2018 Volume 43 Issue 11 Pages 645-657
2018 Volume 43 Issue 11 Pages 645-657
Previous studies have reported the potential developmental neurobehavioral effects of decabrominated diphenyl ethers (BDE 209) on developing animals, but the effects on adult animals are rare or controversial and the mechanism is not fully understood. In the present study, male adult Sprague-Dawley rats performed poor spatial learning and memory in Morris water maze after exposure to BDE 209 by gavage for 30 days. The expression of hippocampal glutamate receptor subunits NR1, NR2B and GluR1, the phosphorylation of NR2B subunit at Ser1301 (p-NR2B Ser1303) and GluR1 subunit at Ser831 (p-GluR1 Ser831) were all decreased, and the phosphorylation ratio of NR2B revealed an increasing trend after BDE 209 exposure. The present study provided evidence that BDE 209 could induce spatial learning and memory deficits in adult rats, and further explored the potential mechanism.
Polybrominated diphenyl ethers (PBDEs) are a class of important additive brominated flame retardants applied in textiles, fabrics, foams and electronic products. Due to the characteristic that PBDEs are not chemically bonded to the consumer products and can be released into the environment along with the using or recycling of products, the detected concentration of PBDEs in the environment revealed an increasing trend in the past few decades (Costa et al., 2008; Ni et al., 2013). All three main commercial formulations, penta-BDE, octa-BDE, and deca-BDE, were banned in the developed countries and areas such as the United States and Europe; however, the 3,3,4,4,5,5,6,6-decabromodiphenyl ether (BDE 209), which dominates the formulation deca-BDE, is still manufactured and used in some developing countries such as China (Costa and Giordano, 2011; Lenters et al., 2013). BDE 209 has been reported to be the main congener of PBDEs in the environment (Chen et al., 2018; Li et al., 2015).
Human exposure to BDE 209 mainly occurs through the respiratory and digestive tracts. Similar to other PBDEs, BDE 209 also has the ability to accumulate in various human tissues or organs and causes adverse effects, especially in the central nervous system (Costa et al., 2008). Since children were considered to have more BDE 209 inhalation and be more susceptible to exogenous chemicals than general population, the most concerning issue in prior studies was the potential developmental neurotoxicity of BDE 209 (Landrigan and Garg, 2005; Costa and Giordano, 2011). For example, epidemiological studies have shown that early-life BDE 209 exposure could cause adverse effects on the cognitive development and behavioral performance in children (Gascon et al., 2012; Chevrier et al., 2016). Several studies have also indicated that animals exposed to BDE 209 during developmental period showed poor performance in behavioral tasks (Viberg et al., 2007; Johansson et al., 2008; Buratovic et al., 2014). Impairment spatial learning and memory ability of rats after development exposure to BDE 209 was also shown in our recent study (Li et al., 2017).
A high level of BDE 209 exposure is also estimated in some adults such as occupational personnel who engage in the manufacturing, using or recycling of products containing this chemical (Feng et al., 2016). A high concentration of environmental BDE 209 in the workplace often results in high body burden in the occupational population. For example, in an electronic waste dismantling area in Guangdong, China, the highest serum concentration of BDE 209 in associated workers was 3436 ng/g lipid, which is much higher than in residents living 50 kilometers away from the region and the general population with no potential occupational exposure (Qu et al., 2007). The relative high serum level of BDE 209 was also reported in workers manufacturing or handling rubber containing BDE 209 (Thuresson et al., 2005). Therefore, the occupational exposure to BDE 209 is also worthy of attention and the information about the adverse effects of BDE 209 on adults is needed. To date, previous studies mostly investigated the thyroid endocrine toxicity, reproductive toxicity or immunotoxicity but rarely examined the potential neurobehavioral effects of BDE 209 on adults (Curčić et al., 2012; Sarkar et al., 2016; Feng et al., 2016).
An earlier study indicated that BDE 209 exposure could cause irreversible nervous system damage by affecting cholinergic system enzyme activity and inducing lipid peroxidation in adult mice (Liang et al., 2010); hippocampal neuronal damage and degeneration were also observed in adult mice after BDE 209 exposure (Feng et al., 2015). These findings agreed with the potential mechanism underlying the developmental neurotoxicity of PBDEs (Costa et al., 2014) and were also associated with learning and memory (Lu et al., 2014; Jung et al., 2012). However, when Heredia et al. treated young adult mice with low-dose PBDE-209 for 15 days, the mice showed some anxiety behavioral changes in the zero maze but no significant spatial learning and memory deficit in the water maze (Heredia et al., 2012). Therefore, there still seems to be controversy regarding whether BDE 209 exposure can cause neurobehavioral alterations such as spatial learning and memory impairment in adult animals.
