2018 Volume 43 Issue 4 Pages 247-256
2018 Volume 43 Issue 4 Pages 247-256
Photoreactive compounds that may experience exposure to ultraviolet (UV) radiation can lead to the intracellular production of reactive oxygen species (ROS), which may cause phototoxic and photoallergenic responses. Here, we developed a novel in vitro photosafety assay and investigated whether it could be used to predict phototoxicity and photosensitivity by measuring changes in intracellular ROS production. THP-1 cells that had previously taken up 5-(and-6)-carboxy-2’,7’-difluorodihydrofluorescein diacetate (carboxy-H2DFFDA), a ROS-sensitive fluorescent reagent, were exposed to photoreactive substances such as phototoxic and photoallergenic materials and then subjected to with UV-A irradiation (5 J/cm2). The fluorescence intensity was subsequently measured using a flow cytometer, and the intracellular ROS production was calculated. A statistically significant increase in ROS following treatment with photoreactive substances was observed in cells irradiated with UV-A. In contrast, no significant increase was observed for non-photoreactive substances in comparison to the control solution. Next, to confirm the impact of intracellular ROS on the photosensitive response, changes in CD86 and CD54 expression were measured following quencher addition during the photo human cell line activation test (photo h-CLAT). The results confirmed the reduction of CD86 and CD54 expression in response to photoallergenic substances following quencher addition. Together, these findings suggest that intracellular ROS production is involved in photosensitizing reactions. Therefore, we suggest that the developed method utilizing intracellular ROS production as an index may be useful as a novel in vitro evaluation tool for photoreactive substances.
Assessing the photosafety, such as phototoxicity and photoallergenicity, of ingredients constitutes a vital and indispensable task for ensuring the increased safety of cosmetic products that are exposed to ultraviolet radiation during use. Photoreactive materials that cause these phototoxic and photoallergic properties generally produce reactive oxygen species (ROS), such as singlet oxygen and superoxide anions that can lead to the oxidation of biological substances and various associated toxicities, through an energy conversion mechanism that occurs during exposure to ultraviolet light (Moore, 2002; Tokura, 2009; Onoue et al., 2017). Accordingly, the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) recently released guidelines (ICH S10, 2014) regarding the ROS assay, which is used to evaluate photosafety, mainly with respect to active oxygen species. The ROS assay is a method for in chemico evaluation of photoreactivity and is currently used as the first-line screening method for photosafety evaluation. Specifically, the assay uses colorimetry to measure the presence of two types of ROS (singlet oxygen and superoxide anions) produced as a result of irradiating chemical substances with simulated sunlight. In particular, this test method is highly sensitive for evaluating direct phototoxic substances in vivo. However, compounds exhibiting light-stability problems might also be judged as positive; therefore, one of the characteristics of this method is that it shows a high number of false-positive results (ICH S10, 2014).
Measurement of intracellular ROS has been studied in vitro using cultured cells. Intracellular ROS are reportedly involved in the expression of photosensitivity in response to various skin-sensitizing substances, with the biochemical trigger for this effect involving the impact of intracellular redox imbalances caused by ROS (Sasaki and Aiba, 2007; Mizuashi et al., 2005). Moreover, a recent study reported the production of intracellular ROS following exposure to sodium lauryl sulfate (SLS), an irritant (Mizutani et al., 2016), suggesting that intracellular ROS production plays a major role in the manifestation of toxicity, such as irritation and sensitization. Therefore, by combining these procedures, in the present study the intracellular ROS-measurement technique was applied to evaluate in vitro photostability using cultured cells for the first time.
Additionally, we confirmed the effect of intracellular ROS on the expression of phototoxicity and photoallergenicity. As previous studies reported reduced phototoxic responses from phototoxic substances following the addition of a quencher that captures and eliminates ROS using the 3T3NRU PT method, which is an in vitro phototoxicity test method (Okamoto, 2001), in the present study, we examined reductions in photosensitive response for photoallergic substances following quencher addition. In addition, we used a photo human Cell-Line-Activation Test (photo h-CLAT) for photoallergic substances to confirm the presence or absence of photoallergic activity in dendritic cells as an index and evaluated this activity by focusing on the elevated expression of the cell-surface antigens CD86 and CD54, which are important for dendritic cell presentation of antigens to T cells. Using photo h-CLAT, we confirmed changes in CD86 and CD54 expression following addition of quenchers to capture and eliminate ROS, allowing examination of the involvement of intracellular ROS in photosensitivity.
