2018 Volume 43 Issue 7 Pages 407-422
2018 Volume 43 Issue 7 Pages 407-422
The Short Time Exposure (STE) test method is an in vitro method for assessing the eye irritation potential of chemicals and is used to classify the Globally Harmonized System of Classification and Labelling of Chemicals (GHS) Category 1 and No Category (NC). The method has been adopted by the Organisation for Economic Co-operation and Development (OECD) as test guideline (TG) 491 since 2015. While this method can be used to classify GHS NC, it is not suitable for testing highly volatile substances and solids other than surfactants. Here we evaluated highly volatile substances to expand the applicability domain. According to TG 491, acetone, ethanol, iso-propanol, and methyl acetate as highly volatile substances resulted in false negatives. Saline was selected as a solvent of these false negatives. In this study, mineral oil was used as the solvent, because these false negatives were amphiphilic. Based on this change, four highly volatile substances were correctly evaluated. The predictive performance for classifying GHS NC was then verified using a substance dataset constructed in reference to the Draize eye test Reference Database and STE Summary Review Document. The accuracy and false-negative rate were 86.6% (194/224) and 3.8% (3/80), respectively. Collectively, the applicability domain was expanded by changing the solvent to mineral oil for highly volatile substances, and the predictive performance for the new applicability domain including highly volatile substances was excellent. The STE test method is suitable to classify GHS NC, indicating its applicability as a test method in a bottom-up approach.
Alternatives to the in vivo Draize eye irritation test have been developed over the last 40 years (Eskes et al., 2005). Meanwhile, some alternative methods have been adopted as test guideline (TG) by the Organisation for Economic Co-operation and Development (OECD), namely the Bovine Corneal Opacity and Permeability (BCOP) test method as TG 437, the Isolated Chicken Eye (ICE) test method as TG 438, the Fluorescein Leakage (FL) test method utilizing Madin-Darby canine kidney cells as TG 460, the Short Time Exposure (STE) test method utilizing Statens Seruminstitut rabbit cornea (SIRC) cells as TG 491, and the Reconstructed human Cornea-like Epithelium (RhCE) test method utilizing three-dimensional cultures of corneal epithelial or epidermal keratinocyte cells as TG 492. The BCOP, ICE, and STE test methods can identify chemicals that induce serious eye damage, defined as United Nations (UN) Globally Harmonized System of Classification and Labelling of Chemicals (GHS) Category 1 (Cat. 1), as well as chemicals that do not cause eye irritation or serious eye damage, defined as GHS No Category (NC) (United Nations, 2015; OECD, 2013a, 2013b, 2015a). The FL test method can identify GHS Cat. 1 chemicals (OECD, 2012), and the RhCE test method can identify GHS NC chemicals (OECD, 2015b).
Due to its short exposure time compared to other tests using monolayer cells (Eskes et al., 2005; Takahashi et al., 2008), the STE test method can use mineral oil as an oil-soluble solvent. Therefore, this method can be applied for oil-soluble chemicals. The STE test method has been validated by the Japanese Society for Alternatives to Animal Experiments and the Japanese Center for the Validation of Alternative Methods (Sakaguchi et al., 2011; Kojima et al., 2013). Furthermore, its applicability domain was determined via a peer review organized by the Interagency Coordinating Committee on the Validation of Alternative Methods (Hayashi et al., 2013; NICEATM, 2013). Although the STE test method is used to identify GHS NC for chemicals, the exceptions are as follows: i) highly volatile substances (vapor pressure > 6 kPa, 25°C) and ii) solids other than surfactants. This is the reason why i) highly volatile substances are expected to be at a low concentration due to volatilization during test system preparation or cell culture exposure, and ii) solids may have physical irritation properties using the Draize eye test, which cannot be predicted by in vitro assays. The STE test method is easy to implement and perform routinely using monolayer cells obtained from well-qualified cell banks, such as the American Type Culture Collection (ATCC, Manassas, VA, USA). On the other hand, Adriaens et al. (2018a) reported that the number of evaluated substances by the STE test method was not high because of its restricted applicability domain, although the other TGs, such as BCOP, ICE, and RhCE test methods, are not restricted particularly for the evaluation to classify GHS NC.
