2018 Volume 43 Issue 10 Pages 579-586
2018 Volume 43 Issue 10 Pages 579-586
Bisphenol A (BPA) is an endocrine disrupting chemical used on a wide range in industry. This compound has been used in the production of polycarbonate plastics and epoxy resins. For this reason and their global use, BPA is one of the most common environmental chemicals to which humans are exposed. This exposure can cause several adverse health outcomes, including at the cardiovascular level. The regulation of ion channels in vascular smooth muscle is pivotal and important for vasoreactivity, and changes in their flux can be involved in the pathophysiology of some cardiovascular diseases. This study aims to analyse in rat aorta whether the vasorelaxant effect of BPA is mediated by L-type Ca2+ channels inhibition. Using male Wistar rat aorta artery rings in the organ bath we analysed the contractility, and to study the activity of calcium current in A7r5 cells we used the whole cell configuration of Patch Clamp technique. Regarding the contractility experiences we observed that in both NA and KCl contraction, BPA caused a rapid and concentration-dependent relaxation. The electrophysiology experiments showed that BPA inhibited the basal and BAY K8644-stimulated whole-cell L-type Ca2+ channel (W-CLTCC) currents, indicating that this drug blocks the L-type Ca2+ channels. Our results suggest that BPA inhibits the W-CLTCC, leading to the relaxation of vascular smooth muscle.
Bisphenol A (BPA; 4,40-dihydroxy-2,2-diphenylpropane; CAS80-05-7) has a long history in science. The high human exposure to BPA is due to the ubiquitous, extensive and indiscriminate use of plastic material (Dekant and Völkel, 2008). The global population is subject to repeated exposure to BPA, with the main paths of BPA exposure being oral intake, inhalation, and dermal absorption (Braunrath et al., 2005; Vandenberg et al., 2013; Biedermann et al., 2010; Loganathan and Kannan, 2011; Aboul Ezz et al., 2015). Most studies based on the analysis of blood samples from adults found that the concentration of unconjugated BPA, which is the biologically active form, ranges from 0.2 to 10 ng/mL (0.9-44 nmol/L) (Vandenberg et al., 2010, 2012a; Alonso-Magdalena et al., 2012). However, other study reported that unconjugated BPA represents no more than 2% of the total BPA in blood, leading to a plasma concentration of unconjugated BPA < 0.02 ng/mL (< 0.1 nmol/L) (Teeguarden et al., 2011). BPA has been demonstrated both in vivo and in vitro to act as an endocrine disrupting chemical (Fenichel et al., 2013). The actions of this kind of chemicals are mediated by endocrine-signaling pathways that act as powerful amplifiers, leading to changes in the cell function in response to extremely low and/or high concentrations (Welshons et al., 2003).
The effects of chronic exposure to BPA have been extensively investigated, but relatively little is known about the physiological response of the cardiovascular system (Posnack et al., 2015; Michaela et al., 2014). Some authors suggested that BPA can directly bind to both α and β estrogen receptors and imitate some actions of estrogen (Gould et al., 1998; Kuiper et al., 1998; Markey et al., 2001). On the other hand, other studies have demonstrated that it can act as an antiestrogen, by blocking the estrogenic response by competing with 17-β-estradiol (Richter et al., 2007; Bonefeld-Jørgensen et al., 2007). Furthermore, BPA blocks the action of endogenous androgen, being considered as an antiandrogenic (Sohoni and Sumpter, 1998; Wetherill et al., 2007), and it can have an agonist and antagonist effect on thyroid function (Wetherill et al., 2007; Moriyama et al., 2002). Besides these long-term actions, a few studies have shown that BPA also exerts short-term effects, but these effects are still contradictory. Walsh et al. have demonstrated that nanomolar concentration of BPA caused rapid increase of intracellular calcium concentration in human breast cancer cells (Nadal et al., 2000; Walsh et al., 2005). On the other hand, BPA can also induce an inhibition of both T-type and L-type calcium channels (Deutschmann et al., 2013), being responsible for the development of some cardiovascular pathologies (Hajszan and Leranth, 2010; Aloisi et al., 2002; Rubin, 2011).
