2018 Volume 43 Issue 9 Pages 531-536
2018 Volume 43 Issue 9 Pages 531-536
Perinatal exposure to bisphenol A (BPA) causes several alterations in brain function and behavior. In previous studies, we showed that prenatal treatment with low-level BPA impaired gender-specific behavior, enhanced depression-like behavior, and augmented behavioral responses to predator odor in rats. On this premise, we hypothesized that BPA-treated rats were more susceptible to predator odor stress. To test the potential neural mechanism underlying this effect, we conducted an electrophysiological study of neurons in the medial amygdala—a regional component of the olfactory pathway with high estrogen and androgen receptor expression, and thus a potential target of BPA—in rats exposed to BPA. Extracellular recordings were obtained during the presentation of 3 plant odors and 3 predator odorants. Odor-responsive neurons in BPA-exposed rats showed greater activity in response to fox odor than did those in control rats. This finding complements the results of our previous behavioral study in which BPA-exposed rats exhibited enhanced avoidance behavior in response to fox odor. Given the close relationship between olfactory signaling and the stress response system, we suspect that BPA modifies the olfactory pathway at the level of the medial amygdala and thus modulates the corresponding stress response.
Bisphenol A (BPA) is an environmental endocrine disrupter that is released from widely used polycarbonate plastics and some dental sealants (Geens et al., 2012; Krishnan et al., 1993; Valentino et al., 2016). Various recent studies have examined the effects of BPA on the central nervous system. For example, our group reported that perinatal BPA exposure impaired gender differences in locomotion, exploratory behavior, and the size of the locus coeruleus in rats (Kubo et al., 2001, 2003). We also studied depression-like behavior using the forced swim test and found that BPA-exposed rats exhibited longer average immobility times than control rats (Fujimoto et al., 2006, 2013). Additionally, we evaluated the effects of BPA on behavioral responses to fox odor (2,4,5-trimethylthiazoline, TMT) using a novel apparatus (Fujimoto et al., 2015) and observed elevated odor avoidance behavior in BPA-exposed rats relative to control rats, indicating increased susceptibility to predator odor. We hypothesized that the prolonged immobility in the forced swim test (Fujimoto et al., 2006) and enhanced susceptibility to TMT (Fujimoto et al., 2015) were the result of the neurological effects of prenatal BPA exposure.
Estrogen and androgen receptors are expressed in several regions of the olfactory pathway in the brain, including the main olfactory bulb (MOB), accessory olfactory bulb (AOB), medial amygdala (MeA), and bed nucleus of the stria terminalis (BNST) (Simerly et al., 1990; Shughrue et al., 1997). Sex steroid hormones induce neurotransmitter synthesis and associated receptor expression (Biegon and McEwen, 1982; Donner and Handa, 2009), and then facilitate neuronal branching and synapse formation (García-Segura et al., 1994; Wood, 1998). It is known that BPA exerts estrogenic and anti-androgenic effects by competing with endogenous hormones for cognate receptor binding (Krishnan et al., 1993). Yet, it is unclear whether prenatal BPA exposure perturbs the normal actions of sex hormones to alter associated neural networks. In the present study, we performed an electrophysiological study of olfactory neurons in and around the MeA, an area of high sex hormone receptor expression, using several odors including TMT, in rats with and without prenatal BPA exposure. We hypothesized that BPA exposure would be associated with neuron hyperresponsiveness to predator odor as a mechanism underlying the influence of BPA on olfactory information processing and the stress response.
Male and female adult rats were purchased from Kyudo Corp. (Kud:Wistar, Saga, Japan). After copulation, pregnant females were randomly divided into control and BPA exposure groups (n = 5 per group). Animals were kept in a closed colony room with controlled temperature and humidity (23 ± 1°C, 60 ± 10% humidity) on a 12-hr light-dark cycle with free access to laboratory chow (CE-2; CLEA Japan, Inc., Tokyo, Japan) and tap water. All experiments were performed in accordance with the Guidelines for Animal Experiments of the Graduate School of Life Science and Systems Engineering at the Kyushu Institute of Technology and in compliance with pertinent governmental regulations (Law No. 105 and Notification No. 6).
