2018 Volume 43 Issue 5 Pages 353-358
2018 Volume 43 Issue 5 Pages 353-358
Photodynamic therapy (PDT) using talaporfin sodium (TS) is tumor cell-selective less invasive therapy for the treatment of malignant glioma. We previously demonstrated that PDT using TS (TS-PDT) treatment exhibits anti-tumor activity against not only glioblastoma cells but also malignant meningioma cells. In general, various stress response proteins have been reported to affect the sensitivity determination for anticancer agents against tumor cells. However, the relationship between the therapeutic effect of TS-PDT and stress response systems in tumor cells is not adequately investigated. In this study, we investigated the gene expression of stress response proteins, including Sod1, Cat1, Gstp1, Gpx1, Nqo1, and Hmox1, in rat malignant meningioma KMY-J cells after treatment of TS-PDT. TS-PDT treatment significantly decreased the cell viability when compared with the no laser irradiation group. In morphological observation, TS at 25.6 µM treatment exhibited a significant cytotoxic effect after 12 hr of laser irradiation to KMY-J cells. After 3 and 6 hr of TS-PDT treatment, mRNA expression of heme oxygenase-1 (HO-1, encoded by Hmox1) was significantly increased by TS-PDT treatment. We also demonstrated that zinc protoporphyrin IX (ZnPPIX), a HO-1 inhibitor, significantly augmented the cytotoxic effect of TS-PDT treatment. These data suggest that HO-1 induction may contribute to a protective response against TS-PDT treatment in the malignant meningioma cells and may attenuate the therapeutic effect for TS-PDT treatment.
Photodynamic therapy (PDT) is a light-mediated treatment method and exhibits anti-tumor activity via cytotoxic reactive oxygen species (ROS), predominantly singlet oxygen, which are generated by activation of a photosensitizer (Dougherty et al., 1998; Dolmans et al., 2003; Agostinis et al., 2011). PDT is widely used both clinically and preclinically to treat a range of tumor types, including those in the lung, skin, liver, and brain neoplasms (Agostinis et al., 2011; Akimoto 2016). Talaporfin sodium (mono-L-aspartyl chlorine, Laserphyrin®, TS) is a water-soluble second-generation photosensitizer with a shorter period of light shielding and lower incidence of skin toxicity compared with porfimer sodium, a first-generation photosensitizer (Nelson et al., 1987; Horimatsu et al., 2012). We have previously reported that the efficacy of PDT using the photosensitizer TS (TS-PDT) to glioma patients in a clinical study (Akimoto et al., 2012). In vivo and in vitro studies have shown that the treatment of TS-PDT induced apoptotic and necrotic cell death in transplanted glioma (Matsumura et al., 2008; Namatame et al., 2008) and cultured glioma cells (Miki et al., 2013, 2014). In addition, we have also demonstrated that the treatment of TS-PDT has anti-tumor activity against not only glioblastoma cells but also malignant meningioma cells (Shinoda et al., 2017).
The anti-tumor effect of PDT is a consequence of production of cytotoxic ROS. ROS, including singlet oxygen, superoxide, hydroxyl radicals and hydrogen peroxide, are responsible for cell death via oxidation of DNA, proteins, lipids, and almost any other biomolecules and are implicated in various disease states such as atherosclerosis, diabetes, cancer, neurodegeneration, and aging (Wu et al., 2013; Patlevič et al., 2016). In contrast, anti-cancer chemotherapy and radiotherapy have been shown to induce several oxidative stress responsive proteins (antioxidant enzymes) and resulted in suppression of the therapeutic effect (Duru et al., 2015; Kang and Hyun, 2017; Taguchi and Yamamoto, 2017). For instance, under oxidative stress conditions, a group of antioxidant detoxification genes such as glutathione S-transferase (GST), NADPH quinone oxidoreductase-1 (NQO-1) and heme oxygenase-1 (HO-1) are transcriptionally activated in an NF-E2 related factor 2 (Nrf2)-antioxidant response element (ARE)-dependent manner (Zhang, 2006; Kensler et al., 2007; Ray et al., 2012). However, the relationship between the therapeutic effect of TS-PDT treatment and stress response mechanism in tumor cells has not been adequately investigated. In the present study, we examined the intracellular stress response, including the expression of antioxidant detoxification genes, and its defensive effect in rat malignant meningioma KMY-J cells after treatment of TS-PDT.