The THP-1 clone A31 cell line was obtained from the American Type Culture Collection (Manassas, VA, USA). The cells were cultured in RPMI 1640 supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA, USA), 0.05 mM 2-mercaptoethanol, and 1% penicillin-streptomycin (Invitrogen).
ChemicalsChlorpromazine HCl (CPZ), 8-methoxypsoralen (8MOP), hexachlorophene (HCP), sulfanilamide (SA), SLS, and methyl salicylate (MS) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Bithionol (BN) and ketoprofen (KP) were purchased from Wako Pure Chemical Industries (Osaka, Japan). Promethazine HCl (PMZ) and penicillin G (PG) were purchased from Tokyo Chemical Industry (Tokyo, Japan). All chemicals were solvated in saline or dimethylsulfoxide (Sigma-Aldrich) and then added to Hanks’ balanced salt solution (Invitrogen).
Four quenchers were used to investigate the effects of intracellular ROS on the photosensitive response. D(−)-mannitol, L-histidine, and N-acetyl-L-cysteine (NAC) were purchased from Wako Pure Chemical Industries. Catalase was purchased from MP Biomedicals, LLC (Santa Ana, CA, USA).
Ultraviolet (UV) irradiation sourceA mercury metal-halide lamp (SOL500; Dr. Hönle AG, Martinsried, Germany) that simulates the spectral distribution of natural sunlight was used as the source of UV-A irradiation. In this study, an H1 filter was used to attenuate UV-B wavelength with high cytotoxicity using a filter. A UV-meter (part no. 0037; Dr. Hönle AG) was used to measure UV-A intensity. The irradiation test was carried out with an irradiance of 1.7 mW/cm2 as equivalent value of the UV-meter.
Determination of intracellular ROS5-(and-6)-Carboxy-2′,7′-difluorodihydrofluorescein diacetate (carboxy-H2DFFDA; Thermo Fisher Scientific, Waltham, MA, USA) is an indicator of cell-permeable active oxygen and a dye that emits fluorescence following the removal of acetate via decomposition or oxidation by intracellular esterase. Here, the quantity of the resulting fluorescent dye was measured using a flow cytometer, after which the extent of intracellular ROS production was also measured.
Prior to measuring intracellular ROS production, a cytotoxicity test was performed to establish dosage. However, as cells in this study were exposed to UV light for set periods of time, these cells were assumed to have sustained damage owing to irradiation. Therefore, it was decided that 80% cell viability (CV80) would be used with respect to the established dose to account for light-induced cellular damage. For the cytotoxicity test, test chemicals were prepared at six concentrations (1/25, 1/24, 1/23, 1/22, 1/2, and 1 × 1,000 μg/mL). THP-1 cells (2 × 106 cells/mL) were incubated in the presence of 2 μM carboxy-H2DFFDA for 30 min at 37°C. After washing twice with Dulbecco’s phosphate-buffered saline (DPBS; Gibco, Gaithersburg, MD, USA), the THP-1 cells that had absorbed carboxy-H2DFFDA were seeded onto a 24-well plate and exposed to the test substance. Subsequently, one plate was irradiated with UV-A (irradiation conditions: UV-A dose of 5 J/cm2 at an intensity of 1.7 mW/cm2, at 28°C for 50 min), whereas the other plate was left to react in the dark room for approximately 50 min. Following UV irradiation, the cells were collected into a test tube, rinsed with DPBS, and then measured using a BD FACS Calibur flow cytometer (Becton Dickinson, San Jose, CA, USA). Dead cells were gated by staining with propidium iodide (PI; Sigma-Aldrich). A CV80 was calculated based on the linearity of the collected data.
Six doses at CV80 (1, 1.2, 1/1.2, 1/1.22, 1/1.23, and 1/1.24 × CV80 μg/mL) were used as reference doses for the test substance. THP-1 cells (2 × 106 cells/mL) that had incorporated carboxy-H2DFFDA were seeded onto a 24-well plate for 30 min at 37°C, followed by exposure to the test substance. The cells were subsequently irradiated with UV-A at a constant intensity for a set time period with irradiation condition as for the cytotoxicity test, washed with DPBS, and the fluorescence intensity was measured via flow cytometry after PI staining according to the same method used for the cytotoxicity test. Three independent tests were carried out, and ROS production was then calculated from the ratio of the results of each test to those of the solvent control. Photoreactivity was determined based on the values for peak ROS production after UV-A (+) or UV-A (−) exposure at test-substance concentrations that resulted in ≥ CV80. A 95% confidence interval (CI) was used for these determinations.
