2018 Volume 68 Issue 2 Pages 284-288
2018 Volume 68 Issue 2 Pages 284-288
‘Harimaru’ is a new potato variety bred from a cross between ‘Saikai 35’ as a female parent and ‘Pike’ as a male parent. Marker selection was performed for 1,647 seedlings to combine resistance genes to late blight (R1), Potato virus Y (Rychc), Potato virus X (Rx1), and golden cyst nematode (H1). In total, 194 selected clones were evaluated in the field, among which the best clone was officially released as ‘Harimaru’. Its yield was slightly lower than the local standard variety, ‘May Queen’. However, it produces tasty potatoes, that do not become mushy with long boiling times despite its high starch content. ‘Harimaru’ may become a local specialty potato and its multiple resistance to potato viruses may allow cultivation using homemade seed tubers from the previous season’s crop.
The production and distribution of seed potato tubers is strictly regulated by the Plant Protection Act, and seed tubers must be certified by government officials in Japan, which often hampers the quick spread of new varieties and launching of seed potato-related businesses by private companies. A small lot of seed tubers of a new variety are hated at any stages from foundation seed to certified seed production and to commercial seed production and in seed tuber distribution. To avoid this well-established but strictly regulated seed tuber production system, a scientific approach might involve the use of true botanical seeds (Lindhout et al. 2011). An alternative is the use of a virus-resistant variety grown using homemade seed tubers from the previous season’s crop. Twelve viruses are known to infect and damage potatoes in Japan (Maoka et al. 2010). Among them, Potato virus Y (PVY) is the most important, causing particularly severe damage as a mixed infection with Potato virus X (PVX).
Disease resistance genes and DNA markers linked to these genes have been developed in potato (Ramakrishnan et al. 2015). Among them, resistance genes to PVX (Rx1), PVY (Rychc), and golden cyst nematode [Globodera rostochiensis (Woll.) Behrens] (H1) are available in Japan, and their DNA markers have been developed (Mori et al. 2010, 2011). The Rx1 and Rychc genes show strain-non-specific resistance. The H1 gene shows strain-specific resistance to pathotypes Ro1 and Ro4. However, only pathotype Ro1 has been found in Japan. Late blight caused by Phytophthora infestans (Montague) de Bary is the most devastating disease in potato cultivation worldwide. One late blight resistance gene, R1, has been cloned, and the gene-specific marker is available (Ballvora et al. 2002). However, R1-mediated resistance has already been broken, and the chemical control has become routine practice in Japan.
In this study, we tried to combine R1, Rx1, Rychc, and H1 genes into one clone using marker selection technology at the seedling stage. The R1 gene, though not useful as mentioned above, was included to evaluate the efficiency of marker-assisted selection in a practical breeding program. Although the agronomically best clone lacked the R1 gene, we successfully bred it as a locally adapted, tasty potato resistant to PVX, PVY, and golden cyst nematode. It was named ‘Harimaru’ and officially released in 2017.
The pedigree of ‘Harimaru’ is shown in Fig. 1. ‘Saikai 35’ is a male and female fertile breeding clone carrying Solanum phureja-derived cytoplasm (P type, as defined by Hosaka and Sanetomo 2012), the PVY resistance gene (Rychc) derived from ‘Konafubuki’, and the golden cyst nematode resistance (H1) gene derived from Tunika, as well as resistance to bacterial wilt (Mori et al. 2012). ‘Pike’ is a US chip processing variety and possesses the H1 gene derived from either ‘Allegany’ or ‘Atlantic’ and common scab resistance (Plaisted et al. 1998); it also contains the PVX resistance gene (Rx1) derived from ‘Atlantic’ (Ross 1986).
