2019 年 69 巻 4 号 p. 688-695
2019 年 69 巻 4 号 p. 688-695
Carrot (Daucus carota) is cultivated in temperate regions for its taproot. Eastern and Western types have been differentiated. In Japan, the former type is categorized into Kintoki, Takinogawa oonaga, and Toso, with a few local cultivars. However, their genetic relationships are unclear because of the paucity of reports. We classified the Japanese Eastern and selected Western types based on simple sequence repeat (SSR) markers. Field traits, including root weight, length, diameter, and skin color, were also examined. Our field tests showed clear differences between the Kintoki and Western-type cultivars, confirming their differentiation. A phylogram based on nine SSRs classified 24 cultivars into groups I and II. Group I included all Eastern-type carrots examined (Kintoki and Toso groups, plus two local and two foreign cultivars), with the exception of an Indian cultivar (‘Pusa rudhira red’). Among them, red carrots including Kintoki were clustered into two subgroups. Western-type, Eastern-Western hybrid, and ‘Pusa rudhira red’ were included in group II. A population structure analysis revealed the split between the Eastern and the other types. This study elucidates the genetic characteristics of the Eastern type of carrot, which will be valuable information for carrot breeding, especially when using the Eastern type as a source.
Carrot is a biannual plant belonging to the genus Daucus in the family Apiaceae or Umbelliferae. This species includes cultivated carrot (D. carota var. sativus) and several wild subspecies, such as D. carota var. carota (see Simon and Goldman 2007 for a review). Cultivated carrot is presently produced in temperate regions as an annual crop before flowering, for its thick and fleshy taproot, which is generally a rich source of carotenoids. The first cultivation of carrot for its storage root is thought to have occurred before the 10th century around Afghanistan (Banga 1957a). The early carrots had purple, red-purple, or yellow roots according to ancient literature (Banga 1957b). The Eastern and Western types of carrot have been differentiated from early carrots, the origins of which are predicted to be around the Central Asiatic Center (northwestern India, Afghanistan, Tajikistan, etc.) and the Near-Eastern Center (Asia Minor, Transcaucasia, Iran, etc.), respectively (Vavilov 1951). There are a few hypotheses regarding the origin of the Western orange carrot (Banga 1957a, 1957b, Heywood 1983, Small 1978), some of which proposed the involvement of hybridization between cultivated and wild carrots. The early carrots were then replaced by the Western orange carrots in Europe. Several Western orange carrot varieties have been bred in European countries.
The Eastern type of carrot was first introduced in Japan at the latest in the 17th century, via China (see Kumazawa and Katsumata 1965, Katsumata 1968a, 1989 for reviews). The Eastern and Western types of modern Japanese carrot have generally been categorized into three and four cultivar groups, respectively (Kumazawa and Katsumata 1965). The Eastern type includes the “Kintoki”, “Takinogawa oonaga”, and “Toso” groups. Kintoki is one of the oldest known carrot cultivar groups in Japan and is regarded as an heirloom vegetable in Kyoto (Kyo-yasai) (Takashima 2003). This group is the only Eastern type that is currently popular in Japan. Several Kintoki cultivars have been developed by seed companies and are grown in western Japan. This cultivar group has a slightly long and tapered root with a red pigment that is mostly derived from lycopene (Yamaguchi and Sugiyama 1960). The ‘Manpukuji oonaga’ was derived from a cross between ‘Takinogawa oonaga’ and the Western-type ‘Sapporo oonaga’ cultivar (Katsumata 1989, Ohara 1985). A Toso group cultivar ‘Yoshino’ is produced in southwestern Japan, Kagoshima Prefecture (Ohara 1985, Okubo 1988). In addition, a few local Eastern-type cultivars, ‘Naga ninjin (Kumamoto naga ninjin)’ and ‘Shima ninjin (chide-kuni)’, exist in Kumamoto and Okinawa Prefectures, respectively (Kumamoto City 2011, Sakamoto 2002). The Western type of Japanese carrot has been classified into the “Sanzun”, “Gosun”, “Danvers”, and “Long orange” groups by Kumazawa and Katsumata (1965). Several of the Western-type cultivars have been bred in Japan (see Ohara 1985 for a review); their roots exhibit an orange color, which is derived from β-carotene. The Western type is superior to the Eastern type with regard to traits for the compactness of roots and late bolting. The cultivation of the Eastern type has decreased recently, since the introduction of the Western type from Europe in the 18th century and the breeding of the Western type varieties since that time.