In addition to BPA having effects on ion channels, this Endocrine Disrupting Chemical (EDC) can also have effects in some parameters of oxidative stress, namely nitric oxide (NO). Thus, since NO is deeply involved in vascular diseases, such as hypertension, Ezz et al. studied the effect of BPA exposure on NO levels in the heart of male albino rats. The authors suggested that BPA has an adverse effect on the rat hearts, mainly due to the production of reactive oxygen species (ROS). In addition, the decrease of NO levels induced by BPA may be considered the mainly mechanism for cardiovascular diseases (CVD) development (Aboul Ezz et al., 2015).
The vascular nongenomic actions of BPA may be responsible for calcium channels inhibition and can induce vasorelaxation. However, before the real impact of BPA on vascular cells can be clearly assessed, more experimental studies are needed to understand its mechanisms. So, our aim was to analyse the effect of BPA in rat aortic smooth muscle and the mechanisms involved in vasorelaxation effect of BPA. For that purpose, the BPA effect on rat aorta without endothelium was analysed. Furthermore, we used A7r5 cells to analyse BPA effects on whole-cell L-type voltage dependent Ca2+ current (ICa,L).
Male adult Wistar rats (Charles-River, Barcelona, Spain) were used in accordance with European Union regulations on the protection of animals (Directive 86/609) and the Guide for the Care and Use of Laboratory Animals promulgated by the US National Institutes of Health (NIH Publication No 85-23, revised 1996). The treatment of the Wistar rats before decapitation and after thoracotomy was similar to other works published by our group (Cairrão et al., 2012; Mariana et al., 2018). Briefly, the rats weighed 400-500 g and were housed and acclimatized for at least one week before performing the experiments, with a light cycle of 12 hr light: 12 hr dark, and food and water were provided ad libitum. After the thoracic aorta ring isolation, these arteries were equilibrated for 60 min until a resting tension of 1.0 g, in a Krebs’ modified solution at 37ºC. Subsequently, the aortic rings were firstly contracted with noradrenaline (NA; 0.1 μmol/L) and the absence of endothelium functionality was confirmed by the lack of relaxant response to acetylcholine (1 μmol/L). Afterwards, the arteries were washed many times for at least 45 min before the next stimuli. The rings were contracted using NA (0.1 μmol/L) or KCl (60 mmol/L), on these contractions the effect induced by BPA was analysed. The BPA concentrations used ranged from 0.001 μmol/L-100 μmol/L according to Michaela et al. (2014). These concentrations are also in accordance with the ones used for sex steroids, which go from physiologic to pharmacologic concentrations (Cairrão et al., 2012, 2008; Alvarez et al., 2010). The ethanol was used to perform control experiments, at the same percentage that was used dissolve BPA.
Cell culture of vascular smooth muscle cells (VSMC)The cells used to perform electrophysiology experiments were A7r5. These cells are a vascular smooth muscle cell line obtained from embryonic rat aorta (Promochem, Barcelona, Spain). The cells were grown according to Cairrão et al. (2012)
Electrophysiology experimentsThe Patch Clamp technique was used to analyse the W-CLTCC currents. This technique was done according to Alvarez et al. (2010) and Cairrão et al. (2012). Different concentrations of BPA (0.001-100 μM), previously dissolved in external solution, were studied in basal and BAY K8644-stimulated (10 nM) ICa,L.
DrugsAll drugs used in this work were purchased from Sigma-Aldrich Química (Sintra, Portugal). BPA was a generous gift from Quintaneiro C, from the University of Aveiro.
The initial dilutions of BPA and BAY K8644 were done in ethanol, and the next appropriate dilutions were performed in Krebs’ modified solution or in the corresponding electrophysiology external solution. The maximal concentration of ethanol used was 0.01%. Noradrenaline was initially dissolved in distilled water and, as for the other drugs, the subsequently concentration was performed according to the experiment achieved. All the drugs were prepared every day before the experiment.
Statistical analysisThe program SigmaStat Statistical Analysis System version 3.5 (2007) was used to analyse the data statistics. The one-way ANOVA followed Tukey post hoc test was used for the analysis of the mean of multiple groups. Student’s t-test was used to compare two groups. In all tests, P < 0.05 was considered significant. To analyse the Ica,L amplitudes the maximum current peak and the stable current plateau were automatically achieved, every 8 sec pulse. The Ica,l data for all the different drugs studied are expressed as a percentage of the basal or BAY K8644-stimulated ICa,L. All the results are expressed as mean ± SEM of n experiments.