The hippocampus is a basic brain area associated with learning and memory. Long-term potentiation (LTP) is widely recognized as the main cellular mechanism of learning and memory. Inhibition of hippocampal LTP can induce learning and memory impairment (Lynch, 2004). The N-methyl-D-aspartate (NMDA) receptor and α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) receptor are two special types of ionotropic glutamate receptors (iGluRs) that regulate LTP and are therefore significantly associated with learning and memory (Morris et al., 2013; Riedel et al., 2003). NMDA receptors contain several identified subunits. NR1 is essential for the function of NMDA receptors and only NR2A or NR2B can bind with NR1 to form the NMDA receptors in the hippocampus (Ishii et al., 1993; Laurie et al., 1997). NR2B subunit plays a more critical role than NR2A subunit in mature mammals (Gee et al., 2011). NMDA receptors containing NR2A subunit show lower glutamic affinity, mediate smaller and shorter currents and inactivate faster than NMDA receptors containing NR2B subunit (Kutsuwada et al., 1992; Tang et al., 1999). AMPA receptors contain four subunits. Studies have demonstrated that AMPA receptors containing GluR1 subunit were required for the LTP and the GluR1 subunit alone was sufficient for hippocampal synaptic plasticity (Schmitt et al., 2005; Selcher et al., 2012). Furthermore, it is known that subunit phosphorylation plays a critical role in regulating the function of iGluRs (Lee, 2006). Phosphorylation of NR2B subunit at the serine residue can enhance the current through the NMDA receptor channel and regulate the interaction between receptor and some proteins in the synapse then affect the NMDA receptor-mediated LTP (Liao et al., 2001; Chung et al., 2004). In addition, phosphorylation of GluR1 subunit at the serine residue can also enhance the channel conductance of AMPA receptor and is therefore significantly associated with LTP (Derkach et al., 1999; Lee et al., 2003). Ca2+/calmodulin-dependent protein kinases II (CaMKII) is another important molecule involved in synaptic plasticity and learning and memory (Lisman et al., 2012). On one hand, CaMKII and its phosphorylation at Thr286 are associated with the downstream signal pathways involved in learning and memory (Giese et al., 1998). On the other hand, CaMKII is one of the main protein kinases, which phosphorylates NR2B subunit at Ser1303 and GluR1 subunit at Ser831 (Lee, 2006). Moreover, the interaction between CaMKII and NR2B, which is regulated by the phosphorylation of NR2B at Ser1303, is considered a critical mechanism of the NMDA receptor-mediated LTP (Meng et al., 2003; Strack et al., 2000).
The present study was aimed at investigating the effects of subchronic BDE 209 exposure on the spatial learning and memory in adult rats, for which the information is not sufficiently available and the current evidence seems to be controversial. Therefore, the adult Sprague-Dawley rats were administered BDE 209 for 30 consecutive days and then were tested in the Morris water maze (MWM) for spatial learning and memory. Moreover, to gain insight into the potential mechanism, the expression of hippocampal glutamate receptor subunits NR1, NR2B and GluR1, the phosphorylation of NR2B subunit at Ser1303 (p-NR2B Ser1303) and GluR1 subunit at Ser831 (p-GluR1 Ser831), the expression of αCaMKII and its phosphorylation at Thr286 (p-αCaMKII Thr286) were examined.
Ten-week-old healthy male Sprague-Dawley rats (N = 32, 300 ± 50 g) obtained from the lab animal center of Southwest Medical University were housed in polypropylene cages (four rats per cage) and maintained in a well-ventilated and hygienic room, in which the temperature was 22°C ± 2°C and the relative humidity was 50% ± 10%. The light in the room was on from 8:00 to 20:00. The rats were fed fully nutritious rodent food and sterilized water. After a 7-day acclimatization period, the rats were randomly divided into four groups with 8 rats in each. All the procedures on animal care and experiment were guided and approved by the ethics committee of Southwest Medical University.
Chemical and treatmentDecabromodiphenyl ether (BDE 209, purity exceeded 98%) was purchased from J&K Technology Co., Ltd. (Beijing, China) and dissolved in peanut oil and then sonicated for 30 min to formulate the BDE 209 emulsions. The emulsions were freshly prepared every day.
The results of one of our previous studies showed no significant difference between blank controls (rats received no oral administration) and vehicle controls (rats received peanut oil). Therefore, we cancelled the blank controls in the present study (Yan et al., 2012). Rats were divided into four groups randomly (n = 8), and were given BDE 209 at doses of 250, 500 and 1,000 mg/kg body weight or an equal volume of peanut oil respectively by oral gavage once daily for 30 continuous days, a subchronic exposure design.
