2019 Volume 44 Issue 10 Pages 643-655
2019 Volume 44 Issue 10 Pages 643-655
Rodent in vivo carcinogenicity bioassays are required for human risk assessment and have been utilized in this capacity for decades. Accordingly, there is an abundance of data that could be accessed and analyzed to better understand the translatability of xenobiotic-induced rodent tumors to human risk assessment. In the past decade, various groups have published assessments of the value garnered by these life-time rodent studies. Results and recommendations from the International Council for Harmonization Expert Working Group (ICH-S1 EWG) on the predictability of the current testing paradigm and proposal for an integrated approach to human carcinogenicity risk assessment are pending. Central nervous system (CNS) tumors in rats are rare and translatability to human remains unknown. This review focuses on microglial cell tumors (MCT) of the CNS in rats including its classification, nomenclature, incidence and translatability to human risk assessment. Based on emerging immunohistochemistry (IHC) characterization, glial tumors previously thought of astrocytic origin are more likely MCTs. These may be considered rodent specific and glucose dysregulation may be one component contributing to their formation. Based on review of the literature, MCTs are rarely diagnosed in humans, thus this tumor type may be rat-specific. We propose to include MCTs as a tumor type in revised International Harmonization of Nomenclature and Diagnostic Criteria (INHAND) classification and all glial tumors to be classified as MCTs unless proven otherwise by IHC.
Based on regulations formulated in the 1960s with modifications overtime, a two-year carcinogenicity study in rats with either a two-year carcinogenicity study in mice, or an alternative six-month transgenic study in mice are usually requested by health authorities across the globe as part of drug registration intended for chronic use. The purpose of these studies is to assess the oncogenic potential of novel pharmaceuticals (ICH guidelines S1A, S1B, S1C). Numerous groups have investigated the predictivity and translatability of xenobiotic-induced tumors in rodents for human risk assessment (Melnick et al., 1996; Allen et al., 2004; Jacobs, 2005) or provided a framework to assess human relevance of observed rodent tumors (Meek et al., 2003). Building on these findings, Sistare et al. published a seminal article summarizing the experience of various pharmaceutical companies with rodent carcinogenicity studies (Sistare et al., 2011). Whereas prior articles focused on how to interpret the results of carcinogenicity studies, Sistare et al. attempted to shift the focus to the actual value obtained from such studies. They concluded by proposing a new framework for carcinogenicity assessment, arguing for a prospective case by case approach guided by available toxicity study results, genotoxicity profile and known pharmacological activity of each drug to justify the need for conducting these studies (Sistare et al., 2011). Another retrospective study by van der Laan et al. further tested the weight of evidence approach in predicting carcinogenicity of 289 non-genotoxic pharmaceuticals and concluded that sub-chronic toxicity studies in conjunction with known pharmacological activities can help categorize pharmaceuticals as likely or not likely to be carcinogenic in rodents, however the full translational value to human risk assessment cannot be quantified (van der Laan et al., 2016). In 2012, an Expert Working Group (S1-EWG) was formed and initiated in 2013 a prospective evaluation of the predictability of two-year rat carcinogenicity study results, and to determine when a single rodent carcinogenicity study (such as a six-month study in TgRasH2 transgenic mice) would be sufficient. The EWG published a status update in (2016) and a final analysis of their work is expected by the end of 2019. Until a change to the guideline is published, non-genotoxic small molecule therapeutics will continue to be evaluated using existing two species carcinogenicity assessment paradigm.
Increased incidence of CNS tumors following treatment with biopharmaceuticals is infrequently reported and their relevance to humans remain unknown. Here, we summarize the available information on CNS tumors documented in carcinogenicity studies for marketed drugs and discuss the nomenclature, locations, and cellular type considering reported incidences in patients following up to decades of drug use.
Two-year rodent bioassays are long in duration (lifespan, usually 104 weeks) and are encumbered with the burden of assessing what generally is a very rare occurrence of test article-induced tumorigenicity. Many animals are needed to provide the statistical power to interpret the results. In many cases with events of rare occurrence, the concurrent control data are insufficient, and interpretation of the results must be complemented using historical incidence of tumors in the species/strain of interest to ascertain if they developed spontaneously or were induced by the test drug. Tumors are routinely categorized as common (incidence ≥ 1%) or rare (incidence < 1%) based on their spontaneous incidence to guide statistical evaluations (Statistics Guidance). Accordingly, pituitary, mammary, and lymphohematopoietic tumors are considered common in life-time rodent carcinogenicity studies while tumors of the CNS are rare. The occurrence of spontaneous CNS tumors also varies by species with higher incidences in rats followed by mice, dogs, non-human primates and humans (Weber et al., 2011). The United States National Toxicology Program (NTP) tested hundreds of chemicals for carcinogenicity potential in the rat. Only two chemicals produced clear evidence of increased incidence of brain tumors; whereas 9 showed equivocal increases (NTP). A review of the product inserts of pharmaceutical drugs also revealed very few instances of potentially drug-related CNS carcinogenicity. In this review, we focused on understanding the potential translatability of CNS tumors especially MCTs in 2-year rat carcinogenicity studies to human risk assessment in biopharmaceutical drug development.