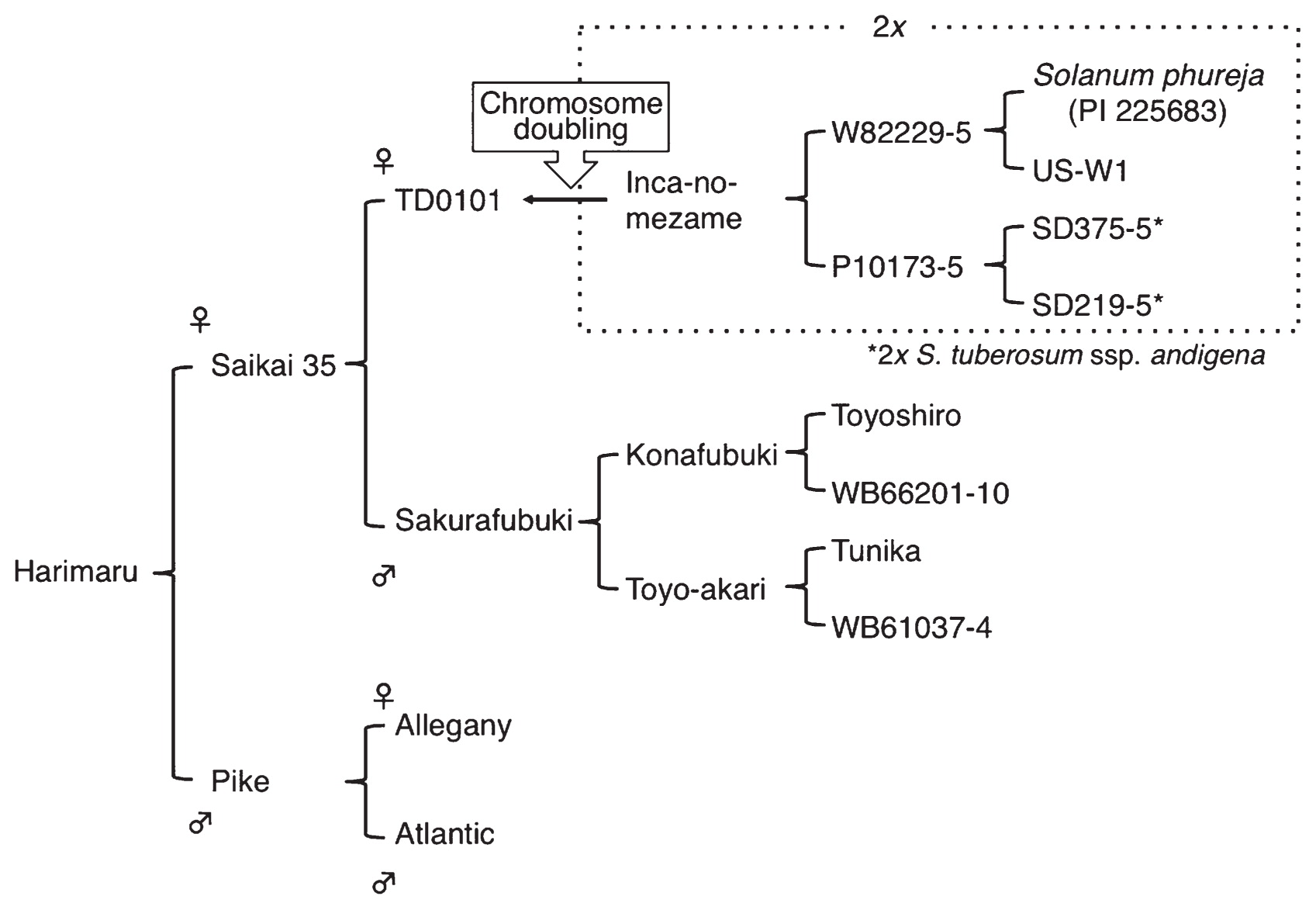
Pedigree of ‘Harimaru’.
Seed tubers were planted in the field in early March 30-cm apart in 1-m-wide rows and fertilized with 18 kg 10 a−1 N, 18 kg 10 a−1 P, and 18 kg 10 a−1 K. Ordinary cultural practices were used until natural senescence in July.