Based on the low absorption rate of BDE 209 through gastrointestinal tract in adult rodents, which was estimated to be approximately only 0.3% of the treated dose (Feng et al., 2015), and the highest serum burden of BDE 209 in workers at an electronic waste dismantling area in Southern China, which was estimated to be 3,436 ng/g (Qu et al., 2007), we designed the exposure dose up to 1,000 mg/kg in the present study. Moreover, the same dose was applied in our previous lab work, and similar doses were also used in other previous studies that treated the adult or pregnant rats/mice with BDE 209 to investigate the thyroid endocrine toxicity, reproductive toxicity, immunotoxicity or developmental neurotoxicity (Biesemeier et al., 2011; Sarkar et al., 2016; Feng et al., 2016).
Morris water mazeSeven days after BDE 209 exposure, rats were tested in the Morris water maze (MWM) for spatial learning and memory according to the procedure in our previous study (Yan et al., 2012). The Morris water maze consisted of a circular black plastic pool (1.6 m in diameter, 50 cm in height), a circular transparent escape platform (10 cm in diameter) and the SuperMaze path tracking software system (Version 3.3.0.0, Xinruan Information Technology, Shanghai, China). Before testing, the pool was filled with clean tap water (22°C ± 2°C, 30 cm in deep). Rats were allowed to swim freely in the pool for 90 sec without the platform.
Hidden platform trialThe hidden platform trial began on the day after free-swimming. The pool was divided into four identical quadrants, the midpoint of the boundary line between pool wall and water surface in each quadrant was designed as the start point. The escape platform was placed 1-2 cm below the water surface in the center of one quadrant and the location of the platform was never changed throughout the trials. Rats were gently placed into water individually from the starting point facing the pool wall, and were allowed to swim in the pool to search for the platform for maximum of 90 sec. The escape latencies to reach the platform and the average swimming speed were recorded. Rats were allowed to have a rest for 30 sec on the platform after escape and were dried with a towel before returned into original cages. If a rat failed to find the platform within 90 sec, it was guided to the platform by the experimenter. The hidden platform trial was conducted for four consecutive days. In each day, rats were placed into water from four different starting point (four trials per day) and there was an interval for at least 30 min between each two tests for the same rat. The data of the four trials in each day were averaged.
Probe trialThe probe trial was a single trial conducted on the fifth day after the hidden platform trial. The platform was removed from the pool and the quadrant of the original platform was defined as the target quadrant. During the probe trials, rats were gently placed into water individually from the starting point on the opposite quadrant of the target quadrant, and were allowed to swim in the pool for 90 sec. The number of times the rat crossed the location of the original platform, the number of times the rat entered the target quadrant, the time the rat spent in the target quadrant and the average swimming speed in the target quadrant were recorded.
Measurement of protein and protein phosphorylation in the hippocampi Tissue isolation and sample preparationOne week after the MWM testing, rats were anesthetized with 3% pentobarbital sodium at the volume of 1 mL/kg body weight. Then rats were sacrificed by neck severing, the hippocampi were isolated quickly on the ice and stored at -80°C before used.
The membrane proteins were extracted from the hippocampi of each treatment group using the membrane and cytosol protein extraction kit (Beyotime) and quantified using the enhanced bicinchoninic acid (BCA) protein assay kit (Beyotime, Jiangsu, China) according to the manufacturer's instructions. The proteins were denatured by boiling with 5 × loading buffer for 5 min and cooled on the ice bath then stored at -20°C.
AntibodiesThe primary antibodies used in the study were the rabbit anti-NMDAR1 (1:5,000), rabbit anti-NMDAR2B (1:1,000), rabbit anti-NMDAR2B (phoshpo-Ser1303) (1:1,000), rabbit anti-GluR1 (1:2,000), rabbit anti GluR1 (phospho-Ser831) (1:1,000), rabbit anti-CaMKII (1:5,000) (Abcam, Cambridge, UK), rabbit anti-CaMKII (phospho-Thr286) (1:1,000) (Cell Signal Technology, Danvers, MA, USA), rabbit anti-beta tubulin (1:1,000) (Proteintech, Wuhan, China) and mouse anti-beta tubulin (1:3,000) (Bioss, Beijing, China). The secondary antibodies were horseradish peroxidase (HRP) labeled goat anti-rabbit IgG (1:10,000) and goat anti-mouse IgG (1:5,000) (Bioss). The antibodies were diluted with the primary antibody dilution buffer or secondary antibody dilution buffer (Beyotime) according to the manufacturer’s guidance.