Although the above in vitro test methods have been adopted as TG by OECD, no currently available method can completely replace the Draize eye test. McNamee et al. (2009) reported that a tiered approach combining multiple test methods is useful for evaluating the eye irritation potential of chemicals. Such tiered approaches include the bottom-up approach, which classifies GHS NC chemicals as the first step, and top-down approach, which classifies GHS Cat. 1 chemicals as the first step. Effective approaches are built by combining some test methods with high predictive performance for classifying GHS NC and GHS Cat. 1 chemicals (McNamee et al., 2009; Hayashi et al., 2012; Adriaens et al., 2018b). Therefore, to build the effective tiered approach, an understanding of the features and predictive performances of each test method is required. The test methods adopted as OECD TGs should be helpful for building effective tiered approaches because the TGs were validated using chemicals possessing various organic functional groups and GHS classifications through validation studies and peer reviews. The predictive performance of OECD TGs is good, but the verified chemical dataset differs between test methods. It was difficult to directly compare the predictive performances obtained by each test method. Adriaens et al. (2014) and Barroso et al. (2017) reported that a dataset consisting of chemicals for which large inter-individual and inter-laboratory differences were not observed in the Draize test is needed to verify the predictive performance of in vitro tests. The predictive performance of the STE test method was previously verified in the STE Summary Review Document (SRD) using chemicals confirmed whether GHS classification was appropriate by NICEATM (2013). Recently, Barroso et al. (2017) described the recommended substances in the Draize eye test Reference Database (DRD) to verify the predictive performance of the developed test method. Adriaens et al. (2018b) reported a testing strategy constructed based on the predictive performance of each test method as confirmed using a same substance dataset selected in reference to DRD.
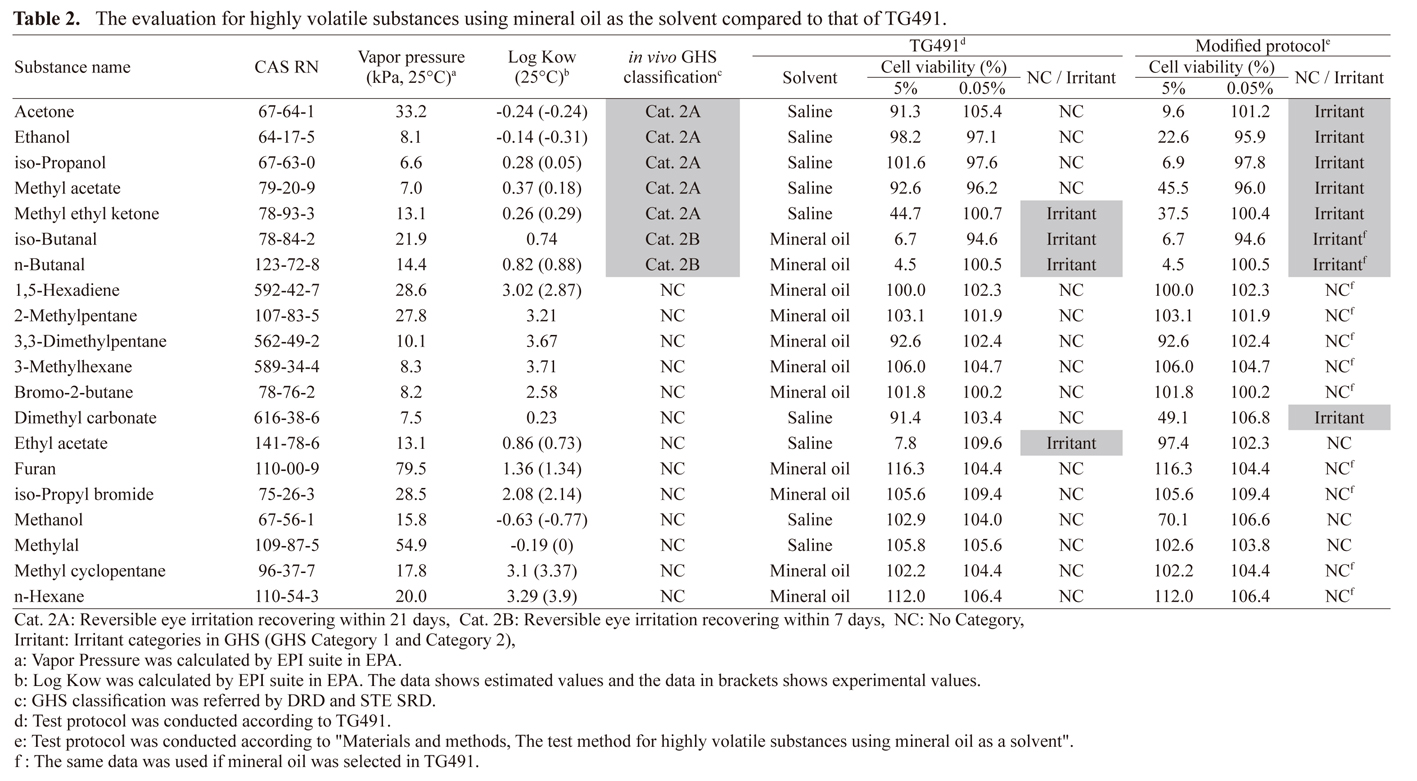