Marker analysisDNA was extracted from fresh leaves using the one-minute DNA extraction method (Hosaka 2004). The presence of four resistance genes (R1, Rx1, Rychc, and H1) were estimated using the following markers: R1-specific marker (76-2sf2: 5′-CACTCGTGACATATCCTCACTA-3′ and 76-2SR: 5′-CAACCCTGGCATGCCACG-3′, Ballvora et al. 2002), Rx1-linked marker (RxSP-S3: 5′-ATCTTGGTTTGAATACATGG-3′ and RxSP-A2: 5′-CACAATATTGGAAGGATTCA-3′, Ohbayashi and Komura 2004), Rychc-linked marker RAPD 38-530 (5′-TTCGAGCCAG-3′, Hosaka et al. 2001), and H1-linked marker (H1SP-S2: 5′-GAGCTCAGAGGTGAAAAATA-3′ and H1SP-A6: 5′-GGAACAATGTTGAATGCAAG-3′, Ohbayashi et al. 2010). Diagnostic marker bands were amplified using polymerase chain reaction (PCR) in a volume of 10 μl consisting of 2 μl of template DNA, 5 μl of 2× Ampdirect® Plus (Shimadzu Co., Japan), 0.25 units of Taq DNA polymerase (NovaTaqTM Hot Start DNA Polymerase, Novagen, USA), and 1 μl each of 3 μM forward and reverse primers. The thermal conditions for PCR were similar to those described in the original literature. The detection procedure for the RAPD marker 38-530 is described in Hosaka (2004). The amplified product was separated on a 1.4% agarose gel in 1× TAE buffer (40 mM Tris-acetate and 1 mM EDTA).
Biological assay for disease resistanceResistance to PVX, PVY, and golden cyst nematode (G. rostochiensis pathotype Ro1) was assayed by inoculation tests courtesy of the Hokkaido Agricultural Research Center, Sapporo.
All breeding processes were performed at the Food Resources Education and Research Center, Kobe University. In November 2007, ‘Saikai 35’ was pollinated with the pollen of ‘Pike’. From 77 pollinations, 33 berries were obtained. On February 10, and September 14, 2008, 1,170 and 1,232 seeds (families 8H14 and 8H18, respectively) were pretreated by soaking in 2,000 ppm gibberellic acid (GA3) solution for 48 hr and sown; from these, 905 and 758 seedlings were raised in 10.5-cm black vinyl pots. Using molecular markers, 194 seedlings were selected as positive for all four resistance genes. In 2009, 2 hills from each of the 194 clones were grown in the field, from which 15 clones were selected based on tuber quality (shape, uniformity and taste). In 2010, 10 hills from each of the 15 clones were grown, from which three clones were selected and named 10H15, 10H16, and 10H17. In 2011, 60 hills per clone were grown, from which 10H16 and 10H17 were selected. In 2012, 324 hills per clone were grown, from which 10H16 was selected as the best. It was named ‘Harimaru’, and official registration paperwork for this clone was filed. ‘Harimaru’ was officially registered on February 24, 2017 and announced as a new variety on March 7, 2017. ‘Harimaru’ is a name coined by the combination of ‘Harima’ (a region where this variety was bred) and ‘maru’ (=‘round’, indicating the tuber shape).
Seedling selection using molecular markersDNA was extracted from 905 seedlings of the family 8H14, of which three DNA samples were accidentally lost during the process. This population was first screened by an R1-specific marker. Then, possible R1 gene holders were analyzed by an Rx1-linked marker. Two hundred thirty-four seedlings were positive for both markers. From the family 8H18, 742 seedlings were surveyed first by an Rx1-linked marker, and then by an R1-specific marker. Two hundred thirteen seedlings were positive for both markers. A total of 447 seedlings likely possessing both R1 and Rx1 markers were surveyed for the presence of the RAPD marker 38-580 for the Rychc gene, resulting in 225 positive seedlings. Then the H1-linked marker was used. Consequently, 194 seedlings were selected as potentially containing the four resistance genes (Fig. 2A).
