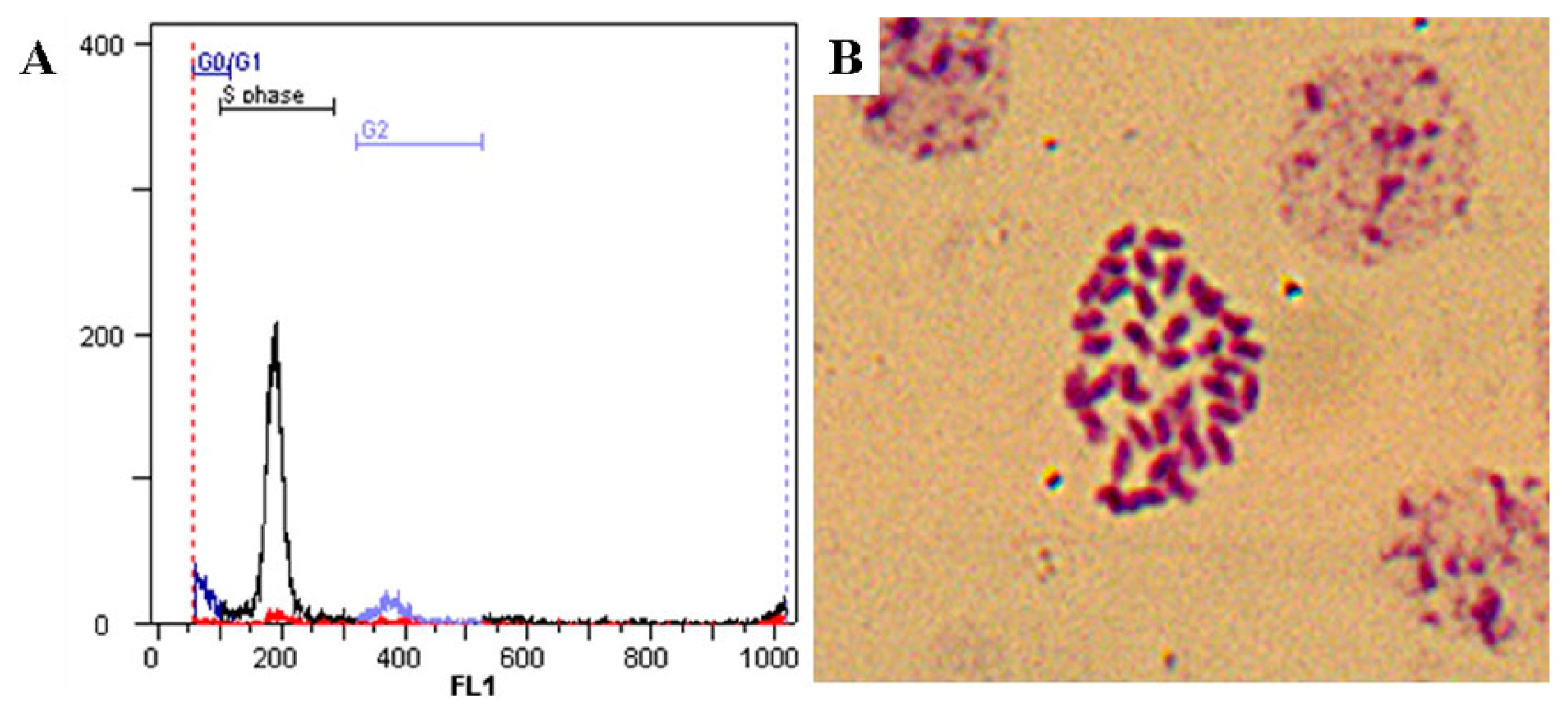
Flow cytometric detection of nuclear DNA and chromosome count in young leaf of a cassava seedling obtained with DMSO application. A: flow cytometric histogram of the nuclear DNA content with a peak at channel 200 corresponding to the diploidy. B: mitotic metaphase chromosomes (2n = 2× = 36).
With the 6 EST-SSR primer pairs, both the 34 DMSO-induced progenies (23 for SC5 and 11 for SC10) and their female parent presented a heterozygous profile with two bands (Fig. 3), demonstrating that these plants impossibly derived from diploid female gametes due to chromosome doubling.
