Abstract
Many different types of chemicals are used in industry, and occupational allergies are becoming a serious problem in the field of industrial hygiene. In this study, we employed a novel enzyme-linked immunosorbent assay (ELISA) with partial peptides of human serum albumin (HSA) to quantify chemical-specific immunoglobulin G (IgG) in serum for evaluating exposure to chemicals. When HSA partial peptides containing lysine residues were mixed with formaldehyde (FA) or phthalic anhydride (PA), almost all lysine residues were lost. Mass spectrometry revealed that PA and FA formed imine and tertiary amine, respectively, with lysine residues in the peptides. Thus, we used FA- or PA-peptide adducts as an artificial antigen to detect FA- and PA-specific IgGs in serum. The concentrations of FA- and PA-specific IgGs in workers at plants utilizing plastic resins were significantly higher than those in general subjects. This method can estimate exposure levels to chemicals and thus be expected to contribute to the diagnosis of allergies in workers and to the prevention of health hazards due to harmful chemicals.
INTRODUCTION
Increasing evidence shows that occupational allergies, such as dermatitis and asthma, could be due to chemicals used for producing plastic polymers; occupational allergies have become a serious problem in the field of industrial hygiene (Bernstein, 1997). Patch test and skin prick tests are commonly used to diagnose causative chemicals for allergic dermatitis. However, these tests have the risk of sensitizing patients (Hillen et al., 2003), and alternative diagnoses are therefore essential. In general, low-molecular-weight compounds are considered haptens and thus cannot elicit an immunoreaction, although reactive substances can bind to carrier proteins to trigger immunogenicity. For instance, several kinds of chemical-protein adducts have been identified in the human body, such as adducts with human serum albumin (HSA), hemoglobin, cytokeratin 18, glucose-regulated protein, actin and tubulin (Day et al., 1996; Lange et al., 1999; Wisnewski et al., 2000; Hettick and Siegel, 2011). Therefore, measurement of chemical-specific immunoglobulin G (IgG) and/or immunoglobulin E (IgE) in the blood is considered an effective approach for diagnosing exposure to chemicals and occupational allergies without sensitization risks. Because blood concentrations of IgG are much higher than those of IgE, specific IgG can be a more useful diagnostic marker of occupational allergy than IgE considering the sensitivity of the measurement. In fact, specific IgE has been shown to identify inhalation challenge-positive workers with 14%-31% sensitivity and 89%-97% specificity, while the corresponding sensitivity and specificity estimates for specific IgG were reported to be 46%-72% and 74%-92%, respectively (Bernstein et al., 2006).
Several methods to detect chemical-specific IgG in the blood have been developed. In particular, measurements targeting toluene-2,4-diisocyanate (TDI) have been widely investigated as diagnostic markers, and enzyme-linked immunosorbent assays (ELISAs) with TDI-HSA adducts have been used as a substrate challenge (Park et al., 2002). An anti-TDI haptenated protein monoclonal antibody was reported as a tool for biomonitoring of TDI exposure (Ruwona et al., 2011), and this antibody was also applied to sandwich ELISAs to detect aromatic diisocyanates adducts (Lemons et al., 2013). An ELISA using formaldehyde (FA) and phthalic anhydride (PA)-HSA adducts as a substrate was also reported (Dykewicz et al., 1991; Nielsen et al., 1988). We previously reported a dot blotting-based method for the detection of chemical-specific IgGs, including TDI, FA and PA (Tsuji et al., 2016; Kawamoto et al., 2015).
ELISAs and dot blotting methods that have been reported for the detection of chemical-specific IgGs employ full-length HSA or other proteins as carriers to produce artificial antigens. Chemicals can react with multiple amino acids in the full-length carrier proteins. For instance, TDI is reported to react with 37 amino acids, including lysine, aspartic acid and glutamine, in HSA (Hettick et al., 2012). Thus, multiple antibodies with different epitopes are detected if full-length carrier proteins are used as an artificial antigen, which could be one reason why it is difficult to determine the relationships between chemical-specific antibodies and occupational allergies.