Rat malignant meningioma KMY-J cells (Riken BRC Cell Bank, Ibaraki, Japan) were cultured as described previously (Shinoda et al., 2017). Briefly, cells were seeded at 1 × 104 cells/well in non-coated cell culture plastic 96-well plates (Thermo Scientific, Waltham, MA, USA) in minimal essential medium (MEM: Nissui, Tokyo, Japan) supplemented with 10% fetal bovine serum (FBS: Nichirei bioscience, Tokyo, Japan) and incubated at 37°C in a 5% CO2 atmosphere for 24 hr. After incubation, cultured cells were treated with appropriate concentrations (0, 6.4, 12.8, 25.6, 32 and 40 µM) of TS (Meiji Seika Pharma, Tokyo, Japan) in fresh 10% FBS-MEM for 4 hr. Then cells were washed with PBS and incubated for 1 hr in fresh 10% FBS-MEM. The cells were subjected to laser irradiation (wave length: 664 nm, laser irradiance: 3.4 mW/cm2, irradiation time: 300 sec, laser fluence: 1 J/cm2) using a semi-conductor laser irradiator ZH-L5011HJP (Meiji Seika Pharma).
Cell viability assayTwenty-four hours after TS-PDT treatment, cell viability was measured using Cell Counting Kit-8 (CCK-8: Dojindo, Kumamoto, Japan) as described previously (Shinoda et al., 2017). Briefly, CCK-8 solution was mixed with culture medium to a final concentration of 10% and the mixture was applied to TS-PDT treated cells for 1 hr at 37°C. After incubation, absorbance was measured at 450 nm (reference wavelength: 600 nm) using a Multiskan FC microplate reader (Thermo Scientific). Phase contrast imaging was performed using a DMi1 inverted microscopy (Leica Microsystems, Wetzlar, Germany). Separately, KMY-J cells were treated with TS in the presence of zinc protoporphyrin IX (ZnPPIX: Cayman Chemical, Ann Arbor, MI, USA), a HO-1 enzyme inhibitor, at 1 µM for 4 hr and then the cells were subjected to laser irradiation. After 24-hr incubation, cell viability was assayed by CCK-8 and evaluated the effect of ZnPPIX against the efficiency of TS-PDT treatment.
Real time RT-PCR analysisTotal RNA extraction from cultured cells and subsequent real-time PCR analysis were performed as described previously (Shinoda et al., 2016). Briefly, 25.6 µM TS-PDT-treated KMY-J cells prepared as described avobe were incubated for 3, 6, 12 or 24 hr after laser irradiation. Then culture medium was removed, washed by cold PBS and added 300 µL cold Isogen II reagent (Nippon Gene, Tokyo, Japan) to each culture well. Cells were collected by scraping and homogenized by pipetting. RNA quality was ensured by spectrophotometric analysis (OD260/280) using NanoDrop Lite spectrophotometry (Thermo Fisher Scientific, Tokyo, Japan). Reverse transcriptome was performed by using qPCR RT Master Mix (Toyobo, Osaka, Japan) and GeneAmp PCR system 9700 (Thermo Fisher Scientific). Real-time PCR was performed by using THUNDERBIRD® SYBR qPCR Mix (Toyobo) using 0.5 µM primers (Table 1) and LightCycler 96 (Roche, Tokyo, Japan). The thermal treatment was 95°C for 10 min, and 45 cycles of 95°C for 10 sec and 60°C for 30 sec. The superoxide dismutase 1 (Sod1), catalase 1 (Cat1), glutathione S-transferase Pi 1 (Gstp1), glutathione peroxidase 1 (Gpx1), quinone oxidoreductase 1 (Nqo1), heme oxygenase 1 (Hmox1), vascular endothelial growth factor A (Vegfa), and beta2-microglobulin (B2m) mRNA levels in each RNA sample were determined using the relative standard curve method. Fold change for each gene was assessed after normalization of the intensity value to that of B2m.