Because the classification described above is mostly based on the morphology, ecology, and pedigree of each cultivar, there are slight differences in group numbers among researchers (see Katsumata 1968b for a review). The problems of such classification stem from the limited number of morphological characteristics that are distinguishable. In contrast, molecular markers are useful tools for genetic analyses. Classification and genetic diversity analyses have been performed using molecular markers (e.g. Arbizu et al. 2014, Baranski et al. 2012, Bradeen et al. 2002, Ellison et al. 2018, Iorizzo et al. 2013, Jhang et al. 2010, Luby et al. 2016, Maksylewicz and Baranski 2013, Mandel et al. 2016, Nakajima et al. 1998, Shim and Jørgensen 2000, Vivek and Simon 1998). These studies focused mostly on the relationships between wild and cultivated carrots, and only a limited number of Eastern-type carrots have been tested. Therefore, the genetic relationships among Kintoki cultivars and among the Eastern type of modern Japanese carrot are unclear.
In this study, we classified carrot cultivars with a special focus on the Eastern type, based on simple sequence repeat (SSR) markers. SSRs are DNA repeats consisting of 1–6 nucleotide repeat units that are frequently used as molecular markers in many eukaryotic organisms (see Vieira et al. 2016 for a review). They are currently available for carrot (Cavagnaro et al. 2011, Iorizzo et al. 2011, Niemann et al. 1997). Root traits, including fresh weight, length, diameter, and skin color, were also examined for Kintoki and other selected cultivars in field tests, because the field data reported for Kintoki cultivars are scarce compared with those for the Western type of carrot. The genetic relationships and diversity of the carrot cultivars are discussed.
A total of 25 lines, 24 carrot cultivars (D. carota var. sativus) and one wild relative (D. carota var. carota), were used in this study (Table 1). Of these, we selected three and 11 carrots for field tests in 2017 and 2018, respectively (Supplemental Table 1), which represented 13 cultivars in total because of a replicate (‘Honbeni kintoki-HS’) in both years. Seeds were sown in mid-August in open fields of Kyoto Prefectural Agriculture, Forestry and Fisheries Technology Center at the following two locations, respectively: Agriculture and Forestry Technology Department (Kameoka, Japan, located at 35°00′59″N, 135°33′45″E, altitude 100 m) and Biotechnology Research Department (Seika-cho, Soraku-gun, Japan, located at 34°46′23″N, 135°45′39″E, altitude 102 m). Starter fertilizer (N, 0.112 kg; P2O5, 0.160 kg; and K2O, 0.104 kg m−2) and compost (20 kg m−2) were applied prior to sowing. Plants were cultivated 7 cm apart under natural conditions in plots with two to four rows of length 1.0–1.5 m, and with 20 cm spacing between rows.
| Type | Groupa | Line nameb | Root skin colorc | Source and note |
|---|---|---|---|---|
| Cultivated carrot (Daucus carota var. sativus) | ||||
| Eastern type | ||||
| Japanese cultivar | ||||
| Kintoki | Kintoki early strain | Red | Accession no. PI 321688, USDA-ARS National Plant Germplasm System (original source: ‘Wase kintoki ninjin’, Fujita Seed Co., Ltd., Osaka, Japan (Fujita Seed, personal communication))d | |
| Kintoki | Kintoki regular strain | Red | Accession no. PI 321689, USDA-ARS National Plant Germplasm System (original source: ‘Itto kintoki ninjin’, Fujita Seed Co., Ltd., Osaka, Japan (Fujita Seed, personal communication))d | |
| Kintoki | Konou nakawase kintoki* | Red | Takayama Seed Co., Ltd., Kyoto, Japan | |