ELISA is convenient for several diagnoses because it can analyze many samples with a brief period. In this study, we aimed to construct an ELISA for determination of chemical-specific IgGs in sera using an HSA partial peptide having a limited reactive amino acid residue as an antigen. This ELISA is expected to detect single chemical-specific antibodies, which could have advantages in exactly estimating exposure levels to chemicals as well as in reducing measurement error. The carbonyl compounds FA and PA were selected for specimens, as large amounts of FA and PA have been used in industry and FA and PA reportedly elicit occupational allergies (Fowler et al., 1992; Wernfors et al., 1986). We designed an HSA partial peptide containing lysine residues because carbonyl compounds can bind to amino groups through a nucleophilic addition reaction. The structure of chemical-peptide adducts was revealed by matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS), and the ELISA was tested with serum samples from general subjects and workers in the plastics industry.
MATERIALS AND METHODS
Preparation of chemical-HSA peptide complexes
Two kinds of biotin-conjugated HSA partial peptides were synthesized and purified to the > 95% level at Eurofins Genomics (Tokyo, Japan): HSA Peptide Lys1: Biotin-ALLVRYTKKVPQVSTP-COOH (MW = 2026.45) and HSA Peptide Lys2: Biotin-ERQIKKQTALVELV-COOH (MW = 1881.24). FA and PA were dissolved in DMSO to a final concentration of 100 mM. HSA partial peptides were dissolved in PBS to a final concentration of 5 mM. Peptides were mixed with FA or PA in 100 mM sodium carbonate buffer, pH 10.8, or phosphate buffer, pH 8.0, respectively, with final concentrations of 0.1 mM peptide and 10 mM FA or PA. The mixture was incubated for 1 hr at 37°C. After centrifugation, the supernatant was used as a chemical-peptide adduct.
Measurement of the amino groups
The reaction of 2,4,6-trinitrobenzenesulfonic acid (TNBS) with primary amino groups within peptides was performed as previously described (Tsuji et al., 2016). The chemical-peptide adducts (25 μL) were mixed with 50 μL of 4% NaHCO3, followed by the addition of 50 μL of 0.01% TNBS. The reaction was carried out at 42°C for 2 hr and then was stopped by the addition of 25 μL HCl. The absorbance was measured at 340 nm. Known concentrations of L-lysine were used as standards.
MALDI-TOF MS analysis
The chemical-peptide adducts were diluted 10-fold in distilled water, and 0.5-μL samples were added on the sample plate. Then, 0.5 μL of 10 mg/mL 2,5-dihydroxy benzoic acid (in 0.1% trifluoroacetic acid: acetonitrile = 3: 2 solution) was plated. After air drying, each sample was analyzed using an AXIMA QIT MALDI TOF mass spectrometer (Shimadzu, Kyoto, Japan). All spectra were acquired in the positive linear mode. Mass calibration was performed using two internal standards, angiotensin II ((M+H)+ at m/z 1046.5423) and ACTH fragment18-39 ((M+H)+ at m/z 2465.1989), with the ProteoMass Peptide and Protein MALDI-MS Calibration Kit (Sigma-Aldrich, St. Louis, MO, USA).
Study subjects
Worker subjects (N = 10) were recruited at a company handling plastic resins (plastic resins mainly used: phenolic resins, polyester resins, polyurethane resins) in Kyushu, Japan from 2013 to 2015. Their mean age was 39.1 years old and the average length of service was 11.5 years. None of the subjects handled plastic resins outside the workplace. General subjects (unexposed during work, N=10) were recruited in Kyushu in 2014. Their mean age was 46.4 years old. The study was approved by the Ethics Committee of the University of Occupational and Environmental Health in 2013 (H25-008).
Serum preparation
After the collection of blood samples, the blood was allowed to clot for approximately 3 hr and was then centrifuged at 2,500 × g for 30 min to separate the serum portion of the blood. The sera were transferred to clean tubes and stored at −80°C until use.