| Gene | Forward Primer (5’-3’) | Reverse Primer (5’-3’) | Product Size (bp) |
|---|---|---|---|
| B2m | GTCGTGCTTGCCATTCAGAA | GGGTGGAACTGAGACACGTA | 107 |
| Cat1 | ACATGGTCTGGGACTTCTGG | CCATTCGCATTAACCAGCTT | 142 |
| Gpx1 | CGGTTTCCCGTGCAATCAGT | ACACCGGGGACCAAATGATG | 245 |
| Gstp1 | CTTTTGAGACCCTGCTGTCC | GAGCCACATAGGCAGAGAGC | 162 |
| Hmox1 | TCTATCGTGCTCGCATGAAC | GAAGGCGGTCTTAGCCTCTT | 80 |
| Nqo1 | TGCTTTCAGTTTTCGCCTTT | GAGGCCCCTAATCTGACCTC | 122 |
| Sod1 | GTCGTCTCCTTGCTTTTTGC | TCTGCTCGAAGTGAATGACG | 131 |
| Vegfa | AAAACACAGACTCGCGTTGC | TGGCTTGTCACATCTGCAAG | 71 |
The data are expressed as the mean ± standard deviation (S.D.) and analyzed for statistical significance by Student’s t-test using Statcel3 (OMS, Tokyo, Japan), when possible. P values less than 0.05 were considered statistically significant.
Figure 1 shows the cell viability of rat malignant meningioma KMY-J cells treated with TS-PDT. After 24 hr of laser irradiation, TS at 12.8 µM and more showed significant decrease of the cell viability when compared with the no laser irradiation group (Figs. 1A and 1B). In addition, time course studies revealed that morphological cell damage was observed after 12 hr of laser irradiation when the cells were treated with TS at 25.6 µM (Fig. 1C). This indicates that TS at 25.6 µM exhibits a significant cytotoxic effect after 12 hr of laser irradiation in KMY-J cells.
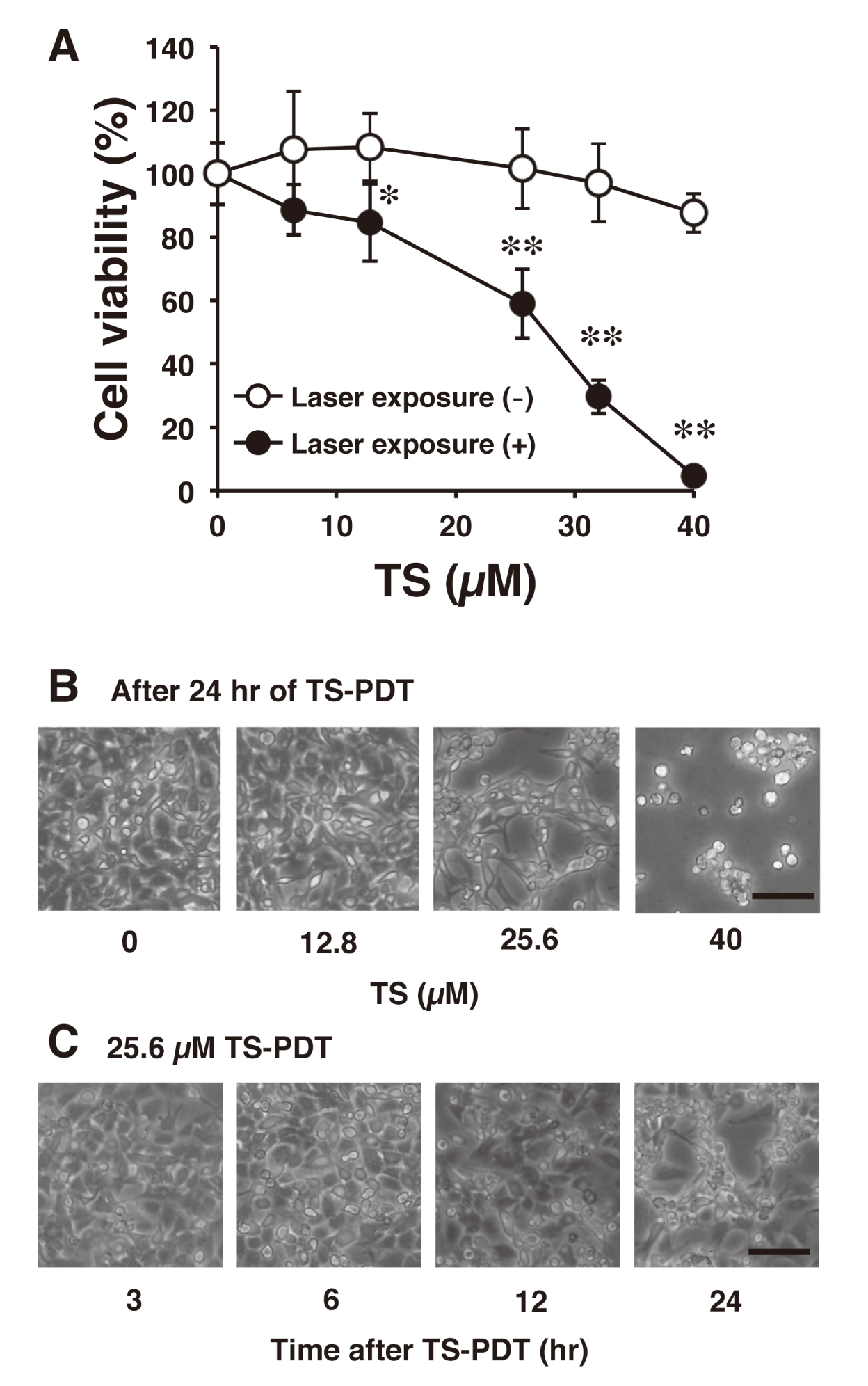