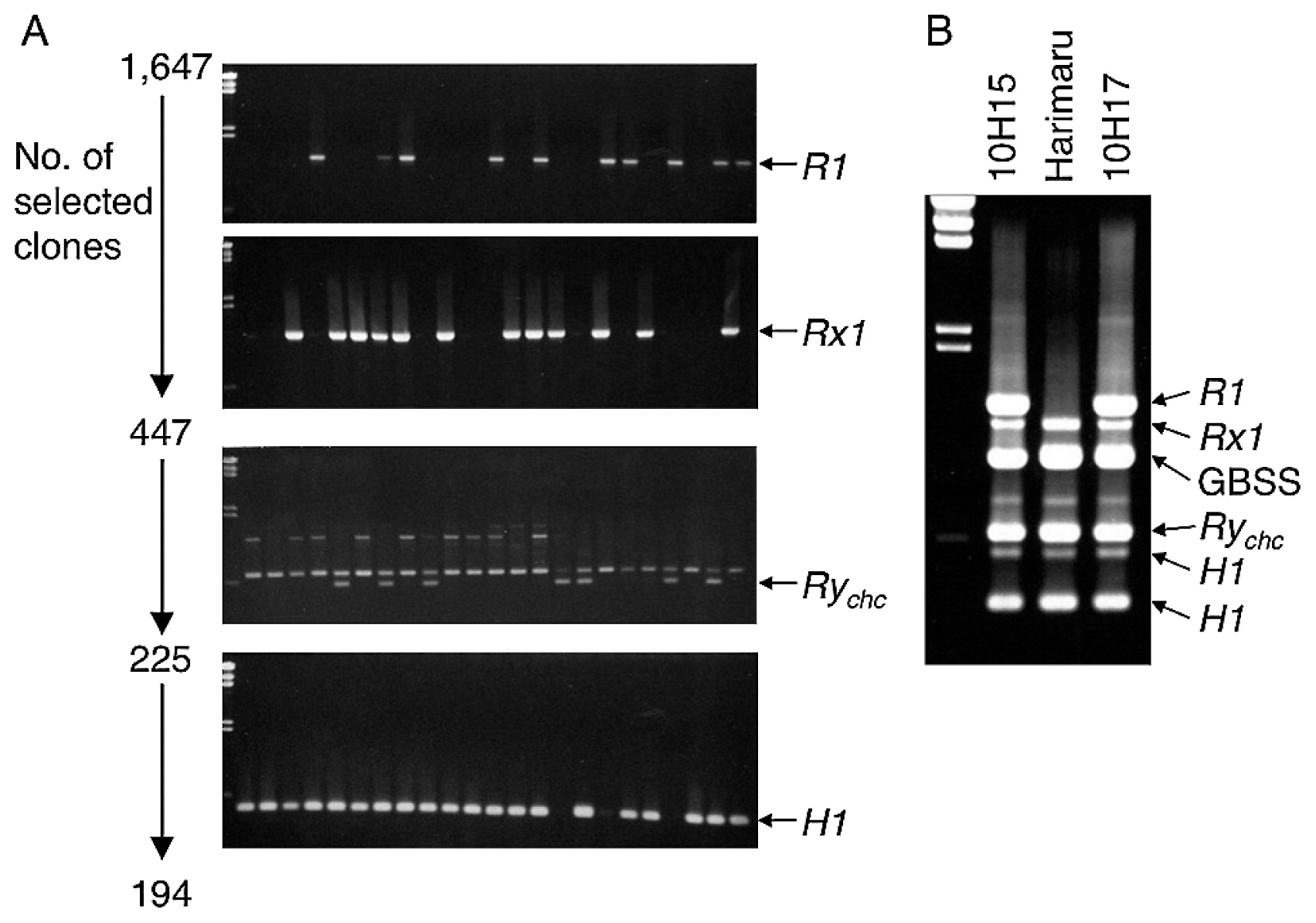
Marker selection. Selection process (A) and reconfirmation of genotypes by the multiplex PCR method of Mori et al. (2011) (B).