There are four false negatives listed in the STE SRD for highly volatile substances, namely acetone, ethanol, iso-propanol, and methyl acetate. These four substances are amphiphilic based on their chemical structures; thus, they dissolved not only in saline but also in mineral oil. When used as a solvent, mineral oil, which is less volatile than saline, might have the potential to suppress the volatilization of substances compared to saline. Therefore, highly volatile substances were evaluated to confirm whether mineral oil is a better solvent. Furthermore, the predictive performance of the STE test method was confirmed using the recommended substances listed in DRD and the substances listed in STE SRD to verify the applicability as a test method in a bottom-up approach for classifying GHS NC substances.
The test substances were selected from the recommended substances for verifying predictive performance in DRD and the substances listed in STE SRD. Furthermore, the substances were narrowed down by focusing on the applicability domain and the highly volatile substances (vapor pressure > 6 kPa, 25°C) defined in the STE test method. Finally, 239 commercially available substances were selected (204 substances in DRD, 104 substances in STE SRD, overlapped 69 substances). The suppliers were Alfa Aesar (Ward Hill, MA, USA), Sigma-Aldrich Co. LLC (St. Louis, MO, USA), Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan), and Wako Pure Chemical Industries, Ltd. (Tokyo, Japan). There were two physical states: one was 16 solids from surfactants, the other were liquids from various organic functional groups. The GHS classification of the selected substances was GHS Cat. 1 for 46 substances, GHS Cat. 2 for 39 substances, and GHS NC for 154 substances. In comparison, the recommended substances (i.e., a total of 375 substances) in DRD included 67 GHS Cat. 1 substances, 53 GHS Cat. 2 substances, and 255 GHS NC substances, indicating that the selected substances had a classification ratio similar to the recommended substances in DRD.
STE test method Cell cultureSIRC cells (CCL-60) were purchased from ATCC and cultured in Eagle’s minimum essential medium (Sigma-Aldrich Co. LLC) containing 10% (v/v) fetal bovine serum, 2 mM L-glutamine, 50 units/mL penicillin containing 50 μg/mL streptomycin (Thermo Fisher Scientific Inc., Waltham, MA, USA). After reaching confluence, the cells were dispersed using trypsin-ethylenediaminetetraacetic acid solution (Sigma-Aldrich Co. LLC) and spread onto 96-well flat-bottomed plates (Corning Costar Co., Cambridge, MA, USA) at 3.0 × 103 cells/well (or at 6.0 × 103 cells/well). After incubation (37°C, 5% CO2) for 5 days (or for 4 days at 6.0 × 103 cells/well), the cells reached confluence.