Enzyme-linked immunosorbent assay (ELISA)
One hundred microliters of HSA peptide Lys1 or 2 at a final concentration of 5 μM were added to a streptavidin-coated 96-well plate (IMMOBILIZER, Thermo Scientific, Waltham, MA, USA), and the plate was incubated for 1 hr at room temperature. The same concentration of biotin was used as a blank control. FA or PA was added to the wells at a final concentration of 0.5 mM, followed by incubation for 1 hr at 37°C. The plate was blocked with protein-free blocking buffer (Pierce), and then human serum was added to each well (1/100 dilution for FA and 1/1,000 dilution for PA). After 3 hr of incubation at room temperature, HRP-conjugated anti-human IgG was added to the wells for 1 hr. Next, 1-Step Ultra TMB-ELISA Substrate Solution (Thermo Scientific) was added to the wells, and then the reaction was stopped with sulfuric acid. The absorbance at 450 nm was measured to determine the chemical-specific IgGs in serum. Human IgG (Human IgG, Whole Molecule Control, Thermo) was biotinylated using the Biotin Labeling Kit-NH2 (DOJINDO LABORATORIES, Kumamoto, Japan) according to the manufacturer’s instruction and then used as a standard.
Statistical methods
Two-group comparisons were performed using Mann-Whitney U test. All analyses were performed by the BellCurve for Excel (Social Survey Research Information, Tokyo, Japan), and statistical significance was assumed when p < 0.05 (two-sided).
RESULTS AND DISCUSSION
Modulation of HSA partial peptide by chemicals
FA and PA can react with lysine residues with high pKa values in proteins because of their nucleophilic carbonyl groups. HSA contains 59 lysine residues with different reactivity with nucleophiles. Hettick et al. (2012) reported that 5 of the lysine residues in HSA, Lys351, Lys413, Lys414, Lys524 and Lys525, were highly reactive with the vapor phase of toluene isocyanate. Therefore, we focused on 2 series of lysine (Lys413-414 and Lys524-525) to design 2 peptides, HSA peptide Lys1 and HSA peptide Lys2 (See Materials and Methods).
We first investigated the number of lysine residues in peptides after reaction with FA and PA. The number of amino groups in HSA peptide Lys1 or HSA peptide Lys2 was 55.7 ± 1.2 and 62.7 ± 8.2 nmol/mg peptide, respectively, indicating that almost all lysine residues were free in each peptide (Fig. 1). When FA and PA were mixed with HSA peptide Lys1 and HSA peptide Lys2 under basic conditions, the lysine residues of both HSA peptides were almost completely lost (Fig. 1). Therefore, lysine residues in the peptides were modified by the reaction with FA or PA. To identify the chemical structure of the reactants, we next measured the molecular mass of the chemical-HSA peptide adducts by MALDI-TOF MS. Comparing the MS spectra of HSA peptide Lys1 with that of its FA adducts, the peaks of the FA adducts were approximately 24 m/z higher than those of unreacted peptide (Fig. 2A). The adducts with HSA peptide Lys2 and FA also showed 24 m/z higher peaks than unreacted HSA peptide Lys2 (data not shown). Therefore, two imines could be produced by the reaction of the carbonyl groups in FA with the primary amino group in HSA peptide Lys1 (Fig. 2C). The peaks of the PA adducts of HSA Peptide Lys1 were higher by approximately 130 m/z than those of unreacted HSA peptide Lys1 (Fig. 2B), and almost the same shift was observed in the case of HSA peptide Lys2 reacted with PA (data not shown). Based on these MS analyses, tertiary amines could be produced by nucleophilic attack of primary amino groups against the carbonyl group of PA (Fig. 2C). The TNBS measurement showed that almost all amino groups reacted with PA (Fig. 1); however, peaks derived from the unreacted HSA peptide Lys1 were observed in the sample of PA-HSA peptide Lys1 adducts. Thus, PA adducts might be degraded by the laser at the step of peptide ionization to generate the original peptide.