| Kintoki | Konou shinbeni kintoki* | Red | Takayama Seed Co., Ltd., Kyoto, Japan | |
| Kintoki | Hakata kintoki ninjin | Red | Derived from a cross between Kintoki variety and a local cultivar ‘Hakata ninjin’ (Ohara 1985, Yamamoto 2002). Nakahara Seed Product Co., Ltd., Fukuoka, Japan | |
| Kintoki | Honbeni kintoki-FT | Red | Fukutane Co., Ltd., Fukui, Japan | |
| Kintoki | Honbeni kintoki-HS | Red | Hashimoto Seeds Co., Ltd., Takamatsu, Japan | |
| Kintoki | Honbeni kintoki-TK | Red | Takii & Co., Ltd., Kyoto, Japan | |
| Kintoki | Honbeni kintoki ninjin | Red | Sanyo Seed Co., Ltd., Himeji, Japan | |
| Kintoki | Shinkou kintoki | Red | Marutane Seed Co., Ltd., Kyoto, Japan | |
| Toso | Yoshino | Orange | Accession no. PI 652138, USDA-ARS National Plant Germplasm System (original source: Matsuwo Seed Co., Ltd., Kagoshima, Japan)e | |
| – | Naga ninjin | Orange | Kiyomasa Farm (Nishiko Co., Ltd.), Kumamoto, Japanf | |
| – | Okinawa shima ninjin | Yellow | Futaba Seed Co., Ltd., Nanjo, Japan | |
| Foreign cultivar | ||||
| USA cultivar | Atomic red | Red | Marche Aozora Co., Ltd., Nagoya, Japan (original source: unknown) | |
| USA cultivar | Nutri-red | Red | Palm Beach Medicinal Herbs, Inc., West Palm Beach, Florida, USA (original source: developed by Robert V. Maxwell, Seminis Vegetable Seeds, Inc./Petoseed Co., Inc., CA, USA in 1996 (Simon 2002)) | |
| Indian cultivar | Pusa rudhira red | Red | Palm Beach Medicinal Herbs, Inc., West Palm Beach, Florida, USA (original source: developed by Indian Agricultural Research Institute, New Delhi, India in 2008 (Indian Agricultural Research Institute 2009, Kalia 2011)) | |
| Hybrid between Eastern and Western types | ||||
| N.A. | Kyokurenai* | Bright red | Hybrid between Kintoki group and ‘Kuroda’-related lines (Sakamaki 2016). Takii & Co., Ltd., Kyoto, Japan | |
| N.A. | Manpukuji oonaga* | Orange | Nippon Norin Seed Co., Ltd., Tokyo, Japan | |
| N.A. | Manpukuji senkou oonaga | Orange | Probable hybrid between ‘Takinogawa oonaga’ and ‘Sapporo oonaga’. Accession no. PI 652129, USDA-ARS National Plant Germplasm System (original source: Kanto-Tozan district, Japan)e | |
| Western type | ||||
| Sanzun | Koyasu sanzun | Orange | Noguchi Seed Co., Ltd., Hanno, Japan | |
| Gosun | Koyo 2 go* | Orange | Takii & Co., Ltd., Kyoto, Japan | |
| Gosun | Tokinashi gosun | Orange | Takii & Co., Ltd., Kyoto, Japan | |
| Danvers | Sannai isshaku | Orange | Noguchi Seed Co., Ltd., Hanno, Japan | |
| Long orange | Kokubu senkou oonaga | Orange | Takii & Co., Ltd., Kyoto, Japan | |
| Wild carrot (D. carora var. carota) | ||||
| N.A. | Robin | Unknown | Fukukaen Nursery & Bulb Co., Ltd., Nagoya, Japan | |
In mid-December (108 and 118–130 days after sowing in 2017 and 2018, respectively), the root fresh weight (RFW), root length (RL), root diameter (RD), root shoulder shape (RSS), and diameter of the collar (petiole base to root) (DC) were measured. Using a colorimeter CR-400 (Konica Minolta Japan, Tokyo, Japan), root skin color was scored at three points (top, middle and lower parts) of the smooth root surface for each individual with regard to the following three parameters: L* (lightness or darkness), a* (red/green coordinate), and b* (yellow/blue coordinate). Subsequently, C* (chroma) and h (hue angle) were calculated according to a previous report (Itle and Kabelka 2009) (Supplemental Table 1). Trait data were subjected to the Tukey-Kramer pairwise multiple comparison test with BellCurve for Excel 2.20 (Social Survey Research Information, Tokyo, Japan) after the removal of outliers. The normality of the trait values in each cultivar was checked prior to the statistical tests.
