2018 Volume 43 Issue 3 Pages 223-227
2018 Volume 43 Issue 3 Pages 223-227
The drug-metabolizing enzyme CYP3A is a heterogeneous enzyme found in the liver that displays local characteristics referred to as “zonation.” Zonation contributes to improved energy efficiency in metabolism. The objective of this study was to determine a scientific basis for the safety of fetuses and nursing infants in cases in which the use of pharmaceuticals by pregnant and nursing mothers is unavoidable. In addition, we analyzed CYP3A zonation in the liver using mice from the fetus stage to the nursing stage. The livers of mice ranging from day 13.5 of the fetal stage to day 7 of the nursing stage were resected and immunostained using rabbit anti-rat CYP3A2 Ab, which can detect CYP3A11, CYP3A13, CYP3A16, CYP3A25, CYP3A41 and CYP3A44. The results indicated that zonation did not begin in the fetus stage up to day 3 of the nursing stage, and began on day 7 of infancy. This study revealed that changes in the metabolic activity of CYP3A in the liver between the fetal and nursing stages are partly related to zonation. Further studies are needed to establish standards for the proper use of pharmaceuticals by pregnant and nursing mothers.
The use of pharmaceuticals by pregnant and nursing mothers is avoided because of the effects the drugs may have on the fetus or nursing infant. However, in cases when the woman suffers from chronic diseases such as hypertension, diabetes, or hyperlipidemia, or when anesthesia is administered as a part of a cesarean section, the administration of pharmaceuticals to pregnant and nursing mothers may be unavoidable. However, the safety of pharmaceuticals utilized in such cases has not been established. To establish safety measures in such cases, we previously conducted a variety of studies from a pharmacokinetics perspective. The results revealed that CYP3A16, which has a low metabolic activity in response to pharmaceuticals, is predominantly expressed in the livers of mice during the fetal stage (Kitaoka et al., 2018). We also found that CYP3A11, which has a high metabolic activity, begins to be expressed on postnatal day 7 (Kitaoka et al., 2018). These factors may explain the low level of drug-metabolic activity during the fetal and nursing stages of development.
However, another explanation for the low level of drug-metabolic activity during the fetal stage was recently revealed. The expression of enzymes involved in drug metabolism, including CYP, is heterogeneous in the liver from the periportal region to the pericentral region. This localized characteristic expression is referred to as zonation (Ring et al., 1999; Braeuning and Schwarz, 2010; Gebhardt and Matz-Soja, 2014; Man et al., 2017). Recently, zonation was suggested to contribute to improved energy efficiency in the metabolism of pharmaceuticals by the liver. However, zonation is not observed in the livers of fetuses (Ring et al., 1999). This may be one factor causing decreased drug-metabolic activity in the livers of fetuses.
Establishment of a relationship between CYP3As (CYP3A16 and CYP3A11) expression and zonation will help the proper administration of pharmaceuticals to pregnant and nursing mothers. Thus, we analyzed CYP zonation in the livers of mice in the fetal and nursing stages of development by immunostaining.
Rabbit anti-rat CYP3A2 antibody was purchased from Nosan Corporation (Kanagawa, Japan). Goat anti PECAM-1 Ab (M-20; sc-1506) was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Alexa Fluor 488 donkey anti-rabbit IgG (A21206) and Alexa Fluor 594 donkey anti-goat IgG (A11058) were purchased from Invitrogen (Carlsbad, CA, USA).
Animal handlingPregnant ICR mice were purchased from Japan SLC, Inc. (Tokyo Laboratory Animals Science Co. Ltd, Tokyo, Japan). The mice were kept at room temperature (24 ± 1°C) and 55 ± 5% humidity with 12 hr of light (artificial illumination; 8:00-20:00). Food and water were available ad libitum. Each animal was used only once. The present study was conducted in accordance with the Guiding Principles for the Care and Use of Laboratory Animals, as adopted by the Committee on Animal Research at Hoshi University.