The addition of NA (1 μmol/L) and the depolarization with isosmotic KCl (60 mmol/L) were used to contract the rat aorta rings devoid of endothelium. The maximal contractions elicited by NA and KCl were 1.57 ± 0.12 g (n = 29) and 2.04 ± 0.18 g (n = 26), respectively, being significantly different (P = 0.044, Student’s t-test). After washing out with Krebs’ solution the contractile effect obtained with the two contractile agents was reversible. Moreover, the solvent used to dissolve BPA, ethanol, did not have significant relaxant effect in all the concentrations analyzed. The effect of increasing concentrations of BPA (0.001-100 μmol/L) was analyzed and we observed that BPA had an effect on the contractions induced by NA and KCl.
As shown in Fig. 1, BPA, 10 and 100 μmol/L (P < 0.05, one-way ANOVA), has a significant vasorelaxant effect in rat aortic rings contracted with NA.

Relaxant effect of BPA (0.001-100 μmol/L) on contraction of endothelium-denuded rat aortic rings induced by NA (0.1 μmol/L). Each column represents the mean values and the lines the S.E.M of number of experiments stated above the columns. *P < 0.05 one-way ANOVA with Tukey post hoc test. The effects are expressed in percent relaxation over the initial area.
Concerning the effect of BPA on KCl-induced contraction, as observed in Fig. 2, higher concentrations of BPA (10 and 100 μmol/L, (P < 0.05, one-way ANOVA)) induced a significant increase in the relaxation of the arteries. So, BPA effect on KCl contraction is concentration dependent, since the percentage of relaxation increases with cumulative concentrations of the compound. Moreover, the vasorelaxant effect of BPA is bigger in KCl contraction than the one induced in NA contraction.

Relaxant effect of BPA (0.001-100 μmol/L) on contraction of endothelium-denuded rat aortic rings induced by KCl (60 mmol/L). Each column represents the mean values and the lines the S.E.M of number of experiments stated above the columns. *P < 0.05 one-way ANOVA with Tukey post hoc test. The effects are expressed in percent relaxation over the initial area.
The activity of W-CLTCC in A7r5 cells was measured with the Patch Clamp technique. The mean value of ICa,L, density was of 0.77 ± 0.27 pA/pF (n = 26). The equilibration between the pipette and the intracellular solutions was achieved after 3-5 min of the patch break, and only after this time the basal current amplitude was measured.