DNA extraction, genotyping, and data analysisUp to 20 individuals per line from the 25 lines (total, 434 individuals) were investigated using SSR analysis. Genomic DNA was extracted from fresh leaf or root as reported previously (Kubo et al. 2019b). Nine carrot SSR markers (Cavagnaro et al. 2011) were used in the analysis (Supplemental Table 2). These markers were chosen based on clear amplification and polymorphisms in samples from a preliminary experiment. SSR fragments were analyzed regarding the number of alleles per locus (A), allelic richness (AR), observed (HO) and expected heterozygosities (HE), fixation index (FIS), and deviation from Hardy-Weinberg equilibrium (HWE), as reported previously (Kubo et al. 2019a). Linkage disequilibrium (LD) for each locus pair across all populations was tested with GENEPOP 4.2 (Rousset 2008). A population-based neighbor-joining (NJ) phylogram was constructed with Populations 1.2.32 (Langella 2011) using a wild carrot (‘Robin’, D. carota var. carota) as an outgroup. The detection of a hierarchical genetic population structure was achieved using STRUCTURE 2.3.4 (Hubisz et al. 2009), as reported previously (Kubo et al. 2019a).
Prior to the genetic analysis, we measured the root traits of the 14 carrots grown at the two locations (Supplemental Table 1). In the Kintoki group, the values of growth-related traits (RFW, RL, RD, RSS, and DC) were in the ranges of 88.66–187.15 g, 206.67–266.56 mm, 31.38–44.34 mm, 2.00–2.65, and 14.74–18.80 mm, respectively. Overall, the Kintoki cultivars tested showed no significant differences in RFW, RL, RD, RSS, and DC, whereas they were significantly different from ‘Koyo 2 go’ and/or ‘Pusa rudhira red’ regarding RFW, RL, RD, and DC (Supplemental Table 1, capital letters). In contrast to the growth-related traits, root skin color was significantly different in many cases, even among the Kintoki cultivars. In particular, the root color parameters of ‘Konou nakawase kintoki’ and ‘Konou shinbeni kintoki’ were significantly different from those of most of the other tested Kintoki cultivars (and also from those of the other cultivar groups). The Western-type cultivar ‘Koyo 2 go’ differed significantly from the Kintoki cultivars regarding all five root color parameters, as expected from the observed difference in their skin colors (orange and red, respectively) (Supplemental Fig. 1).
Polymorphisms of SSR markers and genetic diversity of the carrot linesAmong the nine SSR markers used in this study, A and AR ranged from 6 to 37 and from 3.365 to 7.581, respectively (Supplemental Table 2). HE ranged from 0.233 to 0.477, from 0.233 to 0.575, from 0.435 to 0.698, and from 0.484 to 0.664 in the Kintoki group, Eastern type (Kintoki, other Japanese Eastern type, and foreign cultivars), Eastern-Western hybrids, and Western type, respectively (Supplemental Table 3). The Western type showed an overall higher HE value than the Eastern type. FIS values did not deviate significantly from HWE in the Japanese Eastern type, including the Kintoki group, but deviated significantly from HWE in two of each of the foreign cultivars, Eastern-Western hybrids, and Western type, respectively (Supplemental Table 3, daggers). The present SSR locus did not show any LD, even though three of them (GSSR-088, GSSR-109, and GSSR-125) were localized on the same linkage group (Supplemental Table 2).
Relationships of carrot cultivars based on the NJ phylogram and population structure analysisIn the population-based NJ phylogram (Fig. 1), the 24 ingroup lines were classified into two groups (I and II). Group I included all Eastern-type cultivars (Fig. 1, red, orange, and ocher colors), with the exception of one Indian cultivar (‘Pusa rudhira red’). Among them, carrots with red-colored roots (Fig. 1, red and ocher colors) could be split further into two subgroups (Fig. 1, arrowhead). Group II was a mixture of Western-type cultivars and Eastern-Western hybrids (Fig. 1, blue and purple colors). ‘Pusa rudhira red’ was included in group II (Fig. 1, ocher color). We investigated the population structure of the 25 carrot lines. At the most suitable number of subpopulations (K) at K = 2 (Supplemental Table 4, underlined) after the calculation of K from 1 to 7, clusters 1 and 2 clearly represented Eastern cultivars and other lines (Eastern-Western hybrid, Western type, and wild carrots), respectively (Fig. 2, black and white colors). Overall, the genetic structure of the Eastern-Western hybrid cultivar ‘Kyokurenai’ was equally owned by the two clusters (Fig. 2, black and white colors).