Evaluation and interpretation of resultsThe 95% CIs for ROS production at CV80 concentrations were determined, and at ≥ CV80 concentrations showing 95%CI, the highest value of ROS production (ROS productionmax) was calculated for UV-A(+) and UV-A(−), respectively. Criteria for assessing ROS production were as follows:
Positive : 95% CI [lower-limit value (UV-A (+)) ≥ 2; upper-limit value (UV-A (−)) < 2]
Negative: 95% CI [lower-limit value (UV-A (+)) and upper-limit value (UV-A (−)) < 2]
Photo h-CLATPhoto h-CLAT is a method of in vitro skin-sensitization testing based on the h-CLAT method. In photo h-CLAT, cells are exposed to a test substance while simultaneously being irradiated with UV-A, and photoallergenicity is determined based on dendritic cell activation in response to light (Hoya et al., 2009). In the present study, THP-1 cells were seeded onto a 24-well plate (1 × 106 cells/mL) and gradually exposed to diluted test substances. One plate was exposed to UV-A radiation [UV-A (+)], and another plate was placed in a dark room (non-irradiation). Cells were exposed to irradiation at 5 J/cm2 over approximately 50 min, followed by incubation at 37°C in a CO2 environment. The cells were then washed 24 hr after exposure to the test substance and subsequently divided into three groups after binding of the Fc receptor on the cell surface to antibodies. After cell staining, anti-CD86 (BD PharMingen, San Diego, CA, USA), anti-CD54 (DAKO; Agilent Technologies, Santa Clara, CA, USA), and isotype control fractions (IgG1; DAKO; Agilent Technologies) were measured using a flow cytometer. We confirmed the involvement of ROS in the induction of dendritic cell activation and CD86/CD54 expression by highly reactive photoallergenic substances following UV irradiation, as decreased expression of CD86 and CD54 was observed using chemical quenchers. Photo h-CLAT was conducted on THP-1 cells following exposure to the test substances and during simultaneous culture with one of four chemical quenchers (Okamoto, 2001): mannitol (10 mM), catalase (1,400 U/mL), NAC (10 mM), or histidine (10 mM).
The levels of intracellular ROS produced following exposure to photosensitizing substances (six photoallergenic: CPZ, BN, PMZ, KP, HCP, and SA; one photoirritant: 8MOP, and three non-phototoxic substances: SLS, MS, and PG) were measured using carboxy-H2DFFDA (Table 1 and Fig. 1). Significant intracellular ROS production was confirmed for CPZ, BN, PMZ, KP, HCP, and 8MOP under UV-A(+) conditions, whereas intracellular ROS production under UV-A(−) conditions was not significantly increased relative to the control for any tested substance. Positive results were determined based on a lower limit value of the 95% CI for the UV-A(+) condition of ≥ 2. SA, which is a photoallergic substance, did not show any significant increase under either UV-A(+) or UV-A(−) conditions relative to controls and was judged as a negative result based on 95% CI. Among non-phototoxic substances, SLS resulted in increased intracellular ROS production under UV-A(+) conditions but did not meet the 95% CI criterion for a positive finding (Fig. 2), whereas MS and PG administration did not result in a significant increase in intracellular ROS production as compared with controls under either UV-A(+) or UV-A(−) conditions, and were thus also deemed negative.