Classification of CNS tumors across speciesTumors of the CNS are rare in species commonly used in non-clinical safety testing, including rodent species used for long term assessment of carcinogenicity (Fraser, 1971; Solleveld et al., 1990; Weber et al., 2011; Kaufmann et al., 2012; Bertrand et al., 2014). In these species, CNS tumors are relatively more common in rats than mice, with incidences that vary according to gender, species, breeding lineages and era (Ward and Rice, 1982; Maekawa and Mitsumori, 1990; Bertrand et al., 2014). Time period-associated changes refer primarily to genetic drifting of bred rodents as well as the impact of a Society of Toxicologic Pathology (STP) position paper published in 2013, which recommended expanding the sectioning of the rodent brain from three to seven levels (Bolon et al., 2013). The adoption of this more extensive sectioning of the brain by the industry is dependent on how rapidly it was implemented by each company and the status of ongoing studies at the time the new recommendations were brought forward. A more extensive sampling of the brain is likely to increase the detection of small size tumors. Bertrand et al. offer a relatively recent and robust break down of CNS tumor incidences by species, strain, gender and location (brain, spinal cord), with tumor types broken down into benign and malignant; and low or high grade (Bertrand et al., 2014).
Wide variations in incidence of brain tumors were identified early in the 1980s with the incidence of gliomas in the brain of control male Sprague-Dawley rats varying between 0% and 11% (Swenberg, 1986). Because of this variable incidence, same-sex concurrent (contemporary) controls constitutes the best comparator; while historical control or published data are the least relevant controls, mostly due to the variations in incidence associated with the specific era mentioned above. In the case of rare CNS tumors, it is possible that they may not be detected in control animals, which can complicate the interpretation particularly when the incidences of these tumors in treated animals reach statistical significance. In these instances, it is necessary to widen the pool of control data to provide additional perspective and avoid a false positive assessment of carcinogenicity. The most relevant source of non-contemporary control data is the most recent data available for the same species and strain used at the same facility where the study was conducted and where animals were procured from the same source. If such data are available but do not support the final interpretation of the study, the data cannot be ignored and should be presented as part of the scientific discussion. If, however these preferred sources of historical data are not available, then published literature for same species and strain are most relevant and can be found in relatively recent publications (Krinke et al., 2000; Weber et al., 2011; Bertrand et al., 2014).
The use of non-contemporary control data comes with its share of caveats, which are not limited to the variations in incidence mentioned above. Inconsistencies and changes in the classification and terminology of CNS tumors can cause confusion, and to this day, the exact histogenesis of certain tumors is being revisited (see section on astrocytomas below). For these reasons, it is important to understand the history of tumor classification and preferred terminology to make the most appropriate use of historical control data.
As of the writing of this review, the industry standard for the diagnosis of proliferative lesions in carcinogenicity studies is that published in 2012 by the INHAND project, a joint initiative of the societies of toxicologic pathology from Europe, Great Britain, Japan, and North America, to develop an internationally recognized nomenclature that includes proliferative lesions in rodents (Kaufmann et al., 2012). The recommended classification by INHAND follows various classification systems adapted over time, which parallel the more complex and evolving classification of CNS tumors in humans by the World Health Organization (WHO) (Ward and Rice, 1982; Koestner et al., 1999; Krinke et al., 2000; Louis et al., 2007; Weber et al., 2011; Kaufmann et al., 2012; Louis et al., 2016). Using the INHAND terminology, the histogenic classification of CNS tumors is presented in Table 1. A detailed description of morphologic and biological characteristics of each tumor type is beyond the scope of this review and can be found in various publications, albeit with some discrepancies (Krinke et al., 2000; Weber et al., 2011; Kaufmann et al., 2012). Most commonly, the classification of rodent tumors originates from morphologic similarities with the more robustly defined human counterparts; and the difference between malignant and benign, or high- and low-grade versions of a tumor type is mostly rooted in arbitrary morphologic criteria with variable biological relevance (i.e. size, margin, multicentricity). Conversely, the WHO in its latest classification of CNS tumors in humans now combines phenotypic and genotypic criteria resulting in the generation of “integrated diagnoses” (Louis et al., 2016). As a result, it lists twenty-eight different glial tumors (Louis et al., 2016).