BPA treatmentThe U.S. Environmental Protection Agency (EPA) established a reference dose (RfD) of 50 µg/(kg·day) as the safety standard for BPA (http://www.bisphenol-a.org/). As in previous studies (Fujimoto et al., 2006, 2013, 2015), the dose level was set below the RfD in this study. BPA was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Starting on gestational day 14, dams in the BPA group received BPA solution (0.1 ppm in distilled water) and the control group received distilled water in place of normal tap water. Exposure was continued until the day of birth (postnatal day 0, PND 0). The average BPA intake (calculated from body weight and water intake) was 15 µg/(kg·day). On PND 1, litters were balanced so that each litter contained 4 males and 4 females. On PND 21, all offspring were weaned, classified, and housed in groups consisting of the same sex and litter. Rats were allowed free access to food and tap water until the end of the experiment. Adult male offspring (19 control rats and 17 BPA-treated rats) were used for this study.
Olfactory stimulationPlant odorants included whiskey lactone (WHI) and jasmine lactone (JAS) supplied by Tatasago Koryo Co. (Tokyo, Japan) and trans-2-hexenal (T2H, green odor) supplied by Soda Koryo Co. (Tokyo, Japan). Predator odorants included TMT (fox odor) and isopentenyl methyl sulfide (IPMS, fox odor) purchased from Contech Inc. (Victoria, BC, Canada) and cat odor prepared with an acrylic collar in accordance with Dielenberg’s method (Dielenberg et al., 2001). Triethyl citrate, an odor-free oil, was purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan) and used to dilute the test odorants. With the exception of cat odor, odorants were diluted to 3% solutions with triethyl citrate and 0.05 mL of each solution was dripped onto a piece of cotton using a 5-mL syringe. For the cat odor, pieces of the acrylic collar were placed into a syringe. Each odorant was administered through a separate polyethylene tube (3 mm in diameter) with one end fixed in front of the animal’s nose. Each odor was applied for 3-6 sec and followed by 2 injections of clean air from a separately prepared syringe. The interval between each odor stimulus test was at least 2 min. Air in the experimental room was continuously ventilated.
Electrophysiological recordingAnimals were anesthetized with urethane (1.0 g/kg) and fixed into a stereotaxic instrument (SR-6; Narishige, Tokyo, Japan). A small hole was drilled into the right side of the skull to allow for the insertion of glass microelectrodes filled with 0.5 M sodium acetate and pontamine sky blue. Microelectrodes were created from a glass tube 1.2 mm in diameter using a microelectrode puller (PE-2; Narishige). Recording sites were established to roughly contain the posterodorsal (MePD) and posteroventral (MePV) parts of the MeA. Stereotaxic coordinates for electrode placement were selected using the rat brain atlas (Paxinos and Watson, 1998) as follows: bregma (−2.8 to −3.6 mm) and lateral (2.6 to 4.0 mm) at a depth of 7.8 to 9.2 mm from the brain surface. Extracellular single neuron action potentials were sent to a preamplifier, a high gain amplifier with low- and high-cut filters, and a spike counter (DSE-325P; Dia Medical System, Tokyo, Japan). Neuronal activity was monitored on an oscilloscope and the firing rate (spikes/s) and various marker signals were recorded using a computer-based data acquisition system (UAS1; Unique Medical, Tokyo, Japan).
Data analysisOnly well-isolated and spontaneously active neurons were recorded. The responsiveness of recorded neurons to predator and plant odorants was evaluated; increases and decreases in the firing rate by 20% or more during odor stimulation were defined as excitatory and inhibitory responses, respectively. A typical excitatory response pattern is shown in Fig. 1a. All data were analyzed using Statview 5.0 software for Macintosh (SAS, Inc., Cary, NC, USA). Fisher’s exact tests were used for between-group comparison. Differences were considered to be statistically significant when the P-value was < 0.05.