| Chemical | Photosafety information (in vivo)* |
ROS assay (ICH S10) |
3T3 NRU-PT (OECD TG432) |
Intracellular ROS production test | *Reference | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Photo- irritation |
Photo- allergy |
CV80 (μg/mL) |
ROS production max | Determination 95% CI |
|||||||||
| lower | judge | ||||||||||||
| upper | |||||||||||||
| Chlorpromazine HCl | CPZ | + | + | + | + | UV+ | 0.9 | 2.15 | ± | 0.05** | 2.09 | Positive | Lovell and Jones (2000) |
| UV- | 24.1 | 1.73 | ± | 0.07 | 1.80 | ||||||||
| 8-Methoxypsoralen | 8MOP | + | - | + | + | UV+ | > 150 | 2.71 | ± | 0.03** | 2.68 | Positive | Onoue et al. (2013) |
| UV- | > 150 | 1.08 | ± | 0.06 | 1.14 | ||||||||
| Bithionol | BN | - | + | + | + | UV+ | 5.3 | 5.20 | ± | 0.84** | 4.25 | Positive | Lovell and Jones (2000) |
| UV- | 8.6 | 1.92 | ± | 0.02 | 1.94 | ||||||||
| Promethazine HCl | PMZ | + | + | + | + | UV+ | 9.5 | 3.18 | ± | 0.41** | 2.72 | Positive | Onoue et al. (2013) |
| UV- | 74.7 | 1.54 | ± | 0.05 | 1.59 | ||||||||
| Ketoprofen | KP | - | + | + | + | UV+ | 9.6 | 7.25 | ± | 1.09** | 6.01 | Positive | Lovell and Jones (2000) |
| UV- | > 300 | 1.80 | ± | 0.13 | 1.94 | ||||||||
| Hexachlorophene | HCP | + | + | + | - | UV+ | 4.4 | 2.78 | ± | 0.33** | 2.41 | Positive | Onoue et al. (2013) |
| UV- | 4.2 | 1.80 | ± | 0.12 | 1.93 | ||||||||
| Sulfanilamide | SA | no data | + | + | - | UV+ | > 5000 | 1.79 | ± | 0.12 | 1.65 | Negative | Onoue et al. (2013) |
| UV- | > 5000 | 1.29 | ± | 0.05 | 1.34 | ||||||||
| Sodium lauryl sulfate | SLS | - | - | - | - | UV+ | 29.1 | 2.08 | ± | 0.15 | 1.91 | Negative | Lovell and Jones (2000) |
| UV- | 43.9 | 1.57 | ± | 0.05 | 1.62 | ||||||||
| Methyl salicylate | MS | - | - | + | - | UV+ | > 1000 | 1.28 | ± | 0.26 | 0.98 | Negative | Onoue et al. (2013) |
| UV- | > 1000 | 1.30 | ± | 0.04 | 1.34 | ||||||||
| Penicillin G | PG | - | - | - | - | UV+ | > 1000 | 1.06 | ± | 0.09 | 0.96 | Negative | Onoue et al. (2013) |
| UV- | > 1000 | 1.07 | ± | 0.06 | 1.14 | ||||||||
Statistical analysis using the 95% confidence interval (95% CI). **95% CI > 2.

Intracellular ROS production following treatment with phototoxic and photoallergenic substances (CPZ, 8MOP, BN, PMZ, KP, and HCP) increases as a result of UV-A radiation exposure. For non-photoreactive substances (SLS, MS and PG), intracellular ROS production does not significantly increase as a result of UV-A irradiation. Cell viability 80% (CV80) is calculated based on the cytotoxicity test; the testing focused on CV80, and was conducted at six different concentrations of UV-A (+) and UV-A(−). The relative fluorescence intensity (RFI) represents the mean ± S.D. value calculated from the results of each of the three independent tests. # indicates the 95% confidence interval > 2. CPZ: Chlorpromazine HCl; 8MOP: 8-Methoxypsoralen; BN, Bithionol; PMZ: Promethazine HCl; KP: Ketoprofen; HCP: Hexachlorophene.