A population-based neighbor-joining phylogram of 25 carrot lines based on nine SSR loci. The population names of the Kintoki group, other Japanese Eastern-type carrots, foreign cultivars, Western-type carrots, Eastern-Western hybrids, and a wild carrot are indicated in red, orange, ocher, blue, purple, and black, respectively. Two groups (I and II) are indicated with brackets and roman numerals. The node within the Kintoki cluster that separates the two potential subgroups is indicated by an arrowhead. The numbers on the nodes are the bootstrap values from 1,000 replicates (≥50%). Scale bar, genetic distance (DA) (Nei et al. 1983). See online article for color version of the figure.
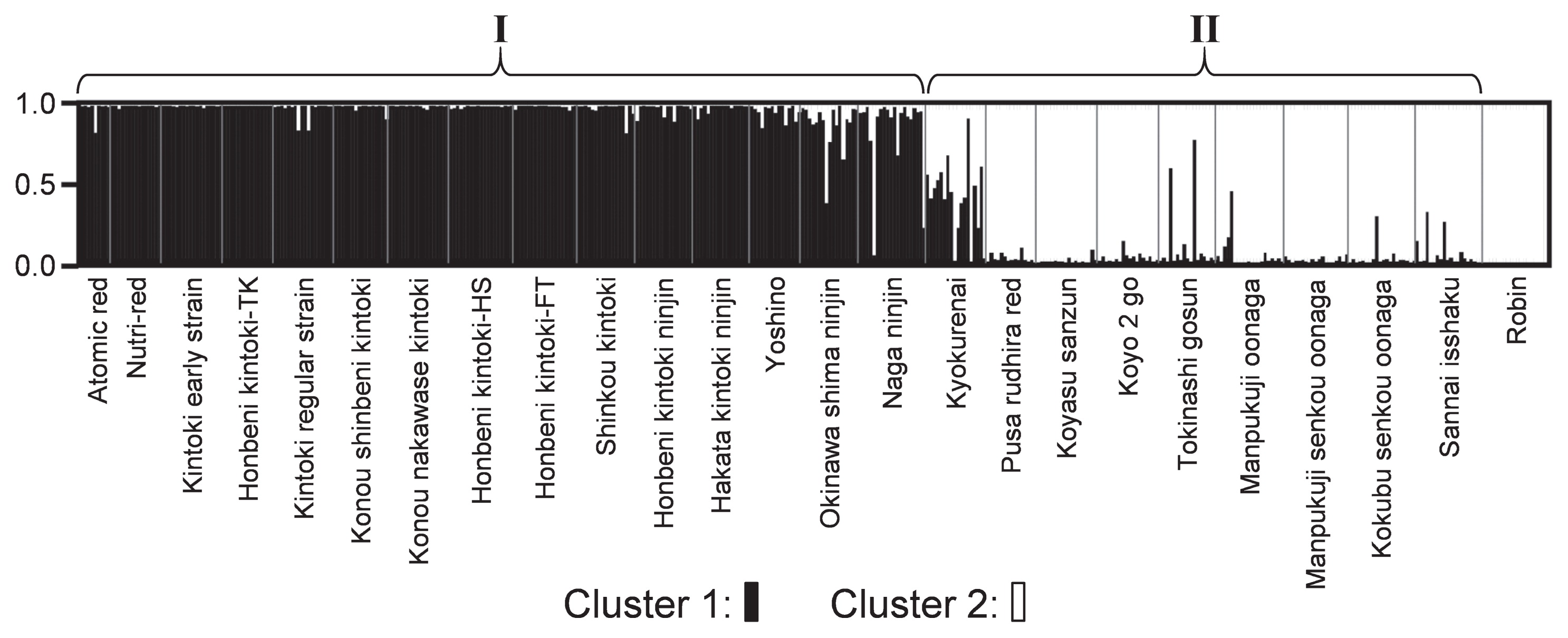
Hierarchical population structure of the 25 carrot lines. The population names are arranged according to the order shown in Fig. 1, from left to right. The populations are separated by gray vertical lines. Individuals within each population are indicated with bar plots, the colors of which correspond to the sum of the assignment probabilities to the two clusters (black and white) at a number of subpopulation (K) = 2. The two potential groups (I and II) observed in Fig. 1 are indicated by brackets and roman numerals at the top.