Test protocolThe STE test method was performed according to OECD TG 491. The test solvent was selected before the test. Saline was selected as the first solvent for each test substance. If the substance was insoluble in saline, then 5% (w/w) dimethyl sulfoxide (DMSO, Sigma-Aldrich Co. LLC) in saline was selected as the second solvent. For substances that were insoluble in both saline and 5% DMSO in saline, mineral oil (Sigma-Aldrich Co. LLC) was selected as the third solvent. In the STE test method, dissolution indicates that the substance is dissolved or becomes a uniform suspension within 5 min at 5% (w/w) and 0.05% (v/v) in the selected solvent. The cells cultured in 96-well plates were exposed to 200 μL of 5% and 0.05% test substance solutions for 5 min. After exposure, the cells were washed twice with Dulbecco’s phosphate buffered saline (−) (Sigma-Aldrich Co. LLC), and 200 μL of 0.5 mg/mL methylthiazolyldiphenyl-tetrazolium bromide (MTT, Sigma-Aldrich Co. LLC) were added. After a 2-hr reaction time, the MTT solution was discarded, MTT formazan was extracted with 0.04 N HCl iso-propanol (Sigma-Aldrich Co. LLC) for 1 hr, and the absorbance of the extract was measured at 570 nm using a plate reader.
The relative cell viability was expressed as the percentage ratio of the MTT formazan absorbance for each test substance to that for the test solvent control (triplicate determination). For each exposure condition for each test substance, the viability from mean absorbance of three wells was calculated, and the calculated result was treated as one independent run. In total, three independent runs were conducted for each exposure condition for each test substance, and the calculated overall mean viability in three independent runs was used to predict the eye irritation potential. If the standard deviation (SD) of cell viabilities was ≥ 15% in three independent runs, three more independent runs were performed.
Prediction modelTable 1 shows the prediction model for the STE test method. The prediction model was assigned to a test substance based on the relative viabilities obtained with 5 and 0.05% solutions of the test substance. On the basis of TG 491, if the relative viabilities exceed 70% under both the 5 and 0.05% exposure conditions, the GHS classification is “NC.” If the relative viability is 70% or less under the 5% exposure condition and greater than 70% under the 0.05% exposure condition, the GHS classification is “No prediction can be made.” If the relative viabilities are 70% or less under both exposure conditions, the GHS classification is “Cat. 1.” In this study, a test substance that had relative viabilities exceeding 70% at both doses were classified as NC, and those with a relative viability of 70% or less at any of the doses was classified as an irritant.
 The test method for highly volatile substances using mineral oil as a solvent
The test method for highly volatile substances using mineral oil as a solvent
Twenty highly volatile substances (vapor pressure > 6 kPa, 25°C) were selected in reference to the recommended substances in DRD and the substances listed in STE SRD. The substances were evaluated according to TG 491. However, the solvent differed. Mineral oil was selected as the solvent to evaluate the highly volatile substances.
Volatility of highly volatile substances in saline and mineral oil as solventThe weights of nine highly volatile substances, namely acetone, dimethyl carbonate, ethanol, ethyl acetate, iso-propanol, methyl acetate, methanol, methylal, and methyl ethyl ketone, dissolved in both saline and mineral oil were measured to confirm the volatile weights. A 5% concentration of each substance was prepared via dissolution in both saline and mineral oil. Then, the weights were measured using an electronic scale at 0, 5, 10, 15, 30, 60, and 120 min. The time point was determined based on the time duration from the preparation to exposure of the test substance solution. Triplicates were used for each measurement. The ratio of volatilization was calculated as the reduced weight of the test substances.
Estimation of the distribution of highly volatile substances in solvents using log KowLog Kow, a physicochemical property related to the affinity between water and oil, was calculated using EPI SuiteTM v4.11, developed by the United States Environmental Protection Agency (EPA, 2012), to estimate the distribution of highly volatile substances in the solvents. Table 2 includes log Kow values for the highly volatile substances. Both the estimated and experimental values are presented as available.