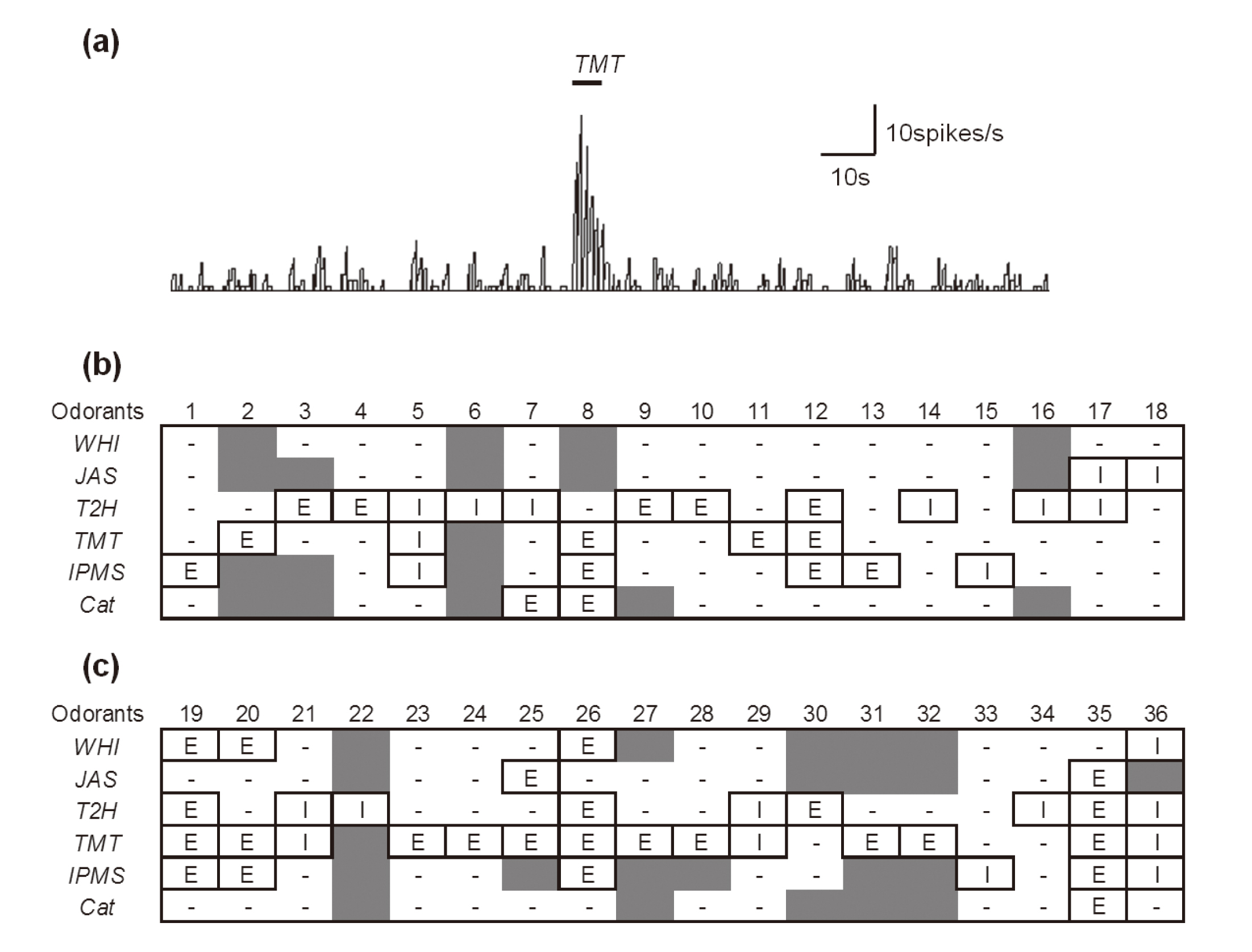
Odor-responsive neurons in the medial amygdala. (a) A typical excitatory response pattern. The thick bar show the duration of odor stimulation by TMT. (b), (c) The numbers across the top indicate the neuron number. (b) Control rats (no. 1-18). (c) BPA-exposed rats (no. 19-36). E: excitatory response; I: inhibitory response; -: no response; gray shading: not tested. WHI, whiskey lactone; JAS, jasmine lactone; T2H, trans-2-hexenal (green odor); TMT, 2,4,5-trimethylthiazoline (fox odor); IPMS, isopentenyl methyl sulfide (fox odor); Cat, cat odor.