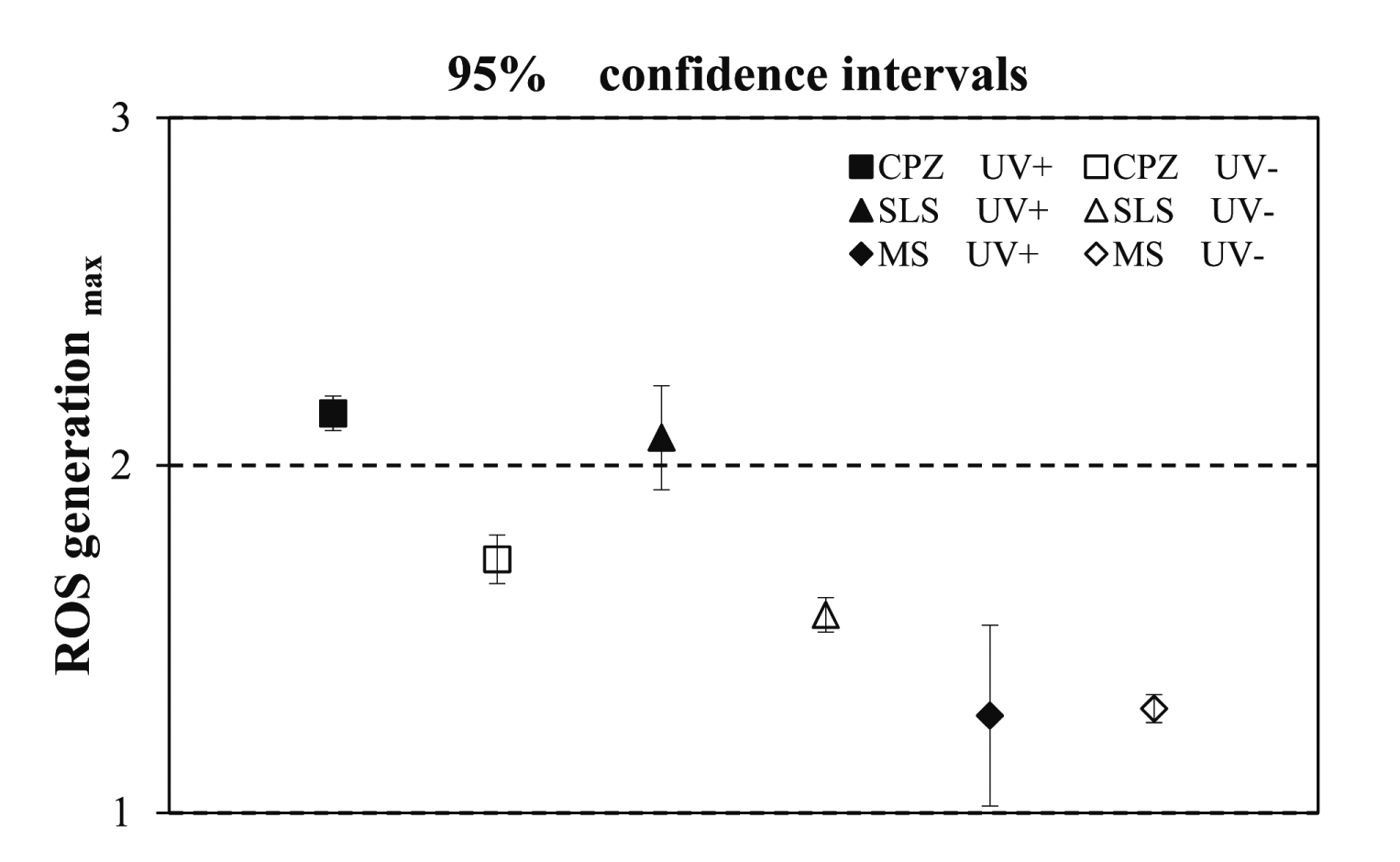
Determination of 95% confidence interval (95%CI). CPZ, SLS, and MS.
CPZ is positive: 95% CI [lower limit value (UV-A (+)) ≥ 2, upper limit value (UV-A(−)) < 2]
MS is negative: 95% CI [upper limit value (UV-A (+) and UV-A(−)) < 2 ]
SLS is negative: 95% CI [lower limit value (UV-A(+)) < 2, upper limit value (UV-A(−)) < 2]
To characterize the involvement of ROS in the manifestation of phototoxicity and photoallergenicity, we investigated whether toxicity development is suppressed by quenchers. A previous study (Okamoto, 2001) examined the extent to which the addition of a quencher reduces phototoxicity using the in vitro 3T3NRU-PT assay (OECD TG 432, 2004). Our results showed that suppression of phototoxicity was observed after the addition of a quencher. Moreover, we investigated the impact of intracellular ROS on the induction of toxicity by photoallergenic substances, as well as the extent of the changes in CD86 and CD54 expression following quencher addition, by performing photo h-CLAT (Fig. 3 and Table 2). Among the various quenchers used, NAC exhibited a particularly strong quenching effect. Furthermore, with the exception of CD54 expression in the presence of KP, the addition of NAC suppressed CD86 and CD54 expression relative to the control and did not exceed the criteria for a positive result from the photo h-CLAT. Histidine, which is known to exhibit a quenching effect on singlet oxygen, showed an inhibitory effect on CD86 and CD54 expression in the presence of CPZ, on CD54 expression in the presence of BN, and on CD54 expression in the presence of KP. Additionally, histidine reduced CPZ-, BN-, and KP-induced photosensitivity; however, the effects of D(−)-mannitol (Smirnoff and Cumbes, 1989) and catalase, both of which exhibit a quenching effect on radical reactions, were more limited. Therefore, although D(−)-mannitol demonstrated a particularly strong capacity to suppress photosensitivity with respect to CD86 expression in response to KP and that of CD54 expression in response to CPZ, no effect was observed in response to other substances. Moreover, catalase exhibited a particularly strong inhibition of photosensitivity with respect to CD54 expression in response to KP and led to a decrease in CD54 expression in response to CPZ.