 Confirmation of the predictive performance of the STE test method using a substance dataset adding highly volatile substances constructed in reference to DRD and STE SRD
Confirmation of the predictive performance of the STE test method using a substance dataset adding highly volatile substances constructed in reference to DRD and STE SRD
The predictive performance of the STE test method was confirmed through the 2 × 2 contingency table. In this study, predictive performance shows the predictivity to classify GHS NC. The accuracy, false-positive rate, false-negative rate, sensitivity, and specificity were calculated and presented in Table 4, according to the results in Table 3. Here, the false-positive rate was represented by the rate identified as irritant incorrectly when classifying GHS NC. The false-negative rate was represented by the rate identified as GHS NC incorrectly when classifying irritant (GHS Cat. 1 and Cat. 2). Sensitivity represented the predictivity by which irritant was correctly evaluated as irritant. Specificity represented the predictivity by which GHS NC was correctly evaluated as GHS NC.


We examined whether the eye irritation potential of 20 highly volatile substances was correctly evaluated when the solvent was changed from saline to mineral oil. Table 2 shows the results using the test protocol in illustrated according to the TG 491 and the protocol in which the solvent was changed to mineral oil. Using TG 491, acetone, ethanol, iso-propanol, and methyl acetate were false negatives, and ethyl acetate was a false positive. Interestingly, by changing the solvent to mineral oil, the eye irritation potential of these five substances was correctly evaluated without any false negative or false positive. Meanwhile, dimethyl carbonate was identified as a false positive. In the TG491, the accuracy, false-positive rate, and false-negative rate for 20 highly volatile substances were 75.0% (15/20), 7.7% (1/13), and 57.1% (4/7), respectively; however, their values for changing the solvent to mineral oil were 95.0% (19/20), 7.7% (1/13), and 0% (0/7), respectively. These results indicate that highly volatile substances can be correctly evaluated without false negatives by changing the solvent to mineral oil.
Evaluation of the volatilization of highly volatile substances in solventsHighly volatile substances were evaluated without false negatives using mineral oil as a solvent (Table 2). The false negatives in highly volatile substances were expected to volatilize when using saline as solvents in the test condition of the STE test method. Therefore, the weights of nine substances, which were dissolved in both saline and mineral oil and were evaluated not only as false negative but also as correct results, were measured. Figure 1 shows the ratio of volatilization of highly volatile substances at 5% concentration in both saline and mineral oil at each time point. Overall, the results showed that when mineral oil was used as the solvent, the volatilization was not only suppressed but also promoted in some substances. Furthermore, each volatilization rate was less than 1% in 120 min. Specifically, for acetone, ethanol, iso-propanol, and methanol, the volatilization ratios were lower in saline than in mineral oil; however, their cell viabilities were higher in saline than in mineral oil (Table 2, Fig. 1). Any results of these substances were not related that volatilization led to a high cell viability when using saline as the solvent (i.e., volatilization did not lead to a low cytotoxicity). Conversely, for dimethyl carbonate, ethyl acetate, methyl acetate, and methylal, the volatilization ratios were higher in saline than in mineral oil (Fig. 1). The cell viability of dimethyl carbonate and methyl acetate were higher in saline, whereas that of ethyl acetate was lower (Table 2). Methylal exhibited similar cell viabilities in both saline and mineral oil (Table 2). High cell viability by volatilization was observed for dimethyl carbonate and methyl acetate in saline, but not ethyl acetate. The relationship could not be examined for methylal because of the absence of cytotoxicity in both test protocols. The volatilization and cytotoxicity of methyl ethyl ketone did not differ between saline and mineral oil (Table 2, Fig. 1).

The volatilization ratio which was calculated by reduced weight for highly volatile substances at 5% concentration in saline and mineral oil as solvent. Each ratio of volatilization was calculated by the reduced weight of the test substances. The weights were measured using an electronic scale at 0, 5, 10, 15, 30, 60, and 120 min in triplicate. The solid line represents the volatilization ratio by the solution in saline. The dotted line represents the volatilization ratio by the solution in mineral oil.