| Cell Type | Tumor | |
|---|---|---|
| Neuronal | Meduloblastoma | |
| Neuromyoblastoma | ||
| Non neuronal | Glia (malignant) | Astrocytoma (low/high grade) * |
| Oligodendroglioma (low/high grade) | ||
| Mixed glioma (low/high grade) | ||
| Nerve sheath | Schwannoma (benign/malignant) | |
| Meninges | Granular cell tumor (benign/malignant) | |
| Meningioangiomatosis | ||
| Meningioma (benign/malignant) | ||
| Ependyma** | Ependymoma (benign/malignant) | |
| Choroid plexus | Papilloma | |
| Carcinoma | ||
| Other cell lineages | Lipomatous hamartoma; malignant reticulosis | |
* Astrocytoma high grade includes glioblastoma/glioblastoma multiforme
**Ependymal cells are considered a type of glial cells
The use of arbitrary criteria in rodent carcinogenicity studies is not without merit. It allows consistency in diagnosis across a global industry using simple standardized methods (standard sectioning of CNS tissues using H&E stain), and whose goal is to identify potential human hazard. However, once a potential hazard is identified, the translatability to a more precise human risk assessment can be difficult, especially if the histogenesis of certain tumor types is uncertain. Additional characterization of CNS tumors in rodents is possible using commercially available antibodies for IHC staining. The cellular targets of commonly used antibodies are listed in Table 2.
| Antibody/Antigen | Cell Type |
|---|---|
| Glial fibrillary acidic protein (GFAP) | Astrocyte, ependymal cell1,2 |
| S100 | Astrocyte, ependymal cell1 |
| Glutamine Synthetase | Astrocyte, ependymal cell1 |
| Vimentin | Astrocyte, ependymal cell2 |
| Oligodendrocyte transcription factor 2 (Olig2) | Oligodendrocyte 1 |
| Leu-7 (HNK-1, CD57) | Oligodendrocyte, choroid plexus2,3,4 |
| Ionized calcium-binding adapter molecule 1 (Iba-1) | Macrophage, microglia1 |
| ED1 (CD68) | Macrophage, microglia, dendritic cell1,5,6 |
| OX-6 (MHC class II) | Macrophage, microglia, dendritic cell1,5 |
| Ricinus communis agglutinin type 1 (RCA-1) | Macrophage, microglia, endothelial cell7 |
| Neurofilament (NF) | Neuron1 |
| Nestin | Neural stem/progenitor cell, endothelial cell8,9 |
1: (Kolenda-Roberts et al., 2013); 2: (Reifenberger et al., 1989); 3: (Motoi et al., 1985); 4: (Galloway et al., 1990); 5: (Sato et al., 1998); 6: (Nagatani et al., 2009); 7: (Krinke and Germer, 1993); 8: (Lendahl et al., 1990); 9: (Suzuki et al., 2010)
Glial tumors in rodent carcinogenicity studies have been diagnosed with inconsistent terminology. The glia (or neuroglia) in the CNS includes oligodendrocytes, astrocytes, microglial and ependymal cells. Glial tumors often include more than one type of cell, which can present with overlapping morphologic features. Consequently, some toxicologic pathologists hesitate to use terminology that refers to a single cell type and prefer the noncommitting term “glioma” or mixed glioma. A consequence of this inconsistent terminology is the distortion of historical incidence of tumors of specific histogenesis (oligodendroglioma, astrocytoma) vs “glioma or mixed glioma” and assessment of relevance to humans in determining potential risks. We experienced the effects of these distortions when reviewing the summary basis of approval (SBA) for various drugs discussed later in Summary of approved drugs associated with glial tumors in rat carcinogenicity studies. When considering study incidences of gliomas, one should consider the possibilities that these may also represent specific glial cell tumors, especially in studies with no incidence of astrocytoma or oligodendroglioma.
Distinguishing between astrocytomas and microglial cell tumors in ratsIn humans, astrocytomas (gliomas of astrocytic origin) are the most common CNS tumor in adults (Louis et al., 2016). By contrast, microglial cell tumors, occasionally referred to as microgliomas, are extremely rare in humans with only two known case reports (Hulette, 1996; Mathews et al., 2016), and they are not included in the current WHO classification of human CNS tumors (Louis et al., 2016); despite listing five different types of histiocytic tumors in the CNS.