Photoallergenicity assessment of CPZ by photo h-CLAT. The upper row displays CD86 and CD54 expression following CPZ treatment under UV-A (+)/UV-A(−) exposure. The positivity criteria were as follows: RFI ≥ 150 for CD86, and RFI ≥ 200 for CD54. The middle row displays the results confirming the changes in CD86 and CD54 expression following histidine (quencher of CPZ) addition, and the testing under UV-A (+) and UV-A(−), respectively. The lower row displays the results confirming the changes in CD86 and CD54 expression following NAC (quencher of CPZ) treatment, and the testing under UV-A (+)/UV-A(−), respectively. CPZ: Chlorpromazine HCl. The relative fluorescene intensity (RFI) represents the mean ± S.D. value calculated from the results of each of the three independent tests.
| Chemicals | Photo h-CLAT EC150 (μg/mL) (UV+) |
Effect of quenchers on CD86 expression | |||
|---|---|---|---|---|---|
| Histidine | Mannitol | Catalase | NAC | ||
| CPZ | 0.42 | + | - | - | ++ |
| BN | N.D. | ∕ | ∕ | ∕ | ∕ |
| PMZ | 1.11 | - | - | - | ++ |
| KP | 15.7 | - | ++ | - | ++ |
| HCP | N.D. | ∕ | ∕ | ∕ | ∕ |
| Chemicals | Photo h-CLAT EC200 (μg/mL) (UV+) |
Effect of quenchers on CD54 expression | |||
|---|---|---|---|---|---|
| Histidine | Mannitol | Catalase | NAC | ||
| CPZ | 0.52 | ++ | ++ | + | ++ |
| BN | 12.6 | ++ | - | - | ++ |
| PMZ | 0.84 | - | - | - | ++ |
| KP | 10.8 | + | - | ++ | - |
| HCP | 1.7 | - | - | - | ++ |
CPZ: Chlorpromazine HCl, BN, Bithionol, PMZ: Promethazine HCl, KP: Ketoprofen, HCP: Hexachlorophene.
++: High quencher effect; CD86/CD54 expression did not exceed the photo h-CLAT positivity criteria.
+: Slight quencher effect; EC150/EC200 for CD86/CD54 exhibited a 1.6-2.0-fold increase.
-: Quencher had no effect; EC150/EC200 for CD86/CD54 was less than 1.5-fold.
To accurately assess the phototoxicity and photoallergenicity of photoreactive compounds, we considered it necessary to evaluate not only the reaction of chemicals but also the reactions occurring in treated cells following exposure to UV irradiation. Therefore, we investigated a method for evaluating intracellular ROS production through the use of seven photoreactive chemicals and three non-photoreactive chemicals, including an additional step of irradiating cells with UV light during test-substance exposure. However, because the widely used reagent for measuring the presence of intracellular ROS, 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA), exhibits poor photostability and reactions using this compound must be performed in a dark room with measurements taken promptly thereafter, in this study we instead utilized carboxy-H2DFFDA (Szivák et al., 2009; Gomes et al., 2017), a chlorinated fluorescein derivative with greater photostability than CM-H2DCFDA, to measure intracellular ROS production.