The segregation ratios of these DNA markers were analyzed (Table 1). The parental clone ‘Saikai 35’ has marker bands for Rychc and H1, while ‘Pike’ has bands for R1, Rx1 and H1. The hybrid seedlings possessing both R1 and Rx1 markers were significantly over-represented, likely because PCR conditions were not optimized for amplification of the R1-specific marker. The H1-linked marker also significantly deviated toward over-representation from the segregation ratios in the progeny of simplex × simplex as expected either by a random chromosome assortment model or by a random chromatid assortment model. If we assume ‘Pike’ is duplex for this marker, a better fitting to the expected ratio can be obtained.
| Gene-specific or linked marker | Observed (+:−) | Postulated genotype for ‘Saikai 35’× ‘Pike’ | Expected ratio (+: )a | P-value by χ2 testa |
|---|---|---|---|---|
| R1 and Rx1 | 447:1197 | Nulliplex × simplex | 1:3 | 0.0403 |
| 169:615 | <0.0001 | |||
| Rychc | 225:222 | Simplex × nulliplex | 1:1 | 0.8872 |
| 13:15 | 0.0977 | |||
| H1 | 194:31 | Simplex × simplex | 3:1 | 0.0001 |
| 559:225 | <0.0001 | |||
| Simplex × duplex | 11:1 | 0.0031 | ||
| 347:45 | 0.2795 |
A later assay using more accurate markers for Rychc and H1 genes (Takeuchi et al. 2008) and a deliberately designed multiplex PCR condition (Mori et al. 2011) indicated ‘Harimaru’ does not have the R1 gene (Fig. 2B).
Growth habit and morphological characteristicsPlant, leaf, flower, sprout, and tuber characteristics of ‘Harimaru’ are shown in Fig. 3. The vine has a spreading shape. The plant height (47.6–68.4 cm) is comparable to that of ‘May Queen’ (52.5–64.5 cm). No anthocyanin coloration was observed in the leaf and stem. The stem is slender, and there are more stems (6.7) than in ‘May Queen’ (3.5–4.8). The flower is pale violet and the number of flowers is intermediate. ‘Harimaru’ matures approximately 140 days after planting on March 5, longer than ‘Kita-akari’ (127 days after planting). Tubers are short ovate with medium-netted yellow skin and shallow pink eyes. The flesh is bright yellow. The tuber dormancy of ‘Harimaru’ is slightly shorter (80 days) than ‘Kita-akari’ (96 days).

Plant, leaf, flower, sprout and tuber characteristics of ‘Harimaru’.
Marketable yield (>30 g) of ‘Harimaru’ was 2,713 kg 10 a−1 (4-year mean in 2014–2017) (Table 2), slightly lower than that of ‘May Queen’, a standard variety in that region (4-year mean of 3,236 kg 10 a−1 in 2006–2009). The mean tuber weight of ‘Harimaru’ (3-year mean = 67 g) was much lower than that of ‘May Queen’ (4-year mean = 88 g). As shown in Fig. 4, tubers smaller than L size (the highest price) predominated the yield. Tubers of ‘Harimaru’ constantly showed high specific gravity (=high starch content) (Table 2). Nevertheless, they do not fall apart with long boiling times. ‘Harimaru’ tubers have a nutty flavor originally derived from the Andean diploid potato (S. phureja). Although a formal sensory evaluation test for the taste was not carried out, we did several taste tests at our institution, restaurants, and grocery stores and always received a favorable review.
| Year | Seed tuber planted (g) | Total yield (kg 10 a−1) | Marketable yield (kg 10 a−1)a | Mean tuber weight (g) | Specific gravity |
|---|---|---|---|---|---|
| 2012 | Whole (30–60 g) | – | 3,150b | 50.6 | 1.097 |
| 2013 | Whole (30–60 g) | – | 3,719b | 56.7 | 1.084 |
| 2014 | Whole (30–50 g) | 3,198 | 2,642c (82.6%) | – | 1.117 |
| 2015 | 1/2 or 1/3-cut (30–50 g) | 3,143 | 2,797c (89.0%) | 67.2 | 1.101 |
| 2016 | Whole (30–50 g) | 2,592 | 2,257c (87.1%) | 65.2 | 1.097 |
| 2017 | Whole (30–50 g) | 3,743 | 3,157c (84.3%) | 67.7 | 1.093 |
aMarketable yield comprises tubers greater than 20 gb or 30 gc and free of external defects. The percentage of marketable yield over total yield is shown in parenthesis.