Until recently, astrocytoma has been the most common CNS tumor diagnosed in Sprague Dawley rats, and by far the most common glial cell tumor in Sprague Dawley and Wistar Han rats (Bertrand et al., 2014). As previously mentioned, they are occasionally diagnosed as glioma, although recent literature clearly recommends differentiation based on cell type as defined by specific morphologic criteria (Weber et al., 2011; Kaufmann et al., 2012; Bertrand et al., 2014). Notably, spontaneous CNS tumors in rats classified as astrocytomas are invariably negative to glial fibrillary acidic protein (GFAP) staining, a well-established marker of astrocytes, although GFAP positive reactive (non-neoplastic) astrocytes are commonly observed in these tumors, particularly at the periphery and around blood vessels. Astrocytomas in rats have long been reported to consistently display positive staining for macrophage lineage markers, and these staining inconsistencies have been attributed to either an immature astrocytic phenotype of neoplastic astrocytes (Krinke et al., 2000; Bertrand et al., 2014) or were left unaddressed (Zook et al., 2000; Weber et al., 2011; Kaufmann et al., 2012). More recent investigation has robustly demonstrated that tumors morphologically diagnosed as astrocytomas in rats are positive for microglial markers and a corrected diagnosis of malignant microglial cell tumor (MCT) has been proposed (Nagatani et al., 2009; Kolenda-Roberts et al., 2013). In the first extensive prospective study of ethylnitrosurea (ENU)-induced brain tumors in rats, Zook et al. reported ENU induction of oligodendrogliomas and mixed gliomas and described the immunohistochemical (IHC) characteristics of astrocytomas, mixed gliomas and oligodendrogliomas (Zook et al., 2000). The IHC signature of neoplastic astrocytes present in these tumors was negative for GFAP (astrocytes, ependymal cells) and LEU-7 (oligodendrocytes) (Motoi et al., 1985; Reifenberger et al., 1989; Galloway et al., 1990). Although not used in that study, IHC staining for ricinus communis agglutinin-1 (RCA-1) was suggested for future use as a specific marker of neoplastic astrocytes, based on the work of Krinke et al. published seven years earlier (Krinke and Germer, 1993). In their work using rat brain tumors, Krinke et al. confirmed reactivity to RCA-1 for all gliomas evaluated and negative reactivity to S-100 and neuron-specific enolase (NSE) for all gliomas evaluated. At that time, RCA-1 was a known marker for human and rat microglial cells. The histogenic discrepancy identified by RCA-1 reactivity of neoplastic astrocytes was attributed to possible glycosylation mimicry, or to the “scavenger activity” of astrocytes and it was suggested that cells previously identified as microglial cells, may in fact be astrocytes (Krinke and Germer, 1993). The assignment of tumors with inconsistent staining properties to the astrocytic lineage may illustrate the depth of the dogma that these rat tumors were the histologic counterpart of human astrocytomas. The incoherent molecular signature of neoplastic astrocytes was also largely ignored or forgiven until the publication of the work by Nagatani et al. in 2009, who clearly questioned the histogenesis of rat astrocytomas, citing a correlation with malignant reticulosis (MR), a tumor of microglial origin; and a molecular signature consistent with microglial cells, suggesting that MR and astrocytomas in rats were derived from microglia (Nagatani et al., 2009). The work by Nagatani et al. originated from the observation of morphological similarities between astrocytomas and MR. They investigated the morphology and molecular signature of sixty-four brain tumors in Sprague-Dawley or F344 rats originally diagnosed as astrocytomas or MR. They observed that morphologic characteristics of astrocytomas and MR were similar; and that there were no differences in immunohistochemical staining as both types of tumors showed positive reactivity for ED-1, RM-4 and vimentin, but not for GFAP or S-100 protein. Subsequently, work by Kolenda-Roberts et al. validated the observations of Nagatani et al., and provided additional terminology recommendations (Kolenda-Roberts et al., 2013). In their work, Kolenda-Roberts et al. investigated the molecular signature of twenty-eight spontaneously occurring glial tumors (astrocytomas, oligodendrogliomas and mixed gliomas); eleven granular cell tumors; and nine tumors from rats treated with acrylonitrile for two years. Their results indicated that most astrocytomas were RCA-1, and Iba-1-positive, and GFAP, S-100 and glutamine synthase-negative, and suggested a diagnosis of malignant microglial tumors for these neoplasms. Three mixed tumors suggested histogenesis composed of oligodendrocytes (Olig-2+) and microglia (Iba-1+) and consequently, the authors suggested to avoid using the term mixed glioma since the astrocytic component appeared to be microglia and because mixed gliomas where often interpreted to be oligoastrocytomas. It is worth noting that although microglial cells do not share the neuroectodermal origin of astrocytes or oligodendrocytes, they are a component of the glial network. Finally, all granular cell tumors and acrylonitrile-induced tumors were RCA-1 and Iba-1 positive. They concluded that acrylonitrile-induced tumors are of microglial cell origin. Additionally, Kolenda-Robert et al. concluded that Iba-1 and RCA-1 are better markers of microglial cells than OX-6/MHC-II, which showed inconsistent staining. The nuclear marker Olig-2 was a reliable marker of neoplastic oligodentrocytes; whereas they did not consider the use of astrocyte markers (GFAP, glutamine synthase or S100) to be crucial in determining the cells of origin in rat brain tumors (Kolenda-Roberts et al., 2013). Subsequently, adding an element of confusion to this evolving tumor diagnosis landscape, Nagatani et al. further investigated the apparent absence of reported GFAP+ astrocytomas by applying a small panel of IHC markers to twenty-six oligodendrogliomas and five mixed gliomas collected from rat brains. The markers included Olig-2, GFAP, and nestin. Their interpretation of results from these stains support the identification of GFAP+ spindle cells that are morphologically different from reactive astrocytes and postulated that these are the first reported GFAP+ neoplastic astrocytes (Nagatani et al., 2013). Figure 1 depicts morphologic and immunohistochemical characteristics of microglial cell tumors.