Cytotoxic effect of TS-PDT on rat malignant meningioma KMY-J cells. [A] Cell viability of KMY-J cells was confirmed by CCK-8 assay 24 hr after TS-PDT treatment. Rat malignant meningioma KMY-J cells were incubated at 37°C for 4 hr in the presence or absence of TS (6.4, 12.8, 25.6, 32 and 40 µM). After TS treatment, the cells were subjected to laser irradiation. Data are represented as mean ± S.D. of four samples. Statistical significance when compared to corresponding “laser exposure (-) group”: * P < 0.05, ** P < 0.01. [B] A representative image of KMY-J cells treated with TS (0, 12.8, 25.6 and 40 µM)-PDT at 24 hr after laser irradiation. Scale bar = 200 µm. [C] A representative image of KMY-J cells treated with TS (25.6 µM)-PDT at 3, 6, 12 or 24 hr after laser irradiation. Scale bar = 100 µm.
We next examined the effects of TS-PDT treatment on the transcriptional induction of several antioxidant enzymes, including superoxide dismutase 1, catalase 1, NQO-1, glutathione peroxidase 1, GST, and HO-1 in KMY-J cells. KMY-J cells were incubated with or without TS (25.6 µM) for 4 hr and then the cells were subjected to laser irradiation. After 3, 6, 12, or 24 hr of TS-PDT treatment, total RNA was extracted from the cells and the mRNA levels of Sod1, Cat1, Nqo1, Gpx1, Gstp1, and Hmox1 were determined by real-time RT-PCR. As shown in Fig. 2, TS-PDT was found to have slightly increased the expression of Cat1 and Gstp1 mRNAs after 12 and 24 hr of laser irradiation, respectively. In addition, the expression of Hmox1 mRNA was significantly increased by TS-PDT after 3, 6, and 12 hr, indicating that TS-PDT induces the expression of Hmox1 at an earlier time point than that of Cat1 and Gstp1. Since non-specific cell damage was not observed after 6 and 12 hr of TS-PDT treatment (Fig. 1C), it indicated that the expression of HO-1 (encoded by Hmox1) is induced by TS-PDT before the appearance of its cytotoxic effect.