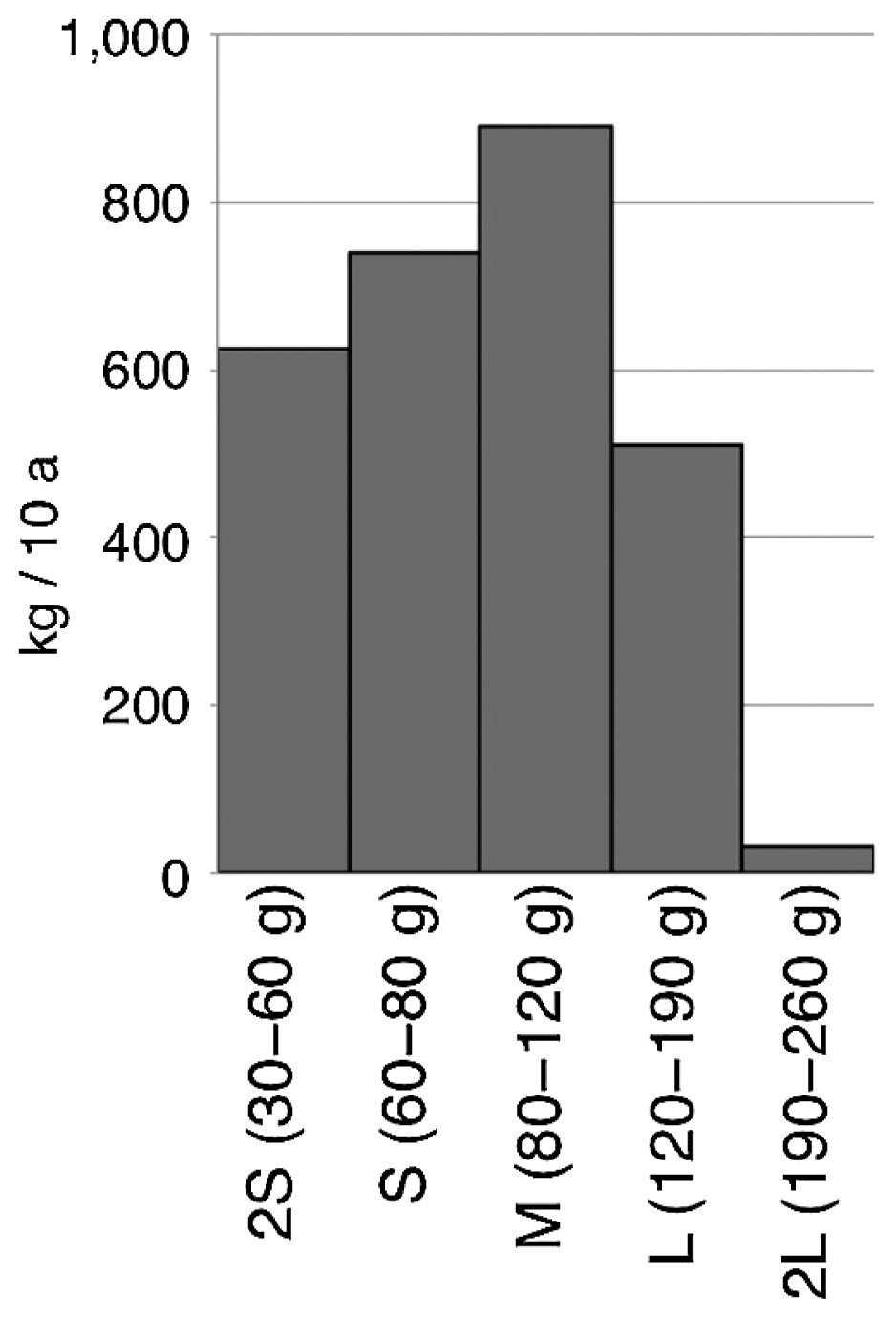
Size distribution for total yield (2,797 kg 10 a−1) in 2015.