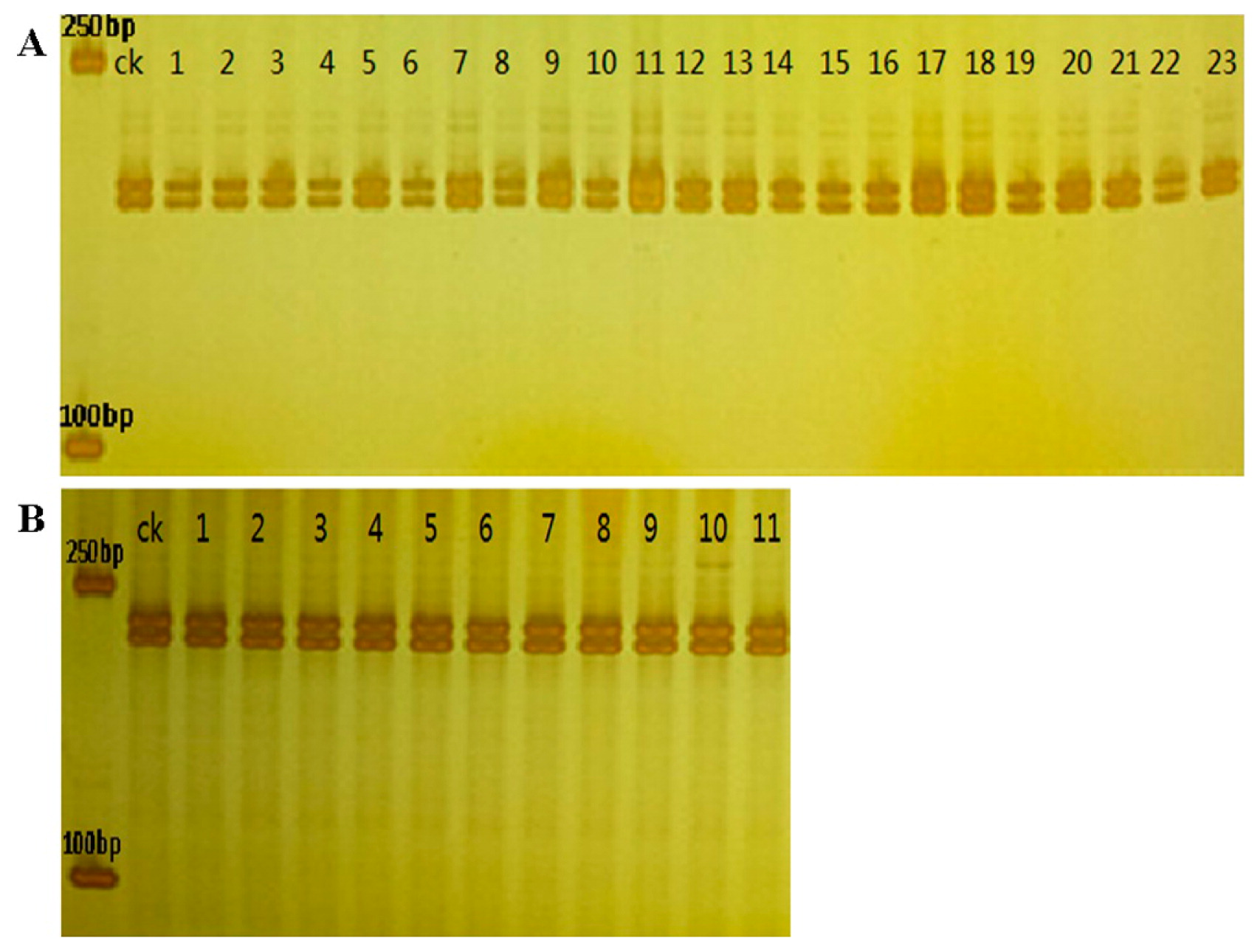
EST-SSR banding pattern of 34 DMSO-induced cassava progenies with primer combination CESR-0831 and CESR-0604. A: CESR-0831. ck SC5 female parent, sample 1–23 DMSO-induced SC5 progenies. B: CESR-0604. ck SC10 female parent, sample 1–11 DMSO-induced SC10 progenies.