In this study, we performed a classification of carrots based on SSR markers especially for Eastern-type cultivars, most of which were derived from Japan. The present study included Eastern-type cultivars of yet-to-be-defined relationship (‘Naga ninjin’ and ‘Yoshino’) as well as several Kintoki cultivars. Selected Western-type cultivars were also included as representatives of Japanese Western-type carrots.
First, we conducted field tests for the selected carrot cultivars, including Kintoki and other groups of cultivars, to obtain primary information, because the field-test information available for Kintoki is limited (Katsumata et al. 1966, Kishimoto 1953). The values of growth-related traits (RFW, RL, RD, RSS, and DC) in the Kintoki group were similar to those reported for comparable cultivation periods (Katsumata et al. 1966, Kishimoto 1953). Thus, our data seem to fit the normal values reported previously. Our field tests yielded relatively stable data regarding the growth-related traits, showing clear differences between Kintoki and Western-type cultivars (Supplemental Table 1). This was in good agreement with their differentiation, which would be derived from the varying processes of establishment (Vavilov 1951). In contrast, significant differences were detected in root skin colors, even among Kintoki cultivars. Such intercultivar differences, even in Kintoki group, were not surprising, because the previous report mentioned that important factors for determining root color were variety (cultivar) and season, followed by maturity at harvest and soil type (Yano et al. 1981). ‘Honbeni kintoki-HS’ showed differences in root skin color between the two locations in the same year. This might be an environmental effect, as carrots generally tend to exhibit a variation in carotenoid content according to conditions such as plant growth, temperature, and soil components (Katsumata et al. 1966). As a result, we confirmed the differentiation between Kintoki and the other carrots regarding the growth related traits.
Next, we performed a genetic analysis of the carrot lines. The average value of A in this study (18.9) was higher than those reported previously (7.6, 9.2, 5.8, and 9.4) (Baranski et al. 2012, Clotault et al. 2010, İpek et al. 2016, Maksylewicz and Baranski 2013). The present FIS values suggest that most of the Japanese Eastern-type cultivars investigated here were under a less stringent selection pressure (Supplemental Table 3). These cultivars seem to be maintained under sufficient population size and genetic diversity, because carrot is an outcrossing species that easily shows inbreeding depression by selfing. In the SSR-based NJ phylogram, the 24 ingroup lines were classified into two groups (I and II), each of which represented the Eastern cultivars and the other types. Although a few terminal nodes were supported with relatively high bootstrap values, no large group was supported by ≥50% bootstrap value. This could be because many of the lines were closely related and because some cultivars might be derived from an intercross between distantly related lines. The number of DNA loci used is of importance in population analyses. Although more than tens of SSR loci result in a good resolution of classification, six or more markers could be sufficient for the detection of population structures (e.g., Politov et al. 2015, Urrestarazu et al. 2015). In fact, the present study, which was based on nine SSR markers, resulted in a clear separation of Eastern-type cultivars from group II, which was composed of Western-type and Eastern-Western hybrid cultivars, with only one exception, ‘Pusa rudhira red’ (Figs. 1, 2). This result confirmed the differentiation of the two types of carrot, as reported previously (Baranski et al. 2012, Iorizzo et al. 2013).