Low power photomicrographs with high power insets of microglial cell tumor stained with hematoxylin and eosin (H&E) (1A); and immunohistochemistry stain for GFAP (1B), Olig-2 (1C) and Iba-1 (1D). Tumor area marked with asterisk (*) and adjacent brain tissue marked with hashtag (#). 1A shows densely packed spindle shaped neoplastic cells with indistinct cell borders. The nuclei are round to elongated with poorly defined nucleoli. Mitotic figures are rare. Neoplastic cells with a more rounded shape infiltrate the adjacent neuropil forming perivascular cuffs. 1B shows several irregularly distributed GFAP positive stellate cells (astrocytes) throughout the neoplasm. These cells have prominent cellular processes and are characteristic of reactive astrocytes (gemistocytes) responding to tissue injury. 1C shows small numbers of evenly distributed Olig-2 positive cells representing pre-existing oligodendrocytes. 1D shows the cytoplasm of neoplastic cells strongly and consistently staining for Iba-1. In the tissue adjacent to neoplasm, Iba-1 positive cells are sparsely and regularly distributed throughout the neuropil with prominent cellular processes typical of normal microglial cells.
Microglial cells are the resident mononuclear phagocytes of the CNS, belonging to the glial system of non-neuronal cells. They form a cell population distinct from other glial cells (astrocytes and oligodendrocytes) and there is broad consensus supporting their myeloid origin, as well as their functional and morphologic similarities to other cells of the monocyte/macrophage lineage (Saijo and Glass, 2011; Ginhoux et al., 2013). In early embryogenesis, primitive macrophages derived from the yolk sac migrate to various tissues, including the brain to generate the initial microglial population. Later during embryonic development, hematopoietic progenitors originating from the mesoderm colonize the fetal liver and serve to generate all hematopoietic lineages, including macrophages. Perinatal recruitment of microglial population occurs through circulating monocytes originating from hematopoietic cells in fetal liver (Ginhoux et al., 2013). During adult life and under physiological conditions, microglia in the CNS is renewed through local proliferation of resident cells, whereas recruitment of circulating precursors occurs in pathological circumstances (Stoll and Jander, 1999; Ginhoux et al., 2013). Microglial cells share markers with other monocyte/macrophage populations in multiple species such as CD11b/c (mice), CD45, complement type 3 receptor, Iba1, OX-42 (rat), CD68, ED-1 (rat), ED-2(rat), F4/80, Mac-1, and Fc receptor (Gehrmann et al., 1995; Fix et al., 1996; Ito et al., 2001; Nagatani et al., 2009).
The concept of MCT in rats has been gaining traction in the toxicologic pathology community. While microglial cell tumors were not a recognized entity until the work by Nagatani et al. published in 2009, the proceeding of the 2011 and 2015 NTP satellite symposium’s “Pathology Potpourri” sessions report that the percentage of participating pathologists shown mystery slides of rat brain tumors before having seen the results of IHC staining supporting a microglial origin were 22% (2011) and 33% (2015) (Boorman et al., 2012; Elmore et al., 2016). The percentages were up to 65% (2011) after revealing the IHC results. Not surprisingly, the diagnosis receiving the most initial votes in each case was astrocytoma.
The case for a microglial origin of conventionally diagnosed astrocytomas is strong and pending additional robust histogenic investigation of these tumors in rodents, it is advisable to complement basic histological assessment of CNS tumors that reach statistical significance in an in vivo carcinogenicity assay with a panel of IHC stains to obtain a more refined diagnosis. The selectivity and availability of Olig-2, Iba-1, and GFAP antibodies for the identification of rat oligodendrocytes, microglial cells and astrocytes, respectively should help pathologists to commit with confidence to a specific diagnosis. Additional characterization of tumors classified as MR and granular cell tumors and their potential histogenic similarity with MCT would also be valuable. Morphologic similarities between astrocytoma, MCT and MR have been repeatedly well described (Maekawa and Mitsumori, 1990; Nagatani et al., 2009; Weber et al., 2011; Boorman et al., 2012; Kaufmann et al., 2012; Elmore et al., 2016). It would be useful to determine if MR is a variant of MCT, which could be combined with MCT for statistical analysis purposes, or if they originate from a different subset of histiocytic cells. Granular cell tumors are also tumors of histiocytic origin associated with the leptomeninges that have distinct morphologic characteristics. It has been suggested that they are derived from a different subset of histiocytic cells, presumably associated with the meninges (Kolenda-Roberts et al., 2013).