Western blottingProtein samples (50 μg/lane) were separated by 8% or 10% sodium dodecyl sulfate gel electrophoresis (SDS-PAGE) in glycine electrophoretic buffer and then transferred onto an equal-sized polyvinyldene fluoride (PVDF) membrane (Merck Millipore, Darmstadt, Germany). After blocking in 5% non-fat milk or albumin from bovine serum (BSA), the membrane was incubated with primary antibody overnight at 4°C, beta-tubulin was used as the loading control. Then the chemiluminescent detection was conducted with a BeyoECL plus kit (Beyotime) following the incubation with secondary antibody and the bands were exposed to the X-ray films. The bands on the films were scanned through a gel imaging system (Bio-Rad, Hercules, CA, USA) and the integrated optical density (IOD) of the bands were detected using the Image J software (NIH, Bethesda, MD, USA). The expression of subunit or level of phosphorylated subunit was calculated as “IOD of subunit/IOD of loading control” or “IOD of phosphorylated subunit/IOD of loading control”, and the phosphorylation ratio of subunit was calculated as “Level of phosphorylated subunit/expression of subunit”.
Statistical analysisThe data from the hidden platform trial in the MWM were analyzed using the two-way repeated measured Analysis of Variance (ANOVA), considering treatment as between-subject fixed factor and time (trial day) as repeated, within-subject factor. The other data were analyzed using one-way ANOVA and the treatment was the between-subject fixed factor. The least significant difference (LSD) post-hoc test was used for the multiple comparisons when ANOVAs were significant. All the procedures of statistical analysis were conducted on the Statistical Product and Service Solutions (SPSS) 19.0 software (IBM, New York, USA) and the results were presented as mean ± S.D.
During the four-day hidden platform trial, the two-way repeated measured ANOVA revealed that no effect of interaction between trial days and treatment on the escape latencies of rats was observed (time × treatment: F9,28 = 1.80, P = 0.08). However, the escape latencies showed significant differences between groups (treatment: F3,28 = 49.73, P < 0.001), and were decreased with the increase of trial days (time: F3,28 = 197.12, P < 0.001). The post-hoc test indicated that rats exposed to 500 or 1,000 mg/kg BDE 209 performed longer escape latencies than controls (500 mg/kg versus control: P < 0.05; 1,000 mg/kg versus control: P < 0.05) and rats exposed to 250 mg/kg BDE 209 (500 mg/kg versus 250 mg/kg: P < 0.05; 1,000 mg/kg versus 250 mg/kg: P < 0.05). Moreover, rats exposed to 1,000 mg/kg BDE 209 had longer escape latencies than rats exposed to 500 mg/kg BDE 209 (1,000 mg/kg versus 500 mg/kg: P < 0.05). However, the average swimming speeds of rats during the hidden platform trial were not influenced by the trial days, treatment or the interaction between trial days and treatment (time: F3,28 = 1.48, P = 0.226; treatment: F3,28 = 0.45, P = 0.718; time × treatment: F9,28 = 0.81, P = 0.611). The results are shown in Fig. 1.

Effects of subchronic BDE 209 exposure on the performance in the MWM during hidden platform trial. Data are presented as mean ± S.D. The statistical differences are indicated as, *significantly different when compared with controls, P < 0.05, #significantly different when compared with rats exposed to 250 mg/kg BDE 209, P < 0.05, &significantly different when compared with rats exposed to 500 mg/kg BDE 209, P < 0.05. (A) The escape latencies rats that found the platform. (B) The average swimming speed of rats swimming in the MWM.
During the probe trial on the fifth day, one-way ANOVA revealed that there were significant differences between groups in the number of times the rats crossed the location of original platform, the number of times the rats entered the target quadrant, and the time the rats spent in the target quadrant (Number of platform crossings: F1,28 = 16.88, P < 0.001; Number of target entries: F1,28 = 15.91, P < 0.001; Time spent in the target: F1,28 = 18.97, P < 0.001). The post-hoc test indicated that rats exposed to 500 or 1,000 mg/kg BDE 209 exhibited fewer platform crossings and target quadrant entries and spent less time in the target quadrant compared with controls (500 mg/kg versus control: P < 0.05; 1,000 mg/kg versus control: P < 0.05) and rats exposed to 250 mg/kg BDE 209 (500 mg/kg versus 250 mg/kg: P < 0.05; 1,000 mg/kg versus 250 mg/kg: P < 0.05). Moreover, rats exposed to 1,000 mg/kg BDE 209 exhibited fewer platform crossings and target quadrant entries and spent less time in the target quadrant compared with the rats exposed to 500 mg/kg BDE 209 (1,000 mg/kg versus 500 mg/kg: P < 0.05). However, the average swimming speeds of rats in the target quadrant showed no significant difference between groups (F3,28 = 0.092, P = 0.964). The results are shown in Fig. 2.

Effects of subchronic BDE 209 exposure on the performance in the MWM during probe trial. Data are presented as mean ± S.D. The statistical differences are indicated as, *significantly different when compared with controls, P < 0.05, #significantly different when compared with rats exposed to 250 mg/kg BDE 209, P < 0.05, &significantly different when compared with rats exposed to 500 mg/kg BDE 209, P < 0.05. (A) The number of times the rats crossed the location of the original platform. (B) The number of times the rats entered the target quadrant. (C) The total time rats spent in the target quadrant. (D) The average swimming speed of rats swimming in the target quadrant.