According to the results of the volatilization of highly volatile substances in solvents, it was suggested that the relationship between volatilization and cytotoxicity was not significant. High vapor pressure describes high volatility in constant temperature. In other words, it is estimated to be a high motion of molecules in constant temperature compared with the motion of other substances. High motion of molecules possibly strongly influences the accessibility to cells by distribution (i.e., the release of the substance from the solvent is difficult) in the test substance solution. Therefore, the distribution was estimated by log Kow. The values were accepted if those were listed in the results of the experiment performed using the EPI SuiteTM v4.11 (Table 2). Overall, most of highly volatile substances were identified to have high log Kow values compared with the false-negative values. The log Kow of the four substances identified as false negatives by TG 491 ranged from 0.18 to −0.31 as an experimental value. Therefore, these results suggest that the low value of log Kow (i.e. < 0.18 in Table 2) leads low accessibility to cells by high affinity to saline compared with mineral oil.
Predictive performance of the STE test method using a substance dataset adding highly volatile substances constructed in reference to the DRD and STE SRDIn total, 239 substances, which were within the applicability domain of the STE test method, and which were highly volatile substances, were selected in reference to the DRD and STE SRD (Table 3). Table 4 shows the predictive performance based on the results in Table 3. Fifteen of 239 substances were not applicable due to insolubility in any solvent. As a result, the accuracy, false-positive rate, and false-negative rate were 86.6% (194/224), 18.8% (27/144), and 3.8% (3/80), respectively, for classifying GHS NC substances. 2,6-dichlorobenzoyl chloride, ethyl trans-3-ethoxyacrylate, and toluene, whose GHS classification was Cat. 2 by the Draize test, were the three false negatives.
In this study, highly volatile substances were added in the substance dataset to expand the applicability domain using the test protocol in which the solvent was changed to mineral oil. Three false negatives and twenty-six false positives except for dimethyl carbonate were obtained using the test protocol in TG491; these were not applied for test protocol in which the solvent was changed to mineral oil because of them not being highly volatile substances.
When classifying GHS NC of chemicals using the STE test method, highly volatile substances are the exceptions of the applicability domain, because four highly volatile substances resulted in false negatives. Here we investigated the way to correctly evaluate highly volatile substances to expand the applicability domain of the STE test method. As a result, the change of solvent from saline to mineral oil resulted in highly volatile substances being correctly evaluated without false negatives. Furthermore, the predictive performance was confirmed using a substance dataset in the applicability domain and highly volatile substances constructed in reference to the DRD and STE SRD. As a result, accuracy and false-negative rate were obtained to be 86.6% (194/224) and 3.8% (3/80), respectively, for classifying GHS NC substances.
For highly volatile substances, it was speculated that the use of mineral oil as the solvent could interfere the volatilization of highly volatile substances. To confirm the volatilization, the weights in the 5% concentration of the substances were measured (Fig. 1). It was found that the difference in volatilization between saline and mineral oil as the solvent did not affect the cytotoxicity in seven out of nine substances in the STE test method. For example, the volatilization of acetone, ethanol, and iso-propanol as false negatives was lower when saline rather than mineral oil was used as the solvent. Therefore, it was suggested that the volatile weight was not related with the cytotoxicity under the test condition for the false-negative substances.
Due to volatilization having no effects on cell viability, investigation regarding the reason why highly volatile substances were obtained as false negative by the STE test method was required. Highly volatile substances were expected to be high motion of molecules compared with the motion of other substances. High motion of molecules possibly strongly influences the accessibility to cells by distribution in the test substance solution. To estimate the distribution of highly volatile substances in the solvents, log Kow was employed. The small values for the four false-negative substances indicated that the affinity of the substances is higher for saline than mineral oil. Thus, when the log Kow is close to 0, it would be difficult for the substances to contact the lipid bilayer membrane on the cell surface due to their high affinity for saline. Conversely, when mineral oil was used as the solvent, the substances could be exposed to the cells by accessibility higher than that of saline. Two substances, namely, methanol and methylal, with log Kow values similar or less than the range of false negatives were correctly classified as GHS NC. However, it was considered that the results were attributable to the low accessibility of the substances to the cell surface by the affinity to saline similarly as the false negatives. Due to the explanation regarding the distribution, further studies are warranted to confirm the current speculation for highly volatile substances using in silico and chemical analysis studies for analyzing the distribution.