Astrocytomas’ being the most common tumors in humans, combined with the extreme rarity of MCTs, highlights the importance of accurately identifying these tumors in rats for a better assessment of human risk. Moreover, misidentification of MCT as astrocytomas in rats may explain the apparent lack of translation of this specific type of tumor to humans.
A comprehensive search of the FDA approved labels (USPI) and the FDA Scientific Basis for Approval (SBA) of drugs identified only a few drugs associated with CNS tumors in chronic toxicity or carcinogenicity studies (2-year rat, 2-year mouse or 6-month transgenic mouse). A total of eight drugs were identified as associated with increased incidence of CNS tumors (astrocytoma, glioma or MCT), which occurred only in the rat.
Despite the rarity of CNS tumors and the fact that the rat is more susceptible to this type of tumor than other species (Weber et al., 2011), it is still reasonable to assume that the mechanisms by which these rare tumors occur are no different than other more common types of tumors. Several factors contribute individually or collectively to the formation of tumors. These include positive genotoxic activity of the molecule; genotoxic compounds interact directly with the DNA through the formation of covalent bonds leading to the formation of DNA adducts and causing DNA modification of the information stored in the DNA which eventually could lead to tumor formation. Hormonal perturbation is another mechanism by which compounds that act on hormonally sensitive tissues such as reproductive organs. Histologic findings in chronic studies can be predictive of tumor development. Such findings include tissue hyperplasia and regeneration associated with degenerative or inflammatory processes. These mechanisms can contribute singly or in combination to tumor formation and may be implicated in drug-induced tumorigenicity. Factors intrinsic to the animal can also increase the risk of tumor development, such as age, where prolonged survival increases the incidence of spontaneous and drug-induced tumors. The following sections attempt to use a weight of evidence approach to understand if one or a combination of factors stand out as the most plausible cause of CNS tumors in the list of drugs outlined in Table 3.

Potential mechanism of tumorigenicity was not reported for any of the 8 drugs listed in Table 3. Our in-depth review did not reveal a common pharmacological mechanism of action, site of action or extent of brain penetration of these drugs to explain CNS tumor observations. Major learnings from each drug are summarized in Tables 3 and 4. CNS tumors when observed were recorded in male rats and there were no concomitant CNS tumorigenicity in mice.
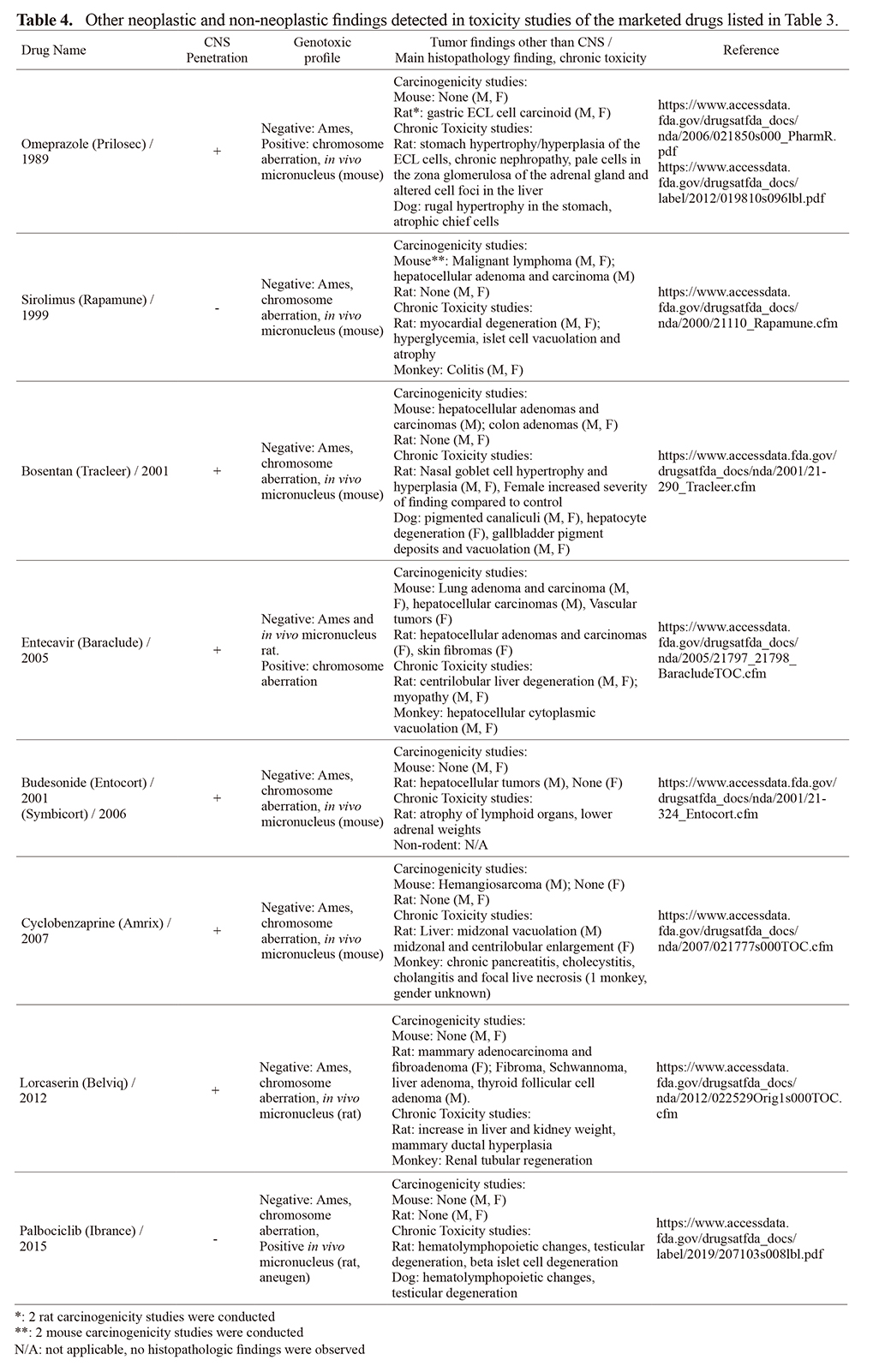