The effects of BPA in basal ICa,L are shown in Fig. 3. At concentrations of 0.05, 1, 10 and 100 μmol/L, BPA inhibits basal ICa,L and this inhibition is rapid and reversible after washout; however, the most pronounced inhibition was at the highest concentrations, 10 and 100 μmol/L.
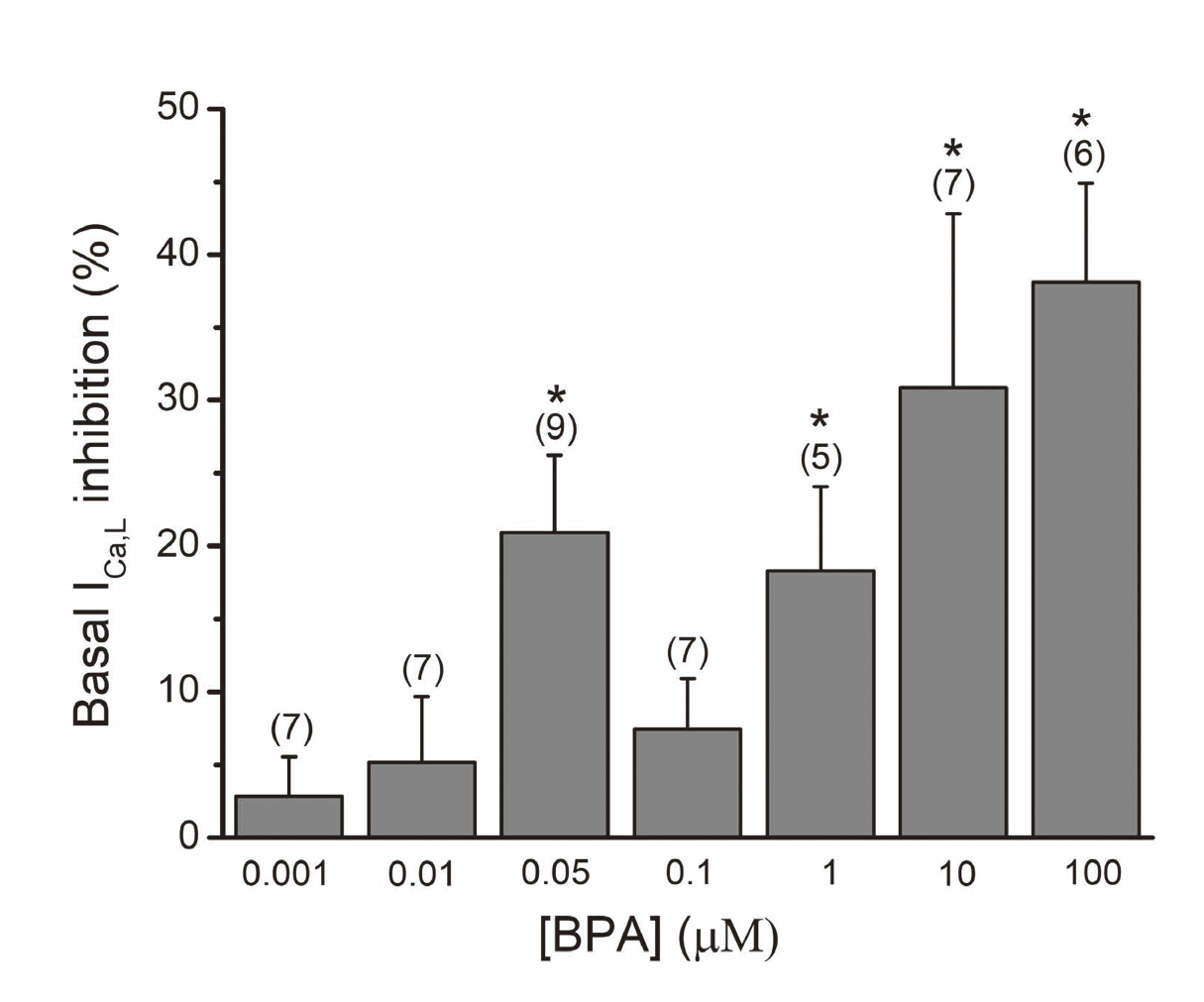
Inhibitory effects of BPA (0.001-100 μmol/L) on A7r5 basal ICa,L. Each column represents the mean values and the lines the S.E.M of number of experiments stated above the columns. *P < 0.05 one-way ANOVA with Dunnett test. The effects are expressed in percent variation over the amplitude of basal ICa.
A typical experiment of one patch clamp experiments showing the effect of increasing concentrations BPA (0.001-100 μmol/L) on basal W-CLTCC is observed in Fig. 4. In this figure the different concentrations of BPA inhibited the basal ICa,L in a reversible way.

Original records of one patch clamp experiments showing the effect of increasing concentrations BPA (0.001-100 μmol/L) on basal L-type Ca2+ channel current (ICa,L) amplitudes in a single A7r5 cell.
Bay K8644, a direct activator of the W-CLTCC, was used to analyze BPA effect on stimulated ICa,L. The application of Bay K8644 (0.01 µmol/L) significantly stimulated the calcium current by 58.69 ± 9.97% (n = 10) above the basal level. The effects of BAY were completely reversible upon washout of the drug. These results indicate that the current analyzed is ICa.L.
Figure 5 shows that BPA (0.1-100 μmol/L) inhibits the stimulated ICa,L in a concentration-dependent manner. This inhibition is higher at 100 µmol/L, with the maximal effect of BPA being 28.05 ± 8.37%. These results show that BPA also inhibits BAY stimulated ICa,L at higher concentrations. A typical experiment of one patch clamp experiments is shown in Fig. 6.

Inhibitory effects of BPA (0.001-100 μmol/L) on ICa,l stimulated by BAY (0.01 µmol/L). Each column represents the mean values and the lines the S.E.M of number of experiments stated above the columns. *P < 0.05 one-way ANOVA with Dunnett test. The effects are expressed in percent variation over the amplitude of of BAY-stimulated ICa,L.