As shown in Table 1 and Fig. 1, exposure to the photoallergenic chemicals (CPZ, BN, PMZ, KP, and HCP) and to 8MOP, a photoirritant, led to a significant increase in intracellular ROS levels upon UV-A(+) irradiation as compared with those observed in the control. Significant increases following UV-A(+) relative to UV-A(−) irradiation, in particular, were considered as critical to identifying photoreactive substances based on statistical analyses using 95% CIs, a criterion that was established as exhibiting good predictability following the assessment of various evaluation methods. The 95% CI method constitutes a statistical method that estimates the true value of compound photoreactivity by assigning a defined range to the mean value of the population. The value used as the 95%CI for a given compound was evaluated as the concentration showing the highest ROS production amount as defined as the concentration value showing the maximum amount of change among concentrations showing a cell viability of 80% or more. For example, some compounds yielded a constant amount of ROS production even when the concentration was changed, whereas one resulted in decreased ROS production as compound concentration was increased. The maximum change amount was utilized because ROS production was not found to increase in a dose-dependent manner by all compounds. Our results showed that the photoallergenic chemicals CPZ, BN, PMZ, KP, and HCP had 95% CIs with a lower-limit value (UV-A(+)) >2 and upper-limit value (UV-A(−)) < 2 and were, therefore, judged positive.
Consistent with previous observations that ROS production is observed in both irritation and allergic reactions (Saito et al., 2013; Mizutani et al., 2016), in the present study, intracellular ROS production was also observed following exposure to phototoxic and photoallergic substances. Attempts were made to distinguish between phototoxicity and photoallergic properties, with increases in intracellular ROS production used as an index; however, for the test substances used in this study, criteria for such a distinction could not be clearly identified. Notably, because ROS production is an early intracellular reaction during the expression of toxicity, it is difficult to distinguish between phototoxicity and photoallergenicity according to ROS production alone. Indeed, among phototoxic and photoallergic substances, few substances can be clearly distinguished from one another in such a manner, because many substances characterized by photoallergic properties often exhibit phototoxic properties. Few photoallergic substances also lack phototoxic properties; therefore, it is difficult to construct an evaluation method capable of distinguishing between phototoxicity and photoallergenicity. Furthermore, we were unable to determine whether a quantitative difference in intracellular ROS levels actually reflects the toxic potency of a substance because of a lack of in vivo data comparing the toxic intensities of photoreactive substances.
Although administration of HCP led to characteristic intracellular ROS production as assessed by the 95% CI method, unlike the many chemicals that showed a tendency toward concentration-dependent increase of intracellular ROS, that by HCP exhibited a concentration-dependent decrease under both UV-A(+) and UV-A(−) conditions. Moreover, BN suppressed ROS production, especially in the presence of UV-A(−) irradiation. These effects might be due to the antioxidant-chemical properties of BN and HCP (Reid et al., 2001). In contrast, no significant increase in intracellular ROS production was observed for SA, which is considered as a photoallergic substance. Previously, it was reported that SA shows high reactivity to UV-B (Onoue et al., 2014; Oeda et al., 2016). However, in the present study, the evaluation was performed with the UV-B wavelengths filtered out in order to reduce high cytotoxic effects. Accordingly, we determined SA as being negative based upon the use of different UV-irradiation conditions. On the other hand, in case of the ROS assay for in chemico evaluation, which used an irradiation source including the wavelength of UV-B, SA was considered positive.
Among the non-photoallergenic chemicals tested, SLS exhibited a tendency to increase intracellular ROS production as compared with the control. Irritants, such as SLS, reportedly increase intracellular ROS production (Mizutani et al., 2016), and our results were consistent with these findings. However, no significant increase in intracellular ROS production was observed under UV-A(+) conditions as compared with UV-A(−) conditions. Moreover, in contrast to the ROS production resulting from photoreactive substances, SLS was determined to be negative based on 95% CI evaluation. However, irritating substances and sensitizing substances that do not show photoreactivity can also sufficiently increase intracellular ROS production. A method using increases in intracellular ROS production as an index for evaluating sensitivity was previously described (Saito et al., 2013); however, for irritants and allergens, when the toxic intensity is high, no significant difference in increased intracellular ROS production between UV-A(+) and UV-A(−) conditions would be expected. Therefore, for photoreactive chemicals, differences in intracellular ROS production between UV-A(+) and UV-A(−) conditions allowed us to appropriately evaluate photoreactive and other substances.