One-way ANOVA revealed that there were significant differences between groups in the protein expression of hippocampal glutamate receptor subunits NR1, NR2B and GluR1 (NR1: F3,28 = 3.64, P < 0.05; NR2B:F3,28 = 21.67, P < 0.001, GluR1: F3,28 = 15.92, P < 0.001). The post-hoc test indicated that the expression of the three subunits were all significantly decreased in the hippocampi of rats exposed to 250, 500 and 1,000 mg/kg BDE 209 when compared with controls. (For all the three subunits: 250 mg/kg versus control: P < 0.05; 500 mg/kg versus control: P < 0.05; 1,000 mg/kg versus control: P < 0.05). The protein expression of NR2B and GluR1 were both significantly decreased in the hippocampi of rats exposed to 500 and 1,000 mg/kg BDE 209 when compared with the rats exposed to 250 mg/kg BDE 209. The protein expression of the two subunits were also significantly decreased in the hippocampi of rats exposed to 1,000 mg/kg BDE 209 when compared with the rats exposed to 500 mg/kg BDE 209 (For both NR2B and GluR1: 500 mg/kg versus 250 mg/kg: P < 0.05; 1,000 mg/kg versus 250 mg/kg: P < 0.05; 1,000 mg/kg versus 500 mg/kg: P < 0.05). The results are shown in Fig. 3.

Effects of subchronic BDE 209 exposure on the protein expression of the glutamate subunits in the hippocampi. Data are presented as mean ± S.D. The statistical differences are indicated as, *significantly different when compared with controls, P < 0.05, #significantly different when compared with rats exposed to 250 mg/kg BDE 209, P < 0.05. (A) The protein expression of NR1 subunit. (B) The protein expression of NR2B subunit. (C) The protein expression of GluR1 subunit. (D) The bands of western blotting.
One-way ANOVA revealed that there were significant differences between groups in the levels of p-NR2B Ser1303 and p-GluR1 Ser831 in the hippocampi (p-NR2B: F3,28 = 9.21, P < 0.001; p-GluR1: F3,28 = 5.86, P < 0.005). The post-hoc test indicated that the levels of p-NR2B Ser1303 and p-GluR1 Ser831 were both significantly decreased in the hippocampi of rats exposed to 250, 500 and 1,000 mg/kg BDE 209 when compared with controls (For p-NR2B and p-GluR1: 250 mg/kg versus control: P < 0.05; 500 mg/kg versus control: P < 0.05; 1,000 mg/kg versus control: P < 0.05). The levels of p-GluR1 Ser831 were significantly decreased in the hippocampi of rats exposed to 500 mg/kg BDE 209 when compared with the rats exposed to 250 mg/kg BDE 209. The levels of p-GluR1 Ser831 were also significantly decreased in the hippocampi of rats exposed to 1,000 mg/kg BDE 209 when compared with the rats exposed to 500 mg/kg BDE 209 (500 mg/kg versus 250 mg/kg: P < 0.05; 1,000 mg/kg versus 500 mg/kg: P < 0.05). The results are shown in Fig. 4.

Effects of subchronic BDE 209 exposure on the levels of p-NR2B Ser1303 and p-GluR1 Ser831 in the hippocampi. Data are presented as mean ± S.D. The statistical differences are indicated as, *significantly different when compared with controls, P < 0.05, #significantly different when compared with rats exposed to 250 mg/kg BDE 209, P < 0.05, &significantly different when compared with rats exposed to 500 mg/kg BDE 209, P < 0.05. (A) The level of p-NR2B Ser1303. (B) The level of p-GluR1 Ser831. (C) The bands of western blotting.
One-way ANOVA revealed that there were significant differences between groups in the phosphorylation ratio of NR2B (F3,28 = 14.96, P < 0.001) but no significant difference in the phosphorylation ratio of GluR1 (F3,28 = 0.06, P = 0.982). The post-hoc test indicated that the phosphorylation ratio of NR2B in the hippocampi of rats exposed to 500 and 1,000 mg/kg BDE 209 were significantly higher than controls and rats exposed to 250 mg/kg BDE 209. The phosphorylation ratio of NR2B in the hippocampi of rats exposed to 1,000 mg/kg BDE 209 were also significantly higher than rats exposed to 500 mg/kg BDE 209 (500 mg/kg versus control: P < 0.05; 1,000 mg/kg versus control: P < 0.05; 500 mg/kg versus 250 mg/kg: P < 0.05; 1,000 mg/kg versus 250 mg/kg: P < 0.05; 1,000 mg/kg versus 500 mg/kg: P < 0.05). The results are shown in Fig. 5.