Ethyl acetate was identified as a false positive using TG 491, whereas it was correctly identified as GHS NC when the solvent was changed to mineral oil. Ethyl acetate exhibited a borderline result between GHS Cat. 2 and NC in the Draize eye test (ECETOC, 1998). Therefore, the classification may change by a slight difference such as changing a solvent. Meanwhile, dimethyl carbonate in saline was correctly evaluated as a true negative using TG 491, whereas it was identified as a false positive when mineral oil was used as the solvent. As discussed for the false negatives, the false-positive result may be attributable to easy access to the cell surface when mineral oil is used as the solvent.
The predictive performance of the STE test method was confirmed using a substance dataset constructed in reference to the DRD and STE SRD. The results illustrated that the predictive performance for classifying GHS NC substances using the 239 substances dataset in this study was similar to the accuracy 90.2% (92/102), false-positive rate 18.8% (9/48), and false-negative rate 1.9% (1/54) of TG 491. Of the 239 substances included in the dataset, 85 were categorized as irritants (GHS Cat. 1 or Cat. 2) by the Draize test. Five out of 85 substances were not applicable because of their insolubility in any solvent. Three out of 80 substances, namely 2,6-dichlorobenzoyl chloride, ethyl trans-3-ethoxyacrylate, and toluene which were GHS Cat. 2 substances, were identified as false negatives. For 2,6-dichlorobenzoyl chloride and ethyl trans-3-ethoxyacrylate, the cell viabilities of both substances were 71.0% and 76.3% at 5% exposure condition (Table 3). The viabilities were close to the threshold (i.e., 70%) of the classification for irritants. For toluene, there were two results obtained using the Draize test in different studies. NICEATM (2013) reported that one was obtained as GHS NC, the other was obtained as GHS Cat. 2B. In conclusion, NICEATM reported that toluene is a mild irritant and mitigates concern about the false-negative classification. Furthermore, Barroso et al. (2017) reported that chemicals for which large inter-individual and inter-laboratory differences were not observed in the Draize test are recommended for verifying the predictive performance of the developed test methods. In other words, toluene was not a recommended substance as reported by Barroso et al. (2017). Also, Adriaens et al. (2018b) reported that the false-negative rate of an in vitro test method should be 0% for GHS Cat. 1 substances, and less than 10% for GHS irritant categories (GHS Cat. 1 and Cat. 2). The false-negative rates for GHS Cat. 1 and GHS irritant categories in the test protocol according to TG 491 and test protocol in which the solvent was changed to mineral oil were 0 and 3.6%, respectively, indicating that the STE test method was a suitable in vitro test method for identifying GHS NC.
Hydroxyethyl acrylate is a GHS Cat. 1 substance. Although the substance was identified as an irritant in this study (Table 3), it was identified as a false negative by Adriaens et al. (2018a). The difference of the results may be related to hydrolysis. Hydroxyethyl acrylate possesses an ester bond in its chemical structure, and it was assumed that hydrolysis occurs at room temperature based on its recommended storage temperature of 2-8°C. To confirm predictively whether hydrolysis occurred, the solvent was changed to mineral oil, which does not contain water. The cell viability was 59.2% when saline was used as the solvent (Table 3), compared to 17.4% when mineral oil served as the solvent (data not shown). In accordance with these findings, Adriaens et al. (2018a) reported that the cell viability was 71.2% when saline was the solvent. The false-negative result by Adriaens et al. (2018a) may affect hydrolysis, owing to a particular reason such as the prolonged time between preparation and exposure, for example. It was suggested that hydrolysis was not promoted when mineral oil served as the solvent and that cytotoxicity was enhanced. Based on these findings, substances that are sensitive to hydrolysis should be evaluated on the condition not promoting hydrolysis such as reducing the time duration from the preparation to exposure.