We detected 44 and 42 spontaneously active neurons in offspring from the control (n = 19) and BPA-treated groups (n = 17), respectively. Of these, neurons responding to at least 1 of the 6 odorants were defined as “odor-responsive neurons.” In these odor-responsive neurons, neurons outside the MePV/PD area were excluded. The coordinates of the recorded data were compared to the rat brain atlas and only neurons with coordinates in the MePV/PD area were selected for analysis. Ultimately, we included data from 18 neurons in offspring from the control group and 18 neurons in offspring form the BPA-treated group. Neuron responses to various odorant stimuli are shown in Fig. 1. It should be noted that not all 36 neurons were exposed to all 6 odorants due to time constraints and other variables. Table 1 summarizes the odor responses of the tested neurons regardless of whether the response was excitatory or inhibitory. There were no significant between-group differences in the ratios of neurons responding to JAS, T2H, IPMS, and cat odor. However, significantly more neurons showed odor sensitivity to WHI and TMT in the BPA group than in the control group. Table 2 summarizes the numbers of neurons showing excitatory and others (inhibitory or no) responses in each group. The number of excitatory responses to TMT was significantly higher in the BPA group than in the control group. Overall, the number of neurons that showed inhibitory responses was small.
| Odorant | Control rats | BPA-exposed rats | Statistics | ||
|---|---|---|---|---|---|
| (+) | (-) | (+) | (-) | ||
| WHI | 0 | 14 | 4 | 9 | * |
| JAS | 2 | 11 | 2 | 11 | n.s. |
| T2H | 11 | 7 | 9 | 9 | n.s. |
| TMT | 5 | 12 | 14 | 3 | ** |
| IPMS | 6 | 9 | 6 | 6 | n.s. |
| Cat | 2 | 11 | 1 | 12 | n.s. |
*, **: Significantly different between control and BPA-exposed rats at P < 0.05, P < 0.01, respectively (Fisher’s exact test), n.s.: not significant. (+): number of neurons showing an odor response (excitatory + inhibitory), (-): number of neurons showing no response.
| Odorant | Control rats | BPA-exposed rats | Statistics | ||
|---|---|---|---|---|---|
| (E) | (- or I) | (E) | (- or I) | ||
| WHI | 0 | 14 | 3 | 10 | n.s. |
| JAS | 0 | 13 | 2 | 11 | n.s. |
| T2H | 5 | 13 | 4 | 14 | n.s. |
| TMT | 4 | 13 | 11 | 6 | * |
| IPMS | 4 | 11 | 4 | 8 | n.s. |
| Cat | 2 | 11 | 1 | 12 | n.s. |
*: Significantly different between control and BPA-exposed rats at P < 0.05 (Fisher’s exact test), n.s.: not significant. (E): number of neurons showing excitatory responses, (- or I): number of neurons showing no response or an inhibitory response.
In this study, we recorded olfactory responses from neurons in the MeA during the presentation of several odors including the predatory odor TMT in order to investigate the neurological effects of prenatal BPA exposure. To the best of our knowledge, this is the first study to investigate the effects of BPA on the central nervous system using extracellular recording methods. Previously, we reported that BPA-exposed rats displayed increased avoidance behavior in response to TMT compared with control rats (Fujimoto et al., 2015). In that study, data from male and female animals were combined and statistically evaluated. Additionally, we evaluated the data from the male animals only and confirmed that the results were similar (data not shown). The present results elucidate one possible mechanism underlying this behavior by demonstrating the increased responsiveness of MeA neurons to predatory odor in BPA-exposed rats compared with control rats.