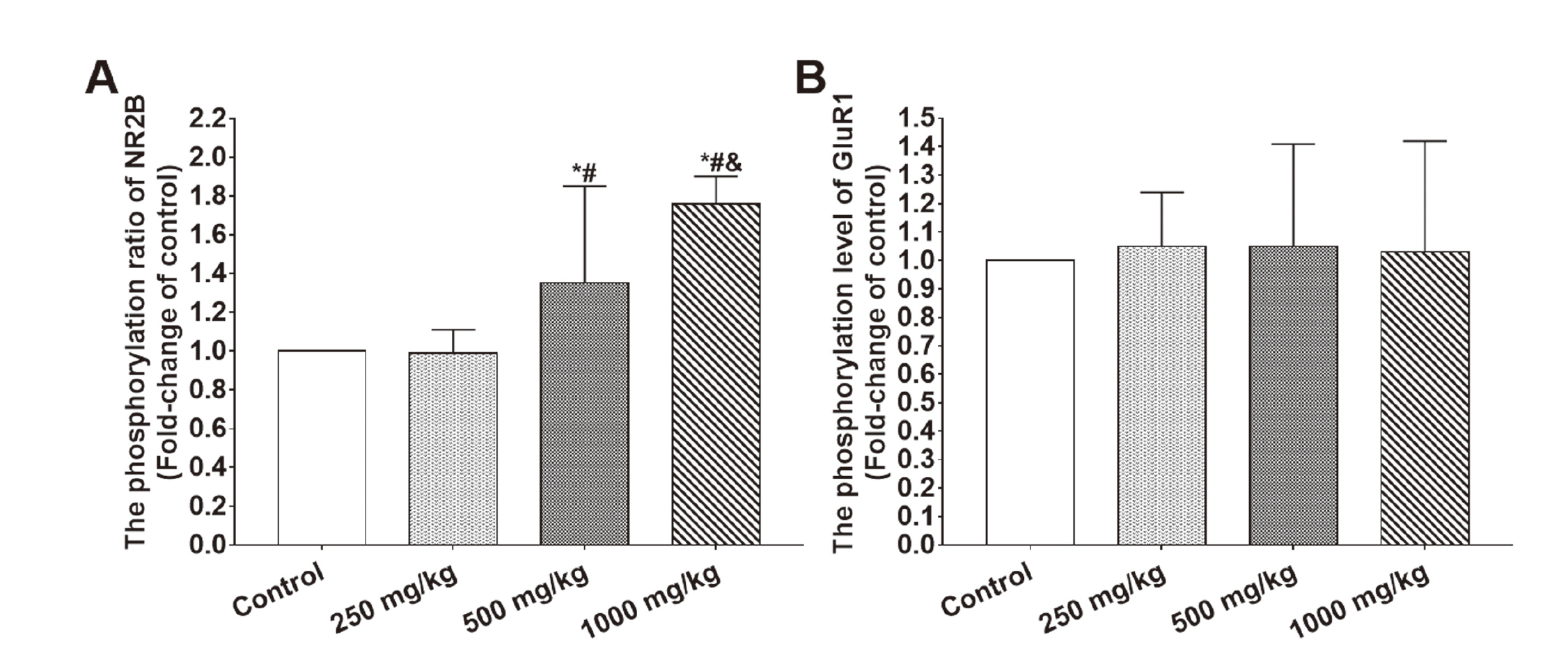
Effects of subchronic BDE 209 exposure on the phosphorylation ratio of NR2B and GluR1 in the hippocampi. Data are presented as mean ± S.D. The statistical differences are indicated as, *significantly different when compared with controls, P < 0.05, #significantly different when compared with rats exposed to 250 mg/kg BDE 209, P < 0.05, &significantly different when compared with rats exposed to 500 mg/kg BDE 209, P < 0.05. (A) The phosphorylation ratio of NR2B. (B) The phosphorylation ratio of GluR1.
One-way ANOVA revealed that there were significant differences between groups in the protein expression of αCaMKII and the level of p-αCaMKII Thr286 in the hippocampi (αCaMKII: F3,28 = 6.65, P < 0.005; p-αCaMKII: F3,28 = 11.87, P < 0.001). The post-hoc test indicated that the protein expression of αCaMKII and the level of p-αCaMKII Thr286 were both significantly decreased in the hippocampi of rats exposed to 250, 500 and 1,000 mg/kg BDE 209 when compared with controls. The protein expression of αCaMKII and the level of p-αCaMKII Thr286 were also both significantly decreased in the hippocampi of rats exposed to 1,000 mg/kg BDE 209 when compared with the rats exposed to 500 mg/kg BDE 209 (For αCaMKII and p-αCaMKII: 250 mg/kg versus control: P < 0.05; 500 mg/kg versus control: P < 0.05; 1,000 mg/kg versus control: P < 0.05; 1,000 mg/kg versus 500 mg/kg: P < 0.05). The results are shown in Fig. 6.