Analysis of 34 DMSO-induced cassava progenies by SRAP showed that the sample 2, 5 and 7 shared the same banding pattern with the SC5 female parent (Fig. 4) and they were from the genuine apomictic plants. The three true apomictic seeds were collected from the SC5 plants treated with 1.5% DMSO. Of 11 DMSO-induced SC10 progenies, no apomictic plant has been identified (Table 4).

Analysis of 34 DMSO-induced cassava progenies by SRAP with primer pair me3-em1, me4-em2 and me5-em4. A: me3-em1. ck SC5 female parent, sample 1–23 DMSO-induced SC5 progenies and the sample 2, 5 and 7 are from the apomictic plants. B: me4-em2. ck SC5 female parent, sample 1–23 DMSO-induced SC5 progenies and the sample 2, 5 and 7 are from the apomictic plants. C: me5-em4. ck SC10 female parent, sample 1–11 DMSO-induced SC10 progenies.
DMSO, a by-product of the wood industry, is used extensively in a variety of fields (Wang et al. 2012). In plant studies, DMSO has been used extensively as an efficient solvent for water-insoluble compounds such as colchicines and oryzalin for polyploid induction (Limera et al. 2016, Thao et al. 2003). Zhao and Gu (1984) reported that parthenogenesis in non-pollinated silk of maize was induced by combinations of DMSO, colchicines and maleic hydrazide (MH), and 19 homozygous diploids were obtained. Hu et al. (1991) explored the chemical induction of apomictic seed formation with different combinations of growth regulators, colchicine, and DMSO in maize. But the highest frequency of seed induction (1.4%) in their study was lower than that of our study and the resulting seeds included diploids, mixoploids and haploids. In addition, the authenticity of their apomictic seeds hadn’t determined due to the lack of molecular marking technology. We have shown that 1.5% DMSO treatment was most effective for the induction of apomictic seed formation in cassava cultivar SC5 with the highest percentages of fruit set and true apomictic seeds. Moreover, the use of DMSO, instead of colchicine, didn’t result in chromosome doubling and polyploid formation described by Nassar (2006). Although its biological effects in apomixis induction have not been known, previous reports that DMSO induced the formation of huge bundles of actin filaments in the nuclei of Dictyostelium mucoroides (Fukui and Katsumaru 1980) and that DMSO could modulate AP-1 activity and lead to cell cycle arrest at the G1 phase and displayed a diversity of antitumor activities (Wang et al. 2012) suggested that the amphipathic molecule DMSO is possibly involved in cell divisions of apomeiosis and/or parthenogenesis.
To the best of our knowledge, this is the first report on induction of apomixis in cassava by DMSO and also we have established a systemic genetic identification of true apomictic plant with the examination of both cytological and molecular evidence. First, flow cytometry and chromosome observation were conducted to determinate the polidy level of the putative plant. Then, EST-SSR was used to assess the heterozygosity and homozygosity of the putative plant. Finally, the true apomictic plant whose genotype is identical to the mother plant was pinpointed with SRAP. Microsatellite or SSR markers are co-dominant in inheritance, reproducible and highly polymorphic, and EST derived SSRs are directly linked to expressed genes although showed less polymorphism (Sraphet et al. 2011). They are efficient tool for the assessment of heterozygosity and homozygosity (Maurya and Yadav 2016). SRAP targets coding sequences in the genome and produces a moderate number of co-dominant markers, with more than ten polymorphic bands per individual of Brassica oleracea L. detected by a single primer combination (Li and Quiros 2001). Overall, the genetic uniformity of the three putative apomictic plants and their female parent unequivocally detected by EST-SSR and SRAP demonstrated the effectiveness of the induction of apomixis by DMSO in cassava though the induction rate needs to be further improved in future. Parenthetically, the three cassava cultivars used in this study can be shared with other cassava genetics and breeding programs, and the transfer of the cassava germplasm collection can be done by in-vtiro culture.
This work is supported by the National Natural Science Foundation of China (No. 31360354) and Central Public-interest Scientific Institution Basal Research Fund for Chinese Academy of Tropical Agricultural Sciences (No. 1630032017089).