Effect of TS-PDT on mRNA levels of antioxidant enzymes and Vegfa in rat malignant meningioma KMY-J cells. KMY-J cells were incubated at 37°C for 4 hr in the presence or absence of TS (25.6 µM). Then, the cells were subjected to laser irradiation and incubated for 3, 6, 12 or 24 hr. mRNA levels of antioxidant enzymes (Sod1, Cat1, Nqo1, Gpx1, Gstp1, Hmox1) and Vegfa were measured by real-time RT-PCR in TS-PDT-treated KMY-J cells. mRNA levels were normalized to B2m levels. Data are represented as mean ± S.D. of three samples. Statistical significance when compared to corresponding “control group” at each time point: ** P < 0.01.
It is known that the expression of HO-1 is regulated by not only Nrf2-ARE pathway but also hypoxia-inducible factor 1 (HIF-1)-Hypoxia Response Element (HRE) pathway (Handy and Loscalzo, 2017). Because the increased expression of Nqo1, Gpx1, and Gstp1, which are downstream target genes of Nrf2-ARE pathway (Taguchi and Yamamoto, 2017), was not observed by TS-PDT treatment at an early time point, it is possible that the expression of HO-1 may be regulated by HIF-1-HRE pathway. To examine its possibility, the expression of Vegfa, which is a downstream target gene of HIF-1-HRE pathway (Courtnay et al., 2015), was also determined. After 6 and 12 hr of TS-PDT treatment, the expression of Vegfa mRNA was significantly increased (Fig. 2). Thus, it is possible that the increased expression of HO-1 was mediated by HIF-1-HRE pathway rather than Nrf2-ARE pathway. However, because it is unclear whether TS-PDT activates HIF-1-HRE pathway, further studies should be performed to clarify the mechanisms by which TS-PDT induces HO-1 expression.
Previous studies have shown that HIF-1 is associated with tumor metastasis, angiogenesis, and chemotherapy resistance (Masoud and Li, 2015; Schito and Semenza, 2016). It has been reported that HO-1 is also involved in the resistance to chemotherapy agents (Liu et al., 2017). Therefore, to examine the relationship between HO-1 induction and susceptibility of TS-PDT, we next examined the effect of ZnPPIX, one of HO-1 