We collected serum from general subjects and workers at plants utilizing plastic resins, and we then determined the concentrations of FA- and PA-specific IgG in sera by ELISA using HSA peptides. HSA peptide Lys1 or Lys2 was bound to the bottom of the plate through a streptavidin-biotin interaction. The lysine residues of the peptides were modified by adding FA or PA to the well to produce artificial antigens on the plate, and then the serum was applied to each well to determine the amount of chemical-specific IgG in the serum (Fig. 3A). Based on control human IgG challenges, the detection limit of this ELISA was 0.1 ng/mL antibody in serum. Anti-FA-HSA peptide Lys1 and anti-FA-HSA peptide Lys2 antibodies were detected in the serum of both general subjects and workers (Fig. 3B and C). However, the levels of anti-FA-HSA peptide Lys1 antibody in the serum of workers were significantly higher than those of the general subjects (Fig. 3B). No difference was detected in the levels of anti-FA-HSA peptide Lys2 antibody between the general subjects and the workers (Fig. 3C). The amount of anti-PA-HSA peptide Lys1 antibody in the serum of the workers was significantly higher than that of general subjects, but there was no significant difference in the levels of anti-PA-HSA peptide Lys2 between the 2 groups (Fig. 3D and E). Interestingly, the concentration of antibodies for HSA peptide Lys1 was much higher than that for HSA peptide Lys2, suggesting that modification of HSA peptide Lys413-414 with chemicals showed robust immunogenicity and that the antibody for HSA peptide Lys1 can reflect exposure more effectively against chemicals than that for HSA peptide Lys2. Taken together, our ELISA with partial HSA, especially HSA peptide Lys1, could measure chemical-specific IgG in serum with high sensitivity.
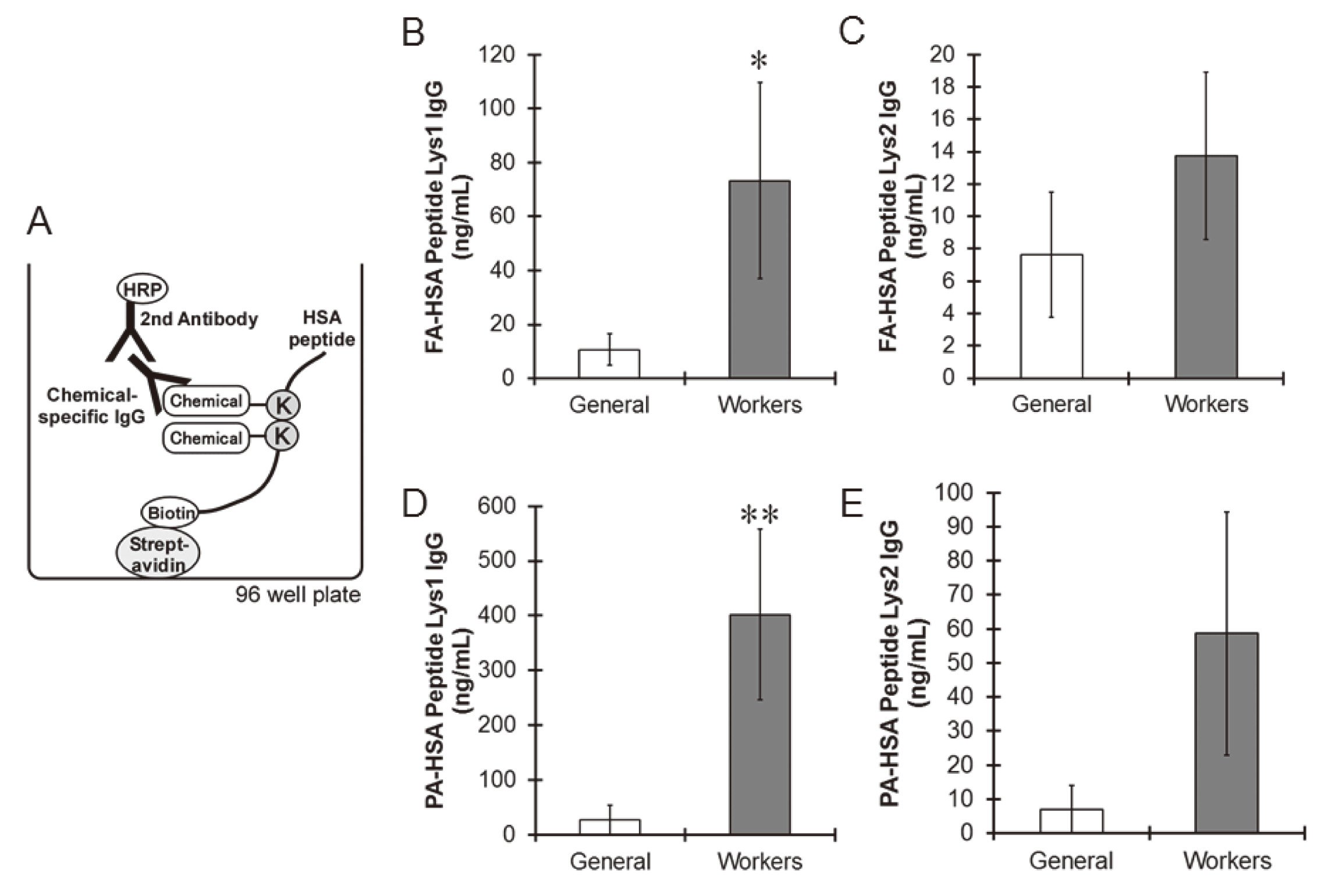
General subjects also may have come into contact with these chemicals in daily life because FA and PA are used as adhesives, lacquers and hardeners, which might be the reason why antibodies for anti-FA HSA peptide and anti-PA-HSA peptide were detected in the serum of the general subjects. Because the workers are exposed to FA and PA due to their usage of FA and PA in the workplace, they showed high concentrations of IgGs specific for FA and PA in serum, indicating the useful application of the ELISA developed here for evaluation of FA and PA exposure. Several workers with high levels of FA- or PA-specific antibody showed allergic symptoms (data not shown). Therefore, this ELISA might be useful for the diagnosis of occupational allergies.