TransfectionAfrican green monkey kidney COS-7 cells (Riken cell bank, Ibaraki, Japan) were cultured in Dulbecco’s modified Eagle’s medium (Nissui Pharmaceutical, Tokyo, Japan) supplemented with 10% fetal bovine serum (Equitech-Bio, Kerriville, TX, USA) in a humidified atmosphere of 5% (v/v) CO2 in air at 37°C (Gluzman, 1981). In total, 1.0 x 105 COS7 cells were dispensed into a 6 cm dish, and after 24 hr, was carried out to transfect each vector (pEF-BOS-CYP3As) with 2 µg of the plasmid containing the mouse CYP3A gene (Mizushima and Nagata, 1990).
ImmunocytochemistryAfter washing with PBS (−), COS7 cells were fixed with 4% paraformaldehyde (PFA) / PBS at r.t. for 10 min. After washing gently with PBS (−) once, the cells were incubated with blocking buffer (0.1% TritonX-100, 3% FBS / PBS (−)) at r.t. for 1 hr and then with the primary antibody (Ab) solution at 4°C overnight. After washing gently with PBS (−) three times, the cells were reacted with the secondary Ab solution in the dark at r.t. for 1 hr. After washing gently with PBS (−) three times, the cells were enclosed with VECTASHIELD with DAPI (H-1200) (Vector Laboratories, Bulingama, CA, USA) using MICRO COVER GLASS. The immunostained sections were detected using FV-1200 (Olympus, Tokyo, Japan).
ImmunohistochemistryICR pregnant mice at 13.5, 15.5 and 17.5 of gestation were anesthetized with isoflurane, and fetuses were removed by cesarean section. And ICR mice (1, 3 and 7 day-old) were similarly dissected. The liver was fixed in 4% PFA at 4°C for 20 min. After washing with PBS (−), the samples were soaked in 10, 20, and 30% sucrose solutions at 4°C in sequence until the tissues sank in each solution. The samples were embedded in Surgipath FSC22 Blue Frozen Section Compound and stored at −80°C. Then, they were sectioned into 12 μm slices using LEICA CM1850 and placed on micro slide glass. Once the sections were sufficiently dried, they were stored at −80°C as frozen sections until use. Immunostaining was then performed in the same manner as immunocytochemistry.
Numerous CYP3A molecules have been identified. The amino acid sequences of CYP3A molecules are extremely homologous. Thus, it is difficult to create an antibody that reacts specifically to any of the CYP3A molecular species. Therefore, it is important to analyze the antibody used experimentally to determine the CYP3A molecular species recognized by the antibody. We first analyzed the reactivity of the antibody (rabbit anti-rat CYP3A2 Ab) used in this study by immunocytochemical staining. In addition, it has been reported that this antibody specifically recognizes the CYP3A molecule among mouse CYP molecular species (Zhao and Ishizaki, 1997).
The expression vector for each CYP3A molecular species was transfected into a COS7 cell, the cell was fixed for 24 hr, and immunocytochemical staining using a rabbit anti-rat CYP3A2 Ab was then performed. This resulted in the acquisition of signals localized to the cytoplasm obtained from all cells except for cells transfected with the control vector (Fig. 1). Although the results indicated that anti-rat CYP3A2 Ab was unable to distinguish the CYP3A molecular species used in this study, it recognized nearly all CYP3A (CYP3A11, 13, 16, 25, 41 and 44) molecular species, including CYP3A16. Therefore, we performed immunostaining using anti-rat CYP3A2 Ab without attempting to distinguish the CYP3A molecular species. We then analyzed CYP3A zonation in the liver.

Analysis of antibody recognition of molecular species by immunocytochemistry. Mouse CYP3As (CYP3A11, CYP3A13, CYP3A16, CYP3A25, CYP3A41 and CYP3A44) expression if forced in cells. After the cells were fixed, the responses were investigated using immunocytochemical staining with anti-rat CYP3A2 Ab. (× 1,200).
First, we confirmed whether anti-rat CYP3A2 Ab could be used to detect CYP3A zonation as reported previously by using the livers of 6-week-old male mice, which are known to have established zonation (Sekine et al., 2009; Walter et al., 2014; Pu et al., 2016). The results indicated characteristic localization of CYP3A near the central vein, where the vascular endothelial cell marker PECAM-1 is expressed. We also observed decreases in this localization phenomenon further from the central vein. This indicates that anti-rat CYP3A2 Ab can confirm CYP3A zonation using this procedure (Fig. 2).