Effects of subchronic BDE 209 exposure on the protein expression of αCaMKII and the level of p-αCaMKII Thr286 in the hippocampi. Data are presented as mean ± S.D. The statistical differences are indicated as, *significantly different when compared with controls, P < 0.05, #significantly different when compared with rats exposed to 250 mg/kg BDE 209, P < 0.05. (A) The protein expression of αCaMKII. (B) The level of p-αCaMKII Thr286. (C) The bands of western blotting.
Decabromodiphenyl ether (BDE 209) is widely manufactured and used as a brominated flame retardant in rapidly developing contries such as China. BDE 209 can be detected at a high level in the evironment and is a important potential risk for the health of human beings. It was reported previously that BDE 209 could induce adverse effects on the neurobehavior such as spatial learning and memory impairment in animals after developmental exposure. However, could BDE 209 exhibit similar effects on spatial learning and memory in adult animals? The evidence was insufficient and controversial. Therefore, in the present study, rats were subchronically exposed to BDE 209 by gavage for 30 consecutive days at the doses of 0, 250, 500 or 1,000 mg/kg body weight, in which the highest dose was based on the highest serum burden of BDE 209 in workers at an electronic waste dismantling area in Southern China and the low absorption rate of BDE 209 through gavage in adult rodents. Moreover, the potential mechanism underlying spatial learning and memory deficits induced by BDE 209 exposure is still not fully understood, so we further investigated the effects of BDE 209 exposure on spatial learning and memory in adult Sprague Dawley rats, and explored the potential mechanism.
Spatial learning and memory are sensitive indicators for the neural effects induced by chemicals, and the Morris water maze (MWM) is a classic and potentially powerful apparatus for examining spatial learning and memory (Li et al., 2017). The results of MWM in the present study revealed a significant decrease in the escape latencies with the increase of trial day, which indicated that all rats improved their performance through learning and memory in the MWM during the four-day period of hidden platform trial. However, rats exposed to 500 or 1,000 mg/kg BDE 209 showed significantly longer escape latencies when compared with the controls, which indicated that the BDE 209 exposure may have impaired spatial learning and memory in adult rats, especially the exposure doses of 500 and 1,000 mg/kg. Interestingly, the average swimming speed showed no change with the increase of trial day and no significant difference between groups, which might suggest that the average swimming speed of rats does not depend on learning and memory, and was not affected by the BDE 209 exposure, or that spatial learning and memory deficits induced by BDE 209 do not depend on the average swimming speed. Moreover, during the probe trial, rats exposed to 500 or 1,000 mg/kg BDE 209 entered the target quadrant and crossed the location of the original platform less frequently and spent shorter time spent in the target quadrant than controls, but the average swimming speed showed no significant difference. These results further indicate that BDE 209 may induce spatial learning and memory deficits in adult rats, and the effect was not dependent on the average swimming speed. The results of MWM were similar to those of our prior study (Yan et al., 2012), in which the adult rats were subchronically exposed to relatively low dose of BDE 47 for 30 consecutive days.
There could be a large difference in the effects of chemicals on animals and human beings, although we observed spatial learning and memory deficits in adult rats induced by BDE 209 or BDE 47 exposure in the present and prior studies. However, to date, there seems to be no studies focused on the neurobehavioral effects of PBDEs for adult human beings. Therefore, the future investigation should pay more attention to adult human beings.
For many years, the potential mechanism of the spatial learning and memory deficits induced by PBDEs (including BDE 209) was not fully explained and was worthy of futher exploration. The N-methyl-D-aspartate (NMDA) receptor is a special subset of ionotropic glutamate receptor (iGluR) which plays a critical role in long-term potentiation (LTP) and regulates the function of learning and memory (Morris et al., 2013). The NMDA receptor contains a variety of subunits. NR1 is the essential subunit of functional NMDA receptor; the stimulation threshold for the induction of LTP in the cultured hippocampal slices from the NR1 knocked out mice was significantly increased, and the mice showed impaired spatial learning and memory in the MWM (Tsien et al., 1996). NR2 is another set of subunits of the NMDA receptor, in which the key subunits in the forebrain, including the hippocampus, are NR2A and NR2B, and the NR2B subunit was more effective for the induction of LTP (Kutsuwada et al., 1992). Rats that received selective NR2B antagonist injection into dorsal hippocampal area exhibited worse spatial working memory than controls in both the T-maze task and the water maze task (Zhang et al., 2013). Furthermore, a study showed that transgenic mice with forebrain NR2B overexpression exhibited enhanced LTP in the prefrontal cortex area, which could be completely blocked by the selective antagonist for NR2B subunit; also, the transgenic mice behaved better than wild-type mice in a series of working memory testing tasks such as the T-maze (Cui et al., 2011). The α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) receptor is another type of iGluR in which the GluR1 subunit plays a key role in the plasticity of mature hippocampal synapses (Mack et al., 2001). GluR1 knocked out mice showed significant LTP deficiency in the CA3-CA1 area of the hippocampus and spatial working memory impairment in the radial maze task, which could be rescued by the transgenosis of GluR1 (Schmitt et al., 2005). The GluR1 subunit is also necessary for the basal synaptic transmission. The hippocampal slices from GluR1 knocked out mice showed impaired LTP in synapses, and the AMPA-mediated evoked currents were down-regulated under baseline conditions (Selcher et al., 2012). In the present study, to gain insight into the potential mechanism underlying the learning and memory deficits induced by BDE 209 exposure, we detected the protein expression of the glutamate subunits NR1, NR2B and GluR1 in the hippocampi of rats by western blotting, and our results showed that the expression of the three subunits were all significantly decreased after BDE 209 exposure. Taking all these results together, we hypothesized that the downregulation of NMDA and AMPA receptors after BDE209 exposure might at least partially result in an increased threshold for the induction of LTP, or the deficit of LTP, subsequently impaired spatial learning and memory ability.