Butyl dipropasol solvent, methyl cyanoacetate, and propasol solvent P are GHS Cat. 2 substances. These substances were identified as irritants in this study and previously by Kojima et al. (2013); however, these substances were reported as false negatives by Adriaens et al. (2018a). The cell viabilities were 0.5%, 39.2%, and 10.6% at 5% exposure conditions for butyl dipropasol solvent, methyl cyanoacetate, and propasol solvent P, respectively in this study (Table 3). Kojima et al. (2013) reported that the average cell viabilities for butyl dipropasol solvent, methyl cyanoacetate, and propasol solvent P were 3.6%, 69.3%, and 11.7%, respectively, at 5% exposure condition obtained by two laboratories in the validation studies. Adriaens et al. (2018a) reported that the cell viabilities were 76.2%, 102.3%, and 81.4% at 5% exposure condition for butyl dipropasol solvent, methyl cyanoacetate, and propasol solvent P, respectively. The cell viabilities for methyl cyanoacetate were substantially different between various studies, including this study (Adriaens et al., 2018, Kojima et al., 2013). However, it can be concluded that butyl dipropasol solvent, methyl cyanoacetate, and propasol solvent P were evaluated as irritants based on the results from the studies reported by Kojima et al. (2013) and this study.
Bis-(3-Aminopropyl)-tetramethyldisiloxane, domiphen bromide (10% in aqueous), and lauric acid were correctly evaluated in this study. However, the results of these substances reported by Adriaens et al. (2018a) did not meet the acceptance criteria, which stated that the SD of the average cell viability derived from three independent repetitions should be <15% for both 5% and 0.05% exposure conditions of the test chemical described in TG491. The three substances should have been assessed as reported in Adriaens et al. for three additional independent repetitions according to TG491. The acceptance criteria for the SD would be met by three additional independent repetitions because the results of those three substances met the acceptance criteria for the SD in this study.
Of the 239 substances in the dataset, 154 were classified as GHS NC substances by the Draize test. Ten out of 154 substances were not applicable because of their insolubility in any solvent. Twenty-seven out of 144 substances were false positives, producing a specificity of 81.3% (Table 4). It was difficult to determine whether particular organic functional groups were related to the likelihood of false positivity. However, as Adriaens et al. (2018b) reported that the specificity of any in vitro test method should exceed 60%, it appears that the STE test method is suitable for GHS NC assessments.
It is noted that 70% substances in this dataset are classified as GHS NC. Meanwhile, 70% substances registered in ECHA from 2008 to 2014 were classified as GHS NC (Luechtefeld et al., 2016). The ratio for GHS NC substances in this dataset would be close to that in the dataset registered by an authority, such as ECHA. In other words, it is important to confirm whether most GHS NC substances can be correctly evaluated. The study results illustrated that the STE test method using the substance dataset in reference to DRD and STE SRD has similarly low false-negative rates as other in vitro test methods and lower false-positive rates than other tests regarding the classification of GHS NC substances. Specifically, the previously reported false-positive rates were 68.5% (61/89) for the BCOP test method, 33.3% (26/78) for the ICE test method, 36.4% (20/55) for the RhCE test method, and 18.8% (9/48) for the STE test method using TG 491 (OECD 2013a, 2013b, 2015a, 2015b). Additionally, the false-positive rate was 18.8% (27/144) in this study. Thus, it was indicated that the STE test method can identify GHS NC substances with a higher performance in safety assessment. Based on this finding, the STE test method is useful for classifying GHS NC substances in the tiered approach. Meanwhile, other tests such as the ICE or RhCE test method are needed to examine substances outside the applicability domain of the STE test method. For example, Hayashi et al. (2012) reported a tiered approach constructed using the STE, RhCE, and BCOP test methods.
In conclusion, highly volatile substances were evaluated without false negatives by changing the solvent to mineral oil. This result can describe the expansion of the applicability domain of the STE test method. Furthermore, the predictive performance of the STE test method for classifying GHS NC was confirmed using a substance dataset constructed in reference to the DRD and STE SRD, with the results illustrating that the good performance was similar to that described for TG 491. Overall, the STE test method is also suitable to classify GHS NC in an expanded applicability domain, indicating the applicability of this test method in a bottom-up approach.
The authors are also grateful to Ms. Kanako Sekine for providing technical assistance at Kao Corporation.
Conflict of interestThe authors declare that there is no conflict of interest.