Immunohistochemical staining of liver tissue segments resected from adult mice. The livers of 6-week-old male mice were resected and tissue segments were created. Then, CYP3A expression and localization were investigated using immunohistochemical staining with anti-rat CYP3A2 Ab. Staining with the vascular endothelial cell marker protein PECAM-1 was also performed. (× 200).
Mice livers were resected from day 13.5 (E13.5) of the fetal stage to day 7 (P7) of the nursing stage. Experiments were carried out using male mice. Thus, genomic DNA was extracted from the tails of mouse fetuses at each stage, and the sex-determining region Y (SRY) gene was used as an indicator to distinguish the sex (data not shown) (Nakagome et al., 1991). Tissue segments were then created and subjected to immunostaining using anti-rat CYP3A2 Ab. A representative photograph is shown in Fig. 3 (n = 3). The results indicated that, as reported previously, CYP3A zonation was not observed during the fetal stage (Ring et al., 1999). CYP3A zonation was also not observed on postnatal day 1 or 3, as was the case during the fetal stage. However, on postnatal day 7, CYP3A expression was increased in cells surrounding the central vein and characteristic localization of CYP3A was observed (Fig. 3). These results indicate that CYP3A zonation in the occurs on postnatal day 7, which is during the pre-weaned period of the infant stage.
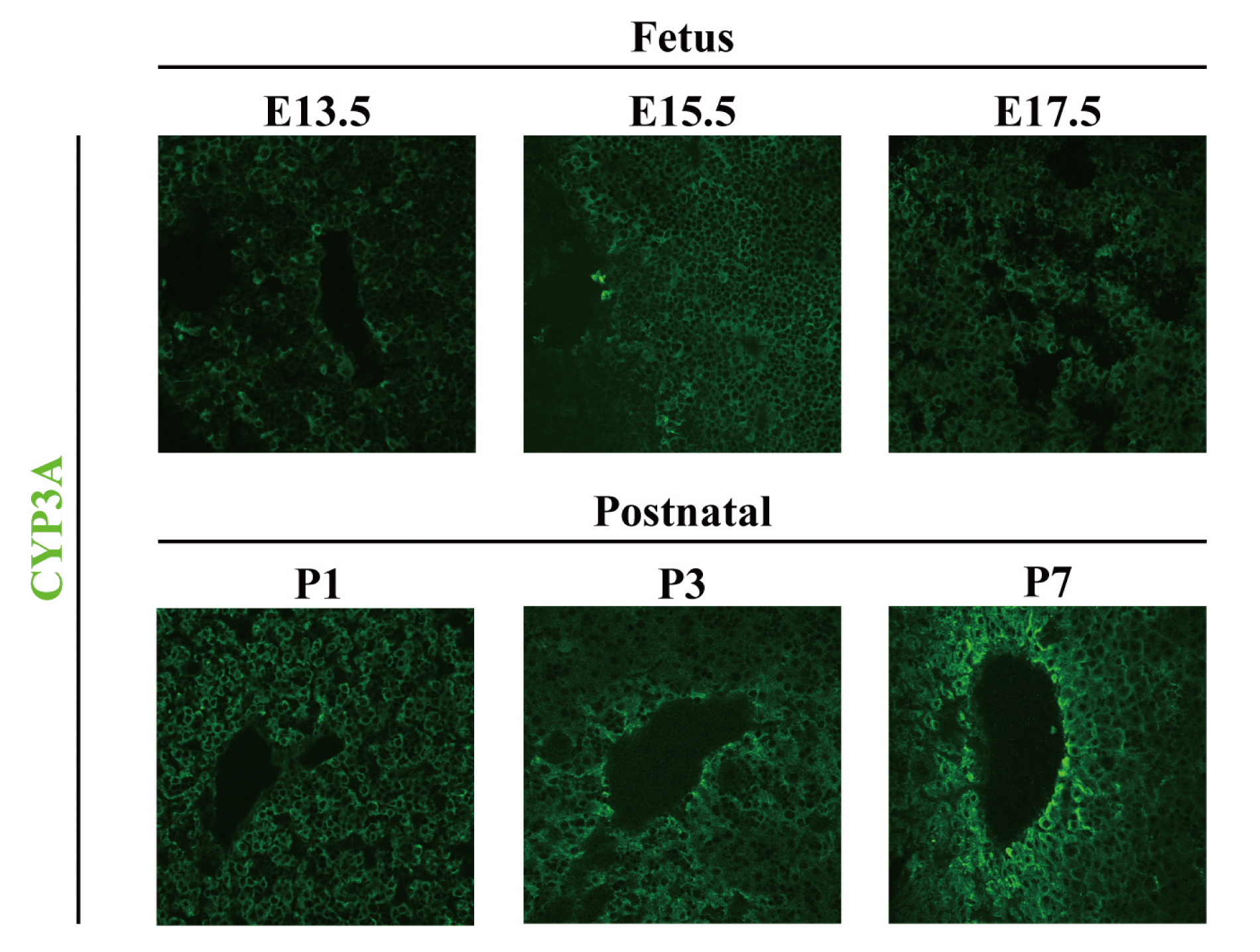
Immunohistochemical staining of liver tissue segments resected from fetal and infant mice. The livers of fetal and infant mice were resected and tissue segments were created. Then, CYP3A expression and localization were investigated using immunohistochemical staining with anti-rat CYP3A2 Ab. (n = 3) (× 400).
Drug-metabolizing enzymes, including CYP, zonate in the liver and are thought to improve the efficiency of substrate metabolism. To establish a scientific basis for the proper use of pharmaceuticals during the fetal and nursing stages, we investigated the relationship between CYP3A expression in the liver and the process of zonation forming. The results indicated that CYP3A zonation did not occur during the fetal stage, when CYP3A16, which has a low degree of drug-metabolizing activity, was predominantly expressed. However, that CYP3A zonation was found to begin during the nursing stage and coincided with