MS was determined as a false-positive substance based on an in chemico ROS assay (Onoue et al., 2013). No intracellular ROS production in response to MS was observed in the present study, and this substance was determined to be negative. This result was also consistent with in vivo findings. However, considering that the in chemico ROS assay is based on changes in ROS caused by UV irradiation and therefore evaluates initial photochemical reactivity, substances that show poor stability to light; that is, that are easily decomposed by light, might also be judged as positive. In contrast, the intracellular ROS assay used in the present study is based on the production of cellular ROS during reactions occurring in association with the expression of toxicity. Therefore, it is considered that a negative result was obtained for MS. Based on the present evaluation of intracellular ROS production in response to seven photoreactive chemicals and three non-photoreactive chemicals using carboxy-H2DFFDA, all chemicals, with the exception of SA that was not susceptible to UV irradiation, presented results reflecting potential in vivo conditions and suggested the potential utility of this method for the practical evaluation of photoreactive substances.
To confirm the involvement of intracellular ROS production in the expression of toxicity, changes in toxicity were examined using active oxygen quenchers, which have been previously used to investigate the involvement of ROS in phototoxicity development in response to phototoxic substances (Okamoto, 2001). When the suppressive effect on phototoxicity was evaluated using the 3T3 NRU-PT method in vitro, quenchers acting on active oxygen and those acting on radical reactions demonstrated characteristic suppressive effects. This confirmed that ROS have a substantial impact on phototoxicity in vitro. Thus, during the present study we focused on photoallergenic substances and confirmed their effects on the expression of toxicity. For this purpose, we used an in vitro photo h-CLAT. h-CLAT, a method of evaluating skin sensitization, was addressed in the OECD testing guidelines in 2016 (OECD TG442E), and is routinely used to check for dendritic cell activation, an event in the skin sensitization adverse outcome pathway (AOP). Photo h-CLAT detects photoreactivity while also incorporating an additional step involving irradiation of test cells with UV-A during exposure to test substances.
Here, changes in CD86 and CD54 expression during the photo h-CLAT were evaluated using chemical quenchers (Table 2 and Fig. 3) including histidine (acting on singlet oxygen), D(−)-mannitol and catalase (acting on radical reactions), and NAC (an oxidizer). Exposure to antioxidant substances BN and HCP resulted in no significant CD86 expression observed in the absence of a quencher. Antioxidant substances affect in vivo redox balance via their own redox reactions and inhibit ROS production. In the present study, the addition of each of these quenchers to the culture media suppressed CD86 and CD54 expression following exposure to CPZ. Mannitol and catalase, which show quenching effects on radical reactions, did not show a quenching effect on CD86, but did show this effect on CD54, whereas histidine, a quencher of singlet oxygen, showed a quenching effect on the photosensitive response associated with CD86 and CD54 expression. Quenchers that suppress the expression of cell-surface antigens differ, and the increased expression of CD86 and CD54 might have been the result of different active oxygen species; however, verification of this will require further research. CD54 expression induced by BN was suppressed by the presence of histidine and NAC, with histidine showing a quenching effect on singlet oxygen and suggesting that BN has reactivity involving singlet oxygen. However, for PMZ and HCP, photosensitivity was only suppressed by NAC. Following exposure to KP, D(−)-mannitol and NAC suppressed CD86 expression, whereas histidine and catalase suppressed CD54 expression. Although quenchers showing effects on photoallergenic substances differed, and differences in quenchers showing effects on CD86 and CD54 were recognized, NAC exhibited strong effects against all substances, except that of KP involving CD54 expression. NAC is a very strong antioxidant substance potentially involved in the prevention of cytotoxicity (Spagnuolo et al, 2006). Because the development of photosensitivity arising from any of the test substances could be suppressed through the addition of a quencher, these results thus supported ROS involvement in the expression of photosensitivity in response to photoallergenic substance exposure.
In conclusion, the photosafety assessment method established during this study and using intracellular ROS production as an indicator of phototoxicity represents a useful mechanistic approach to measuring photoreactivity. To improve the predictive accuracy of intracellular ROS production, it is necessary to evaluate additional test substances in the future. However, given the current absence of an in vitro method to accurately evaluate photoallergenicity, this novel cell-based method shows efficacy for evaluating the photosafety of compounds.
Conflict of interestThe authors declare that there is no conflict of interest.