Six of the 8 compounds were brain penetrant while the other two drugs (palbociclib and sirolimus) did not cross the blood brain barrier at appreciable levels suggesting factors other than local CNS drug concentration may play a role in glioma development. CNS was the primary site of pharmacologic activity for two brain penetrant drugs (lorcaserin and cyclobenzaprine intended for obesity and muscle relaxant, respectively) with likely broad distribution throughout the brain. In the case of lorcaserin for example, preferential distribution in the CNS varied by species (brain: plasma ratio = 26x in the mouse, up to 35x in the rat, and 10x in the monkey). Brain partition in human is not known but using CSF exposure in humans as a surrogate to brain exposure, it was concluded that lorcaserin’s partition into the brain of humans is less than that observed in rats. These data were used to assess the translation of the risk of tumorigenicity from rat to human supporting an adequate therapeutic index based on exposure multiples specific to the CNS, and risk considered to be very low for humans (Lorcaserin SBA). The site of primary pharmacology of the remaining 6 drugs was outside the CNS with variable drug distribution into the brain, thus potential mechanism(s) of CNS tumorigenicity were unlikely related to the presence of the drug in the CNS.
Genotoxic mechanisms, hormonal perturbation and immune suppression are among the most likely causes of tumor formation. Two of 8 drugs (omeprazole and entecavir) were shown to carry clastogenic activity in genotoxicity assays, one (sirolimus) was an immunosuppressant and one (budesonide) has hormonal modulation properties. It is possible these underlying properties may have contributed to the development of glial tumors for these drugs. However, one cannot discount the possibility that given the lack of a common oncogenic mechanism and low incidence, that these CNS tumors could be observed by chance as opposed to being drug-induced. Supporting this hypothesis, in the three rat carcinogenicity studies conducted with budesonide, the CNS tumors were only observed in one study; also, in the case of omeprazole, the tumors were observed in a 52-week chronic toxicity study but not the 2-year carcinogenicity study, suggesting exercising caution in interpreting the data and assessing translation and potential relevance of these tumors to humans.
A disproportionality analysis (DA) was conducted for the drugs listed in Table 3 to assess the translatability of the rat carcinogenicity findings to humans. The results suggested lack of translation since signals of disproportionate reporting (SDS) of CNS tumors in patients taking any of these eight drugs were not observed. Supporting this conclusion is epidemiology work conducted to assess the translation of CNS tumors observed in rat studies induced by the chemical toxicant acrylonitrile (Collins and Strother, 1999). Acrylonitrile is an intermediary chemical used in the manufacture of several products and was extensively studied to understand the translation of rat CNS tumor risk to humans. In rats, acrylonitrile is considered a potent CNS carcinogen causing tumors by selectively inducing oxidative stress in the rat brain at concentrations in the upper range of workplace exposures, raising concern that it may be tumorigenic in humans (Jiang et al., 1998). Several epidemiology studies concluded that translation of rat CNS tumors to human is weak which contrasts with the level of risk that can be predicted based on rat carcinogenicity results, again calling for caution when extrapolating rat CNS tumors to human risk (Collins and Strother, 1999; Schulz et al., 2001).