Original records of one patch clamp experiments showing the effect of increasing concentrations BPA (0.001-100 μmol/L) on BAY- stimulated ICa, L amplitudes in a single A7r5 cell.
As observed in the contractility experiments, the vehicle used to dissolve BPA, ethanol at the maximal concentration 0.01%, did not affect basal or stimulated ICa,L (-2.15 ± 2.90%, n = 7).
The main goal of this study was to analyse the effect of BPA at the vascular level; in this sense we studied the effect of Bisphenol A on endothelium denuded rat aorta contracted arteries and on the ICa,L measured by Patch Clamp in A7r5 cells. Cumulative concentrations of BPA (0.001-100 μmol/L) were administered to aortic rings contracted either with NA and KCl, and for the first time it was observed that BPA induces a rapid and concentration-dependent relaxation. Nevertheless, it is known that the intracellular calcium concentration ([Ca2+]i) is the key of the vascular smooth muscle contraction/relaxation (Stull et al., 1991). So, the next step of this work was to analyse the effect of BPA in the voltage-dependent Ca2+ influx currents, once the L-type Ca2+ channels are the mainly responsible for the aortic rings contractility. Previous works performed in A7r5 cells by our group characterised electrophysiologically (the activation and inactivation kinetic) and pharmacologically (with several concentrations of nifedipine, a specific blocker and with BAY, a specific activator of the W-CLTCC) these voltage-dependent Ca2+ influx currents and showed that this current is ICa,L (Cairrão et al., 2012; Alvarez et al., 2010; Mariana et al., 2018). Basal and BAY-stimulated ICa,L were inhibited by the highest concentration (100 μmol/L) of BPA. These suggested that BPA induces a non-genomic effect responsible for the inhibition of L-type calcium channels and for the endothelium independent vasorelaxant effect of BPA.
Concerning the BPA vasorelaxant effect, we can conclude that this effect observed is not due to NO production, once the endothelium was previously removed, but further studies are necessary to understand if the endothelium is important in intact vessels exposed to BPA. The vasorelaxation in arteries contracted with NA is less pronounced than the effect induced on the arteries contracted with KCl, once the maximum relaxation achieved with NA was 7.78% and with KCl was 17.98%. This difference can be explained by the different vascular action pathway of both contractile agents. The NA pathway involved in the contraction is through the activation of the α1A-, α1B-, α1D-, β1-, and β2-adrenoceptors. The α1- adrenoceptors are associated with the activation of the Gq protein that induce the increase of intracellular calcium from the sarcoplasmic reticulum leading to contraction (Wang et al., 2004; Guimarães and Moura, 2001). The β1- and β2-adrenoceptors are coupled to Gs protein inducing the activation of adenylate cyclase, which increases the intracellular concentration of cAMP and leads to vasorelaxation (Weihua et al., 2000; Traupe et al., 2007). Other pathway associated to the contraction induced by NA is the influx of extracellular calcium via voltage- or receptor-operated calcium channels, which increases intracellular calcium levels (Perusquía et al., 1996). The plasma membrane depolarization is induced by high extracellular KCl concentrations and can activate the voltage-dependent channels, mainly the W-CLTCC (Alvarez et al., 2010). So, these results seem to indicate that the decrease in calcium influx by blocking the W-CLTCC are the main pathway involved in the BPA vasorelaxation. In order to check this pathway, we analysed the BPA effect in the activity of the calcium channels current in A7r5 cells.
Concerning the effect of BPA in the calcium current, the results demonstrated that this compound has the ability to inhibit W-CLTCC, with its higher effect at 0.05 μmol/L and 1-100 μmol/L, with an inhibition of 20.91%, 18.30%, 30.84% and 38.14%, respectively. This effect seems to be consistent with several authors that already demonstrated in the last decades numerous studies have played an important role in creating two great concepts of BPA: low-dose effects, for example the effects observed for concentrations in the range of human exposure and nonmonotonicity, and the nonlinear relationship between dose and effect (Vandenberg et al., 2012b; Richter et al., 2007). Moreover, it also inhibits BAY-stimulated ICa,L, with its maximum effect was observed (28.05% of inhibition) for the concentration of 100 μmol/L.