Several studies have detected chemical-specific IgG by ELISA; in these studies, full-length HSA or other proteins were used as a carrier (Dykewicz et al., 1991; Nielsen et al., 1988). However, in such cases, it is difficult to evaluate the relationships between the concentration of IgG and industrial allergies, as detected IgGs constitute a mixture of different IgGs. Using the ELISA developed in this study, chemical-HSA peptide can be immobilized onto the bottom of a 96-well plate in the same direction because of the streptavidin-biotin interaction, which can contribute to higher reproducibility. Importantly, the advantage of this ELISA is that it can detect a single chemical-specific antibody, which could be useful for understanding the relationships between serum chemical-specific IgG concentrations and allergy in industry. The next step is to examine the relationships between occupational allergy and the IgGs that were measured in this study.
Industrial chemicals other than FA and PA react with specific amino acids in protein; for instance, methyl acrylate, which is a material for acrylic fiber and acrylic resin, strongly reacts with cysteine (Böhme et al., 2009). The HSA molecule includes 35 cysteine residues, but only Cys34 is free, whereas the others form disulfides. Therefore, a partial HSA peptide containing Cys34 might be used for the evaluation of exposure to methyl acrylate. Again, this novel ELISA with HSA peptide can be applied to other chemicals besides FA and PA to evaluate exposure and occupational allergies.
In conclusion, a novel peptide ELISA was established for the detection of chemical-specific IgG in serum. This method can estimate exposure levels to chemicals and thus be expected to contribute to the diagnosis of allergies in workers and to the prevention of health hazards due to harmful chemicals.
ACKNOWLEDGMENTS
This work was partially supported by grants from the Ministry of Education, Culture, Sports, Science and Technology, Japan, KAKENHI for Y.I. and M.T. (Nos. 17H04714 and 17K09174), a grant from Hiroshima University Industry-Academia Collaboration Program for Y.I. and an Industrial Disease Clinical Research Grant to M.T. This manuscript has been checked by a professional language editing service (American Journal Experts).
Conflict of interest
The authors declare that there is no conflict of interest.
REFERENCES
- Bernstein, D.I. (1997): Allergic reactions to workplace allergens. JAMA, 278, 1907-1913.
- Bernstein, D.I., Ott, M.G., Woolhiser, M., Lummus, Z. and Graham, C. (2006): Evaluation of antibody binding to diisocyanate protein conjugates in a general population. Ann. Allergy Asthma Immunol., 97, 357-364.
- Böhme, A., Thaens, D., Paschke, A. and Schüürmann, G. (2009): Kinetic glutathione chemoassay to quantify thiol reactivity of organic electrophiles--application to alpha,beta-unsaturated ketones, acrylates, and propiolates. Chem. Res. Toxicol., 22, 742-750.