The phosphorylation of subunit plays a major role in regulating the function of glutamate receptors, In the present study, we also detected the phosphorylation of NR2B at Ser1303 (p-NR2B Ser1303) and GluR1 at Ser831 (p-GluR1 Ser831), which were closely associated with the currents through the glutamate receptor channels thus regulating LTP (Chung et al., 2004; Liao et al., 2001; Derkach et al., 1999; Lee et al., 2003). Our results showed that the levels of p-GluR1 Ser831 and p-NR2B Ser1303 in the hippocampi of rats after BDE 209 exposure were significantly decreased, which might affect the induction of hippocampal LTP, and could be a potential mechanism of spatial learning and memory deficits caused by BDE 209 exposure. However, interestingly, the phosphorylation ratio of NR2B at Ser1303 was significantly increased and the phosphorylation ratio of GluR1 at Ser1303 showed no siginificant change though the levels of p-NR2B Ser1303 and p-GluR1 Ser831 were decreased. A study showed that NR2B and CaMKII could aggregate to form NR2B-CaMKII complex and transported to the postsynaptic membrane, which was critical for the NMDAR-mediated LTP (Lee, 2006). The interaction between NR2B and CaMKII was regulated by the phosphorylation of NR2B at Ser1303; in brief, the phosphoralytion of NR2B could promote the depolymerization of NR2B-CaMKII complex while the dephosphoralytion of NR2B could prevent the depolymerization (Strack et al., 2000; Raveendran et al., 2009). In the present study, the increaed phosphorylation ratio of NR2B suggested that the stability of the NR2B-CaMKII complex might be affected, and therefore the NMDAR-mediated LTP could be impaired and the BDE 209-exposed rats showed spatial learning and memory deficicts in the MWM. Moreover, in the present study, we also detected the protein expression of CaMKII and its phosphorylation at Thr286. Our results showed that the expression of αCaMKII and the level of p-αCaMKII Thr286 were both significantly decreased in the hippocami of rats after BDE 209 exposure. On one hand, the decrease of the expression of αCaMKII and the level of p-αCaMKII Thr286 might have affected the downstream biochemical reactions of CaMKII involved in LTP or learning and memory (Lisman et al., 2012). On the other hand, since CaMKII is one of the major protein kinases that phosphorylates NR2B at Ser1303 and GluR1 at Ser831 (Lee, 2006), the alteration in the expression of αCaMKII might also be associated with the changes in the phosphorylation of NR2B and GluR1.
In conclusion, our present study further indicated that subchronic BDE 209 exposure could also induce spatial learning and memory impairment in adult rats. The decrease of the protein expression of the glutamate receptor subunits NR1, NR2B and GluR1, the decrease of the levels of p-NR2B Ser1303 and p-GluR1 Ser831, the increase of the phosphorylation ratio of NR2B, the decrease of protein expression of CaMKII and the level of p- CaMKII Thr286 might be the potential mechanism. Moreover, the changes in the phosphorylation of NR2B and GluR1 might be partially associated with the decrease of CaMKII. However, since various kinase and signal pathways are involved in the protein expression and phosphorylation, and the mechanism of neurobehavior effects caused by BDE 209 is still not fully explained, the mechanism of how BDE 209 exposure induces neurobehavioral alterations needs further investigation. Moreover, we think that the potential mechanism of the changes in the protein expression and phosphorylation of glutamate receptor subunits induced by BDE 209 exposure may be a interesting issue.
This work was supported by Fund Project of Sichuan Science and Technology Department, grant number: 2010JY0128, and Fund Project of Luzhou Medical college, grant number: 200917.
Conflict of interestThe authors declare that there is no conflict of interest.