inhibitor (Hirai et al., 2007), on the TS-PDT-induced cytotoxicity in rat malignant meningioma KMY-J cells. KMY-J cells were incubated at 37°C for 4 hr in the presence or absence of TS (6.4, 12.8, 19.2, 25.6 and 32 µM) with or without ZnPPIX (1 µM) and then the cells were subjected to laser irradiation. After 24-hr incubation, cell viability was decreased and morphological cell damage was increased by ZnPPIX treatment when compared with 19.2 and 25.6 µM TS-PDT treated groups without ZnPPIX (Figs. 3A and 3B). It is suggested that HO-1 induced by TS-PDT also may be involved in the resistance to treatment of TS-PDT in the malignant meningioma cells.
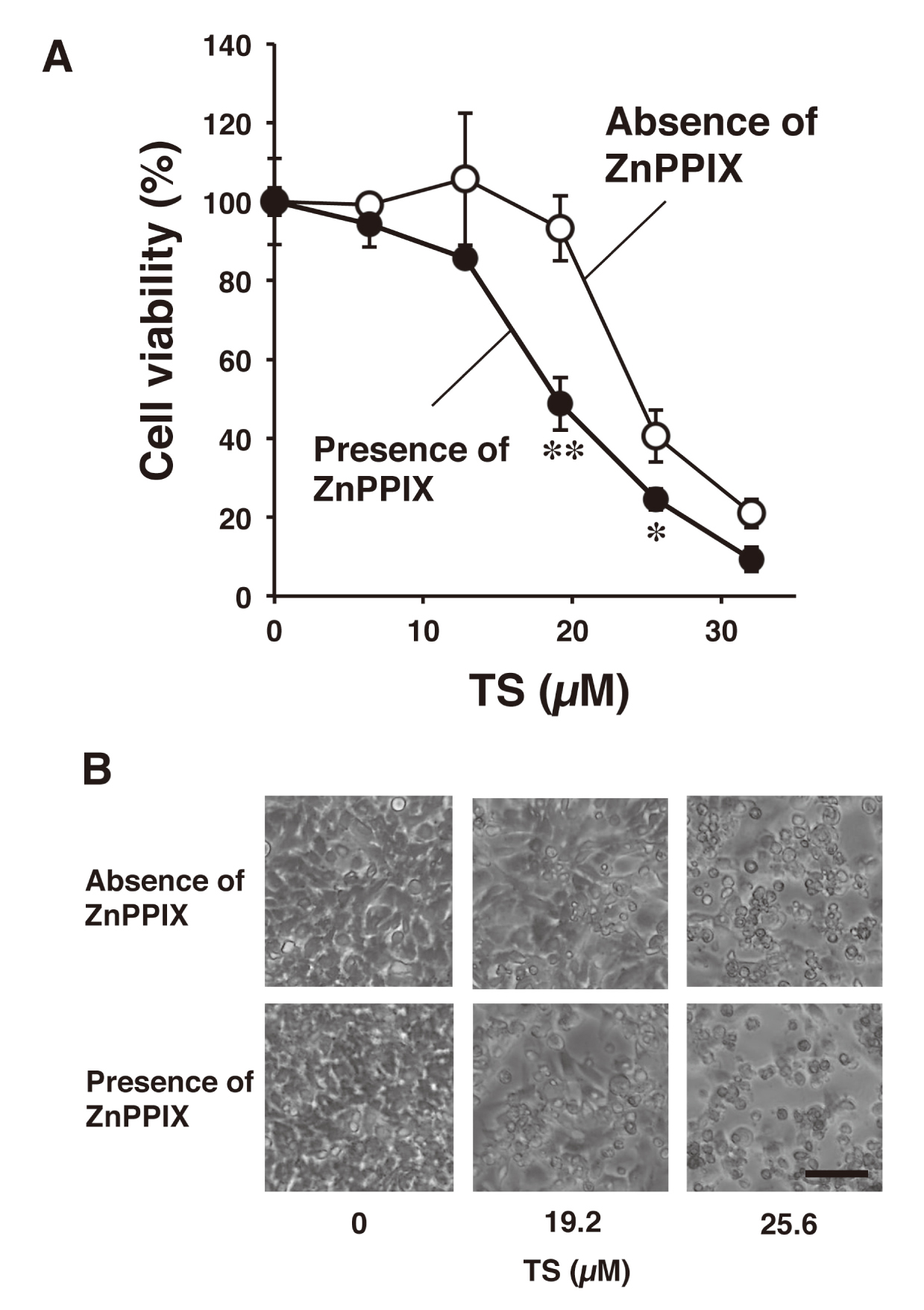
Effect of ZnPPIX, a HO-1 inhibitor, on the TS-PDT-induced cytotoxicity in rat malignant meningioma KMY-J cells. [A] KMY-J cells were incubated at 37°C for 4 hr in the presence or absence of TS (6.4, 12.8, 19.2, 25.6 and 32 µM) with or without ZnPPIX (1 µM), a HO-1 enzyme inhibitor, and then the cells were subjected to laser irradiation. After 24-hr incubation, cell viability was assayed by using CCK-8. Data are represented as mean ± S.D. of four samples. Statistical significance when compared to corresponding “absence of ZnPPIX group”: * P < 0.05, ** P < 0.01. [B] A representative image of KMY-J cells treated with TS (0, 19.2 and 25.6 µM)-PDT and ZnPPIX (0 or 1 µM) at 24 hr after laser irradiation. Scale bar = 100 µm.
In this study, we examined the effect of TS-PDT treatment on the expression of antioxidant detoxification genes in rat malignant meningioma KMY-J cells and revealed that the mRNA expression level of Hmox1 was significantly increased before cytotoxicity was observed. In addition, it is considered that HO-1 induction by TS-PDT treatment may, at least in part, contribute to acquisition of resistance in cancer cells. Therefore, it is possible that effective inhibition of HO-1 expression may contribute to enhancement in the therapeutic effect of TS-PDT treatment against malignant meningioma.
Conflict of interestThe authors declare that there is no conflict of interest.