Inoculated leaves and the upper leaves were sampled 10 days and four weeks after inoculation, respectively, and were examined by enzyme-linked immunosorbent assay (ELISA) and reverse-transcription polymerase chain reaction (RT-PCR) methods, as described by Maoka et al. (2010). PVX was not detected by either method. After inoculation of PVY strain O, hypersensitive reaction was observed on the surface of the inoculated leaves. PVY was not detected by ELISA. However, RT-PCR did produce a faint band, indicating a slight presence of PVY in the inoculated and the upper leaves. This is a normal response of Rychc-mediated resistance (Maoka et al. unpublished). ‘Harimaru’ is highly resistant to golden cyst nematode. It is, however, susceptible to late blight (P. infestans). For other diseases such as common scab and bacterial wilt, ‘Harimaru’ has not been tested.
Recently, a new variety ‘Nagasaki Kogane’ was bred by applying marker-assisted selection at the second field-grown stage and it has H1 and Rychc genes (Sakamoto et al. 2017). We successfully bred a tasty potato, ‘Harimaru’, with multiple disease resistances. This is the first incidence of marker-assisted selection applied at a seedling stage and the first variety possessing H1, Rx1 and Rychc genes in Japan. ‘Harimaru’ produced slightly lower tuber yield than the local standard variety. However, its taste and cooking quality are appreciated by people in the region; thus, its growing area is gradually increasing.
When we started, the marker selection technology was still at a premature stage. We performed PCR only for positive clones for the next marker, resulting in more than 2,500 PCR for 1,647 seedlings, which is less efficient compared to the currently used multiplex PCR technique (Mori et al. 2011). For amplification of the R1 gene-specific marker, we used an annealing temperature of 55°C as described in the literature (Ballvora et al. 2002). This condition might induce unspecific amplification, resulting in over-representation of R1 and Rx1 positive seedlings. The multiplex PCR technique (Mori et al. 2011) modified the annealing temperature to 68°C for the R1-specific marker. The H1-linked marker that we used was perfectly matched with the presence of H1 gene in Japanese potato varieties (K. Hosaka, unpublished data). However, the present data indicated the possibility of duplex status for the marker in the US variety ‘Pike’. According to the biological assay of golden cyst nematode resistance in the progenies of ‘Pike’, ‘Pike’ is undoubtedly simplex for the H1 gene (T. Igarashi, Calbee Potato Inc., personal communication). Thus, of the four homologous chromosomes of potato chromosome 5 in ‘Pike’, one has the marker and H1 gene, while the second has only the marker. The multiplex PCR technique (Mori et al. 2011) uses two more tightly linked markers sandwiching the H1 gene (Takeuchi et al. 2008).
For normal potato breeding, it takes at least ten years from crossing to applying for variety registration. However, the breeding process of ‘Harimaru’ was completed in six years because adaptability in several different locations was not tested. Wide adaptability was not required for local farmers who need specialty potatoes in their regions.
‘Harimaru’ has extreme resistance genes Rx1 and Rychc, which makes it possible to use a part of the product stored for next season’s seed tubers. Such a cultivation system using homemade seed tubers is an old-fashioned or traditional farming system in the Andes. The other viruses rarely occur or, even if infected, cause mild or no symptoms, resulting in no significant yield losses. Potato spindle tuber viroid (PSTV) is another feared pathogen, but its infection of potato has never been reported in Japan. Further incorporation of resistance genes to these viruses and a viroid may allow cultivation using homemade seed tubers, which may become a new, sustainable potato cultivation system.
We thank Dr. Tetsuo Maoka for providing a biological test against PVX and PVY and Dr. Takashi Narabu for providing a biological test against golden cyst nematode; both researchers are based at NARO, Hokkaido Agricultural Research Center, Sapporo. We also thank other members of the Food Resources Education and Research Center, Kobe University, for being involved in a taste test.