As mentioned before, BPA-based products are tough, versatile and water-resistant and are used in a variety of consumer products (Halden, 2010), so the human being is constantly exposed to them. In fact, several studies have shown that the levels of BPA can be detected in over 90% of individuals examined in many populations. The mean concentration of BPA found in human urine samples is around the nmol/L to μmol/L, being this the reason for the concentrations used in this study range from 1 nmol/L to 100 μmol/L. (Calafat et al., 2005; Cantonwine et al., 2010; Itoh et al., 2007; Kim and Park, 2013; Ning et al., 2011).
The effects of EDCs, including bisphenol A are still not fully understood, regarding their mechanisms and/or their doses. However, several studies show that BPA has effects on the functioning of the cardiovascular system, mainly in ion channels (Soriano et al., 2016).
In 2014, Posnack et al. demonstrated that BPA exposure has an impact in cardiac electrical function in ex vivo female rat hearts (Posnack et al., 2014). Later, in 2015, they reported that BPA induced alterations in left ventricular pressure, contractility and lusitropy, leading to the hypothesis that in vivo under physiological conditions the BPA exposure could be a risk factor for populations predisposed to cardiovascular diseases (Posnack et al., 2015).
Regarding ion channels, using human and canine coronary artery smooth muscle cells, Asano and co-workers demonstrated that BPA induce a non-genomic, reversible and dose-dependent effect, since it activates the large conductance Ca2+/voltage-sensitive K channels (Maxi-K channels) (Asano et al., 2010). Later, genomic studies performed by Rottgen et al. corroborated the Asano results, and demonstrated that BPA activates the BK channels through the increase of α- and β1-subunits expression (Rottgen et al., 2014). These studies suggest that BPA induces a vasodilator effect, being in agreement with our results that BPA induces rat aorta vasorelaxation.
In relation to Ca2+ channels, we demonstrated that BPA inhibits the W-CLTCC current in A7r5 cells. These results are in agreement with a study of Deutschmann et al., in which they demonstrated that BPA inhibits the LTCC in rat endocrine GH3 cells, cardiac myocytes and human embryonic kidney (HEK 293 cells). This inhibition is dose-dependent and reversible, and they concluded that BPA possibly exerts its action by binding to channels at the resting state. Furthermore, this study was applied in other voltage-gated Ca2+ channels in native cells and the authors found that BPA also inhibited the N-, P/Q-, and T-type Ca2+ channels (Deutschmann et al., 2013). Recently, Michaela and collaborators suggested that BPA inhibits three subtypes, Cav3.1, Cav3.2 and Cav3.3, of T-type calcium channels in HEK293. They demonstrated that at low concentrations (nmol/L) BPA inhibits the three subtypes without affecting channel gating properties, on the other hand, at high concentrations (µmol/L) besides inhibiting these three subtypes, BPA also changes the channel gating (Michaela et al., 2014). In summary, the concentrations used the electrophysiological experiments are according to the obtained from the sex hormones for A7r5. The pharmacological concentrations used to obtain this ionic modulation observed (W-CLTCC inhibition and potassium channels activation) are due to the low solubility of sex steroids (Alvarez et al., 2010; Cairrão et al., 2012), since pharmacological concentrations are needed in in vitro studies to induce the same effect as physiological concentrations in in vivo studies (Chou et al., 1996; Yildiz and Seyrek, 2007). This hypothesizes that the same happens with endocrine disruptors since they act on the same receptors as sex steroids.
In conclusion, our findings represent the known effects of BPA on rat aortic smooth muscle cells, but other effects on different species and/or arteries should be studied to increase our understanding of cardiovascular BPA toxicity. Given its widespread presence in the environment and its identification in several tissues in human population, studying exposure to BPA may help in the prevention of cardiovascular pathologies later in life.
This study is supported by FEDER funds through the POCI - COMPETE 2020 - Operational Programme Competitiveness and Internationalisation in Axis I - Strengthening research, technological development and innovation (Project POCI-01-0145-FEDER-007491) and National Funds by FCT - Foundation for Science and Technology (Project UID/Multi /00709/2013). We thank the Fundação para a Ciência e a Tecnologia (Portugal) for supporting the grant SFRH/BD/131665/2017.
Conflict of interestThe authors declare that there is no conflict of interest.