All Kintoki and two USA red carrot cultivars (‘Atomic red’ and ‘Nutri-red’) were included in group I (Fig. 1, red color). This strongly suggests a close relationship among the Kintoki (and red carrot) cultivars examined in this study. The two subgroups detected in group I (Fig. 1, arrowhead) suggests a potential differentiation of the Kintoki group cultivars. Their significance is currently unknown. The pedigrees of the two USA red carrots might be a clue to address the potential differentiation, although no detailed information is available (Simon 2002). Concerning the three local Japanese non-red root cultivars, ‘Naga ninjin’, ‘Okinawa shima ninjin’, and ‘Yoshino’, only ‘Shima ninjin (Okinawa shima ninjin)’ has previously been confirmed to be part of the Eastern type (Baranski et al. 2012, Maksylewicz and Baranski 2013). The present study demonstrated that all three cultivars truly belong to the Eastern type, which were separate from the Western type and Eastern-Western hybrids (Figs. 1, 2). It is interesting to note that ‘Hakata kintoki ninjin’ was placed at a relatively basal position among the red carrots and, thus, close to the three non-red root cultivars mentioned above (Fig. 1, orange color). This may reflect the pedigree of ‘Hakata kintoki ninjin’, which is derived from a cross between the Kintoki variety and the yellow root cultivar ‘Hakata ninjin’ (Ohara 1985, Yamamoto 2002) (Table 1). The latter once existed in the Kyushu region until the 1960s (Katsumata 1968a, Ohara 1985). ‘Pusa rudhira red’ was excluded from group I (Fig. 1). This cultivar was also peculiar in the field tests, as it showed significant differences in three growth-related traits: RFW, RD, and DC (Supplemental Table 1, capital letters). ‘Pusa rudhira red’ was developed by the Indian Agricultural Research Institute and is derived from farmer material grown locally in the North Indian plain (Indian Agricultural Research Institute 2009, Kalia 2011, Dr. P. Kalia, personal communication). As India is geographically close to the origin of carrots (Afghanistan), this cultivar might retain a primitive genetic background, prior to the split of the Eastern and Western varieties. In fact, the center of the umbel (flower head) in ‘Pusa rudhira red’ has a few red-purple flowers that are surrounded by white flowers (Supplemental Fig. 2), which is a characteristic that is often observed in wild carrots (e.g. Westmoreland and Muntan 1996). In this sense, the investigation of wild accessions from the Central Asiatic Center might be interesting, but Iorizzo et al. (2013) have already demonstrated that such wild accessions formed a sister clade to all cultivated carrots.
We investigated three cultivars that are derived from a cross between Eastern and Western types: one ‘Kyokurenai’ and two ‘Manpukuji oonaga’ lines. ‘Kyokurenai’ was located at a neighbor cultivar of the Eastern-type group (group I) in the phylogram (Fig. 1) and contained two clusters that would represent the Eastern and Western types identified in the structure analysis (Fig. 2, black and white colors). These results were in good agreement with the pedigree data: ‘Kyokurenai’ was bred by crossing the Kintoki and Gosun group lines (Sakamaki 2016). The genetic background of Kintoki would have placed ‘Kyokurenai’ at the closest position to group I. Two ‘Manpukuji oonaga’ lines formed a cluster with ‘Kokubu senkou oonaga’ and ‘Sannai isshaku’ (Fig. 1). This result was reasonable because one parent of ‘Manpukuji oonaga’ (‘Sapporo oonaga’) belongs to the “Long orange” group, as does ‘Kokubu senkou oonaga’. No genetic information is available for the other parent (‘Takinogawa oonaga’) because of extinction (Ito 1975, Kumazawa and Katsumata 1965, Ohara 1985), although its morphology has been recorded (Matsubara 1951, Matsuoka 1950). The genetic background of the contemporary ‘Manpukuji oonaga’ may be mostly derived from the Western type (Fig. 2, white color). A genetic drift during its breeding process after hybridization might have shifted ‘Manpukuji oonaga’ to a position closer to the Western type.
In conclusion, the present study elucidates the genetic characteristics of the Eastern type of carrot, which were unclear. The properties of their root traits were also investigated. The information obtained in this study will be valuable information for carrot breeding, especially when using the Eastern type as a source.
NK, MT, KO, TF, and YM designed this study. MT, KO, TF, and YM cultivated the carrot samples. NK, RY, MT, and YM evaluated the root traits. NK and RY performed the phylogenetic, genetic diversity, and population structure analyses. NK and YM drafted the manuscript. All authors read and approved the final manuscript.
We thank USDA-ARS National Plant Germplasm System for providing carrot seeds, Kyoto Integrated Science & Technology Bio-Analysis Center for technical support in part of the genotyping analyses, Prof. P.W. Simon, Fujita Seed Co., Ltd., Dr. P. Kalia, Mr. T. Kondo, and Kumamoto City for cultivar information, and Ms. H. Kasaoka for technical assistance. This work was supported by a grant from Kyoto Prefectural Government.