We evaluated odor responsiveness to 6 odorants, 3 of which were plant odorants (WHI, JAS, and T2H). These odorants have been previously associated with anti-stress-like effects in rodents. WHI and JAS are thought to produce anxiolytic and analgesic effects through actions on the gamma-aminobutyric acid (GABA) system (Hossain et al., 2002, 2004). T2H attenuates stress induced by electrical foot shock, TMT odor (Nikaido et al., 2011), and the forced swim test (Kim et al., 2005; Watanabe et al., 2011). Although the current study did not examine the stress-alleviating properties of T2H, we believe that this topic warrants future investigation. In our study, we identified several T2H-responsive neurons in the MeA (11/18 neurons in the control group), this response frequency was relatively high among the plant type odors (Fig. 1 and Table 1). It is suggested that MeA neurons have some specificity for T2H. With regard to predator odors, 5 of 17 neurons responded to TMT while 6 of 15 neurons responded to IPMS in control rats (Fig. 1 and Table 1). Even fewer neurons (2 of 13) responded to cat odor. Cat odor was the only odorant used in this study that was not purely chemical in composition. Such odors (e.g., urine and feces) tend to activate neurons in higher olfactory areas such as the neocortex (Onoda et al., 1984).
In this study, we found that rats in the BPA group had more neurons that were responsive to WHI and TMT than the control group (Fig. 1 and Table 1), and there were more excitatory responses to TMT in the BPA group compared with the control group (Table 2). These findings suggest that BPA exposure induced hypersensitivity to these odors. Interestingly, there was no between-group difference in the responsiveness to IPMS, which is another fox-derived odor. It is unclear whether rats exposed to BPA were stressed by TMT but not by IPMS. It should be noted that in this study, due to constraints on recording time, only 12 neurons in the BPA group were tested with IPMS. Future studies that include behavioral testing and/or hormone level measurements are necessary to clarify our findings.
Our finding of increased odor-induced excitatory responses in the BPA group suggests a relationship with the behavioral data showing increased TMT avoidance in rats prenatally exposed to BPA (Fujimoto et al., 2015). We predict an association between the hypothalamic-pituitary-adrenal (HPA) system and odor-induced avoidance behavior. We did not measure stress hormone levels in the present study, but, it is known that exposure to predator odor leads to HPA axis activation in rats (Adamec et al., 1998; Morrow et al., 2000). Kobayakawa et al. (2007) showed that the “avoidance behavior against the TMT” and “the HPA system” were closely related, in that, when exposed to TMT odor, dorsal-zone-depleted mice lacked the avoidance response and showed no increase in stress hormone levels. The MeA is thought to play an important role in the predator odor-induced stress response. It has been reported that rats with an inactivated MeA showed no activation of the HPA system caused by ferret odor (Masini et al., 2009) and no fear-related behaviors in response to the TMT odor (Müller and Fendt, 2006). The results of this study suggest that BPA causes some modifications to the function of the MeA.
Estrogen and androgen receptors are found in several olfactory pathways and are highly expressed in the MePD (Simerly et al., 1990; Shughrue et al., 1997). The influence of low-dose BPA on the amygdala has been studied extensively in recent years. For example, Patisaul et al. (2012) reported that exposure to BPA decreased estrogen receptor beta expression and had an anxiogenic effect on juvenile rats. Cao et al. (2013) reported increased estrogen receptor alpha and beta expression in the MePD of BPA-exposed rats at PND 1. Yu et al. (2015) reported that PND 90 female mice treated with BPA during puberty showed high estrogen receptor alpha expression in the MeA. Additionally, Ogi et al. (2015) reported increased GABA and glutamatergic neurotransmission in the amygdala of female BPA-treated mice. The findings of our present study further expand the knowledge regarding the function of the amygdala and the stress response in BPA-exposed rats, in that BPA exposure was associated with increased excitatory responses to TMT in the MeA. BPA may exert effects on the stress response pathway at the level of the MeA by modifying olfactory response performance, likely via hormone receptors.
In conclusions, we investigated the MeA olfactory pathway as a potential target of prenatal BPA exposure by recording neuronal responses in the MeA during the presentation of various odors. Using this approach, we found that MeA neurons in BPA-exposed rats showed more excitatory responses to TMT than did control rats, a finding that complimented our behavioral results showing enhanced TMT avoidance in BPA-exposed rats. Taken together, these findings suggest that prenatal BPA exposure increases signaling from the MeA to the hypothalamus in response to predator odor signals, resulting in greater stress levels. These results improve our understanding of the effects of BPA on the function of the amygdala and the stress response.
The study was supported by Grants-in-Aid for Scientific Research (No. 16209006, S.A.) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Conflict of interestThe authors declare that there is no conflict of interest.