the predominant expression of CYP3A11 during this stage of development. The CYP3A substrate, which is involved in the oral ingestion of foods and pharmaceuticals, enters the liver from the portal vein and is then sent throughout the body via the central vein. The substrate concentration of CYP3A near the portal vein differs from that near the central vein, and as a result heterogeneous metabolism occurs. Thus, CYP3A zonation occurs to ensure that metabolism is efficient throughout the liver. The types and amounts of CYP3A substrate are thought to increase as development progresses from the fetal stage to the nursing and weaning stages. In addition, a wide variety of enzymes are expressed in the liver corresponding to the dramatic environmental changes that occur in the weaning stage and later. Thus, it may be necessary to optimize CYP3A metabolic activity in response to the changes that occur during the developmental process. In mice, weaning begins at approximately postnatal week 3. In preparation for weaning, expression of CYP3A11, which is highly metabolically active, may increase and CYP3A zonation begins.
The present study was not focused on zonation to differentiate CYP3A molecular species. Therefore, we only describe the results of immunostaining based on our previous research. The results found in the fetal stage may reflect CYP3A16, while the results found in the nursing stage may reflect CYP3A11. The CYP3A molecular species should be identified in future studies. In our previous studies, we found a relationship between changes in CYP3A metabolic activity and molecular species from the fetal stage to adulthood. In addition, this study revealed a relationship between changes in CYP3A metabolic activity and the presence versus absence of zonation. Further studies are required to establish the proper use of pharmaceuticals in pregnant and nursing mothers.
We thank Misa Iizuka, Osamu Kosaka, Saori Tomita, Tomoka Yasukawa and Hiroyuki Yoshida (Department of Clinical Pharmacokinetics, Hoshi University) for their technical assistance. This work was supported by the MEXT-Supported Program for the Strategic Research Foundation at Private Universities 2014-2018 [MEXT, Grant S1411019]. We would like to thank Editage (www.editage.jp) for English language editing.
Conflict of interestThe authors declare that there is no conflict of interest.