- Day, B.W., Jin, R. and Karol, M.H. (1996): In vivo and in vitro reactions of toluene diisocyanate isomers with guinea pig hemoglobin. Chem. Res. Toxicol., 9, 568-573.
- Dykewicz, M.S., Patterson, R., Cugell, D.W., Harris, K.E. and Wu, A.F. (1991): Serum IgE and IgG to formaldehyde-human serum albumin: lack of relation to gaseous formaldehyde exposure and symptoms. J. Allergy Clin. Immunol., 87, 48-57.
- Fowler, J.F. Jr., Skinner, S.M. and Belsito, D.V. (1992): Allergic contact dermatitis from formaldehyde resins in permanent press clothing: an underdiagnosed cause of generalized dermatitis. J. Am. Acad. Dermatol., 27, 962-968.
- Hettick, J.M. and Siegel, P.D. (2011): Determination of the toluene diisocyanate binding sites on human serum albumin by tandem mass spectrometry. Anal. Biochem., 414, 232-238.
- Hettick, J.M., Siegel, P.D., Green, B.J., Liu, J. and Wisnewski, A.V. (2012): Vapor conjugation of toluene diisocyanate to specific lysines of human albumin. Anal. Biochem., 421, 706-711.
- Hillen, U., Frosch, P.J., Franckson, T., Pirker, C. and Goos, M. (2003): Optimizing the patch-test concentration of para-tertiary-butylcatechol: results of a prospective study with a dilution series. Contact Dermat., 48, 140-143.
- Kawamoto, T., Tsuji, M. and Isse, T. (2015): Comparison of IgG against plastic resin in workers with and without chemical dermatitis. BMC Public Health, 15, 930.
- Lange, R.W., Lantz, R.C., Stolz, D.B., Watkins, S.C., Sundareshan, P., Lemus, R. and Karol, M.H. (1999): Toluene diisocyanate colocalizes with tubulin on cilia of differentiated human airway epithelial cells. Toxicol. Sci., 50, 64-71.
- Lemons, A.R., Bledsoe, T.A., Siegel, P.D., Beezhold, D.H. and Green, B.J. (2013): Development of sandwich ELISAs for the detection of aromatic diisocyanate adducts. J. Immunol. Methods, 397, 66-70.
- Nielsen, J., Welinder, H., Schütz, A. and Skerfving, S. (1988): Specific serum antibodies against phthalic anhydride in occupationally exposed subjects. J. Allergy Clin. Immunol., 82, 126-133.
- Park, H.S., Lee, S.K., Kim, H.Y., Nahm, D.H. and Kim, S.S. (2002): Specific immunoglobulin E and immunoglobulin G antibodies to toluene diisocyanate-human serum albumin conjugate: useful markers for predicting long-term prognosis in toluene diisocyanate-induced asthma. Clin. Exp. Allergy, 32, 551-555.
- Ruwona, T.B., Johnson, V.J., Hettick, J.M., Schmechel, D., Beezhold, D., Wang, W., Simoyi, R.H. and Siegel, P.D. (2011): Production, characterization and utility of a panel of monoclonal antibodies for the detection of toluene diisocyanate haptenated proteins. J. Immunol. Methods, 373, 127-135.
- Tsuji, M., Yu, H.S., Ishihara, Y., Isse, T., Ikeda-Ishihara, N., Tuchiya, T. and Kawamoto, T. (2016): A Simple Method for Detection of Multiple Chemical-Specific IgGs in Serum Based on Dot Blotting. Health, 8, 1645-1653.
- Wernfors, M., Nielsen, J., Schütz, A. and Skerfving, S. (1986): Phthalic anhydride-induced occupational asthma. Int. Arch. Allergy Appl. Immunol., 79, 77-82.
- Wisnewski, A.V., Srivastava, R., Herick, C., Xu, L., Lemus, R., Cain, H., Magoski, N.M., Karol, M.H., Bottomly, K. and Redlich, C.A. (2000): Identification of human lung and skin proteins conjugated with hexamethylene diisocyanate in vitro and in vivo. Am. J. Respir. Crit. Care Med., 162, 2330-2336.