From the list of drugs associated with CNS tumors in the rat (Table 3), palbociclib (a CDK4/6 inhibitor) and sirolimus (an mTOR inhibitor), also resulted in glucose dysregulation in the rat. The similarity of glucose effects and CNS tumor formation between both drugs (palbociclib and sirolimus), which do not share pharmacological action or chemical structure is intriguing. In their respective rat carcinogenicity studies, both drugs caused hyperglycemia, decrease in body weight, increase in survival compared to the vehicle controls, reduced body fat, pancreatic beta cell loss, [diabetic] cataracts, [diabetic] kidney lesions, and decreased incidence of pituitary and pancreatic islet cell tumors; all observed in male rats only. The enhanced susceptibility of male SD rats to diabetes/glucose dysregulation compared to female rats of the same strain was also reported in the spontaneously diabetic Torri rat where 100% of male rats developed diabetes by week 40 compared to only 33% of female rats becoming diabetic by week 65 (Sasase et al., 2013). The mTOR pathway is upstream of the CDK pathway where it facilitates the translation of various proteins most importantly Cyclin D1 (the cognate partner of CDK4) that forms a complex required for the phosphorylation of Rb protein, which subsequently contributes to progression of the cell cycle and DNA replication (Law et al., 2006). In addition, mTOR was shown to regulate glucose uptake (Chen et al., 2013), glycolysis (Bjornsti and Houghton, 2004), as well as proliferation and survival of pancreatic beta cells (Leibowitz et al., 2008). From this small database, it is difficult to ascertain if concomitant occurrence of hyperglycemia and MCTs in these examples is co-incidental or potentially point to a causal relationship.
Based on extensive literature searches and supported by the disproportionality analysis we conducted, there were no reports of increased occurrences of glial cell tumors in humans with any of the above 8 drugs reported to be associated with glial cell tumors in rat carcinogenicity studies.
Because spontaneously diabetic Torii rats exhibit similar diabetic phenotype as with 2 of 8 drugs (sirolimus and palbociclib) associated with MCTs in our database, pathologic evaluations in life-time studies in this strain may provide an opportunity to further understand any link between hyperglycemia, microglial cell activation and MCTs.
Taken together, CNS glial cell tumors are reported for 8 marketed drugs with different indications. Based on emerging IHC characterization, glial tumors previously thought of astrocytic origin are likely to be MCTs. These may be considered as rodent specific since MCTs are extremely rare in humans and at least one mechanism (i.e. glucose dysregulation) may be unique to male rats. We propose to include MCTs as a tumor type in future rat CNS neoplasm classification and that all glial tumors other than oligodendrogliomas (previously diagnosed as astrocytomas or gliomas) in rats be classified as MCTs unless proven otherwise by IHC.
In this review article we described the types of CNS tumors identified in rat carcinogenicity studies with a focus on microglial cell tumor classification, nomenclature, and incidence to better understand this tumor’s potential relevance to human risk assessment. Despite the rarity of brain neoplasms in humans or in animal species used in the 2-year bioassays, brain tumors do occur in all species, with rats displaying the highest incidence. The microglial cell tumor, which until recently has been – in our opinion erroneously - classified as astrocytoma or glioma in rat carcinogenicity studies, is the most common brain tumor in rats, while its occurrence in humans is extremely rare.
Drug-induced CNS tumors are rare in rats. Only eight marketed drugs reported CNS tumors in rat carcinogenicity studies and disproportionate reporting analysis suggested lack of translation since signals of disproportionate reporting of CNS tumors in patients taking any of these eight drugs were not observed. We attempted to identify a potential mechanism or common factor leading to the emergence of MCTs in rat brains treated with these eight drugs. No clear mechanism was identified; however, the similarity of findings with two of these drugs, palbociclib and sirilomus may warrant further experiments to understand potential role of glucose dysregulation on microglial cell activity in rodent models of hyperglycemia.
We would like to thank Dr. Ikuo Horii for review and helpful discussions in preparation of this review article, Drs. Susan Anway and Dina Tresnan for conducting the disproportionality analysis, Dr. Ingrid Pardo for neuropathology consultations, and Shuyan Lu for editorial assistance.
Conflict of interestAll authors are employees of Pfizer, Inc. USA.