Abstract
The purpose of this study was to explore whether renal endothelial cell injury is associated with oxidative stress in trichloroethylene (TCE)-induced immune kidney damage by detecting adhesion molecules and oxidative stress indexes. In this study, a mouse model of skin sensitization with the antioxidant Tempol was used to explore the mechanism. Blood urea nitrogen (BUN), creatinine (Cre), and histological examination were used for kidney function evaluation. Kidney homogenates were used for detecting renal nitric oxide (NO), nitric oxide synthase (NOS), superoxide dismutase (SOD) and malondialdehyde (MDA). Renal endothelial nitric oxide synthase (eNOS), E-selectin, vascular cell adhesion molecule (VCAM-1) and intercellular adhesion molecule (ICAM-1) protein levels were measured by immunohistochemical and Western blot. We found that BUN and Cre levels increased in the TCE sensitization positive group and the TCE+Tempol sensitization positive group. In the TCE sensitization positive group, a partial area of vacuolar degeneration and lysed epithelial cells were observed in renal tubules. In TCE+Tempol sensitization positive group, small areas were also found to be vacuolar degenerated and renal tubules were dissolved. Renal NO, NOS, SOD and eNOS levels decreased and MDA levels increased, renal E-selectin, VCAM-1and ICAM-1 protein levels increased in the TCE sensitization positive group and the TCE+Tempol sensitization positive group. Tempol attenuated TCE induced up-regulation of MDA, E-selectin, VCAM-1and ICAM-1 and down-regulation of NO, NOS, SOD and eNOS. In conclusion, trichloroethylene-sensitized mice renal immune injury is associated with the renal endothelial cells’ oxidative stress state.
INTRODUCTION
Trichloroethylene (TCE) is an important organic solvent widely used as a metal part cleaner, dry cleaning agent and chemical extractant (Jollow et al., 2009), which has been characterized as a human carcinogen in the Final Health Assessment in 2011 (Liu et al., 2015). TCE, usually distributed in air, soil and water, can be absorbed via the respiratory tract and the skin in workplaces (Zhang et al., 2016). Among the patients who are injured by exposure to trichloroethylene, most of the injuries are manifested as liver and kidney damage, and a small number of the patients mainly manifested severe skin lesions. According to the Chinese National Diagnostic Criteria, these skin disorders are grouped under occupational dermatitis medicamentosa-like induced by TCE (ODMLT) (Liu et al., 2015). The latency period of ODMLT ranged from 10 days to three months. ODMLT can be serious and progress rapidly, potentially resulting in death if not treated promptly (Zhao et al., 2012). It has been recognized that some ODMLT patients may suffer from renal damage. Ultrasound examination of clinical ODMLT patients showed enlarged size of both kidneys, enhanced cortical echogenicity and thickening, experimental examination revealed that the serum creatinine (Cre) level of the patients was (125.7 ± 11.3) μmol/L at admission and (112.3 ± 8.9) μmol/L at discharge, and the blood urea nitrogen (BUN) level of the patients was (10.6 ± 4.3) mol/L at admission and (3.2 ± 1.3) mol/L at discharge (Zhang et al., 2011).
The nephron is the physiological function and structural unit of kidneys, and is mainly composed of glomeruli and renal tubules. The glomeruli are composed of blood vessels and renal capsules; there are rich vascular networks distributed near the renal tubules. Abnormal vascular function often leads to kidney damage. The vascular endothelium, which is located on the inner surface of the blood vessel, is an important and essential component of the blood vessel, keeps the blood circulation and the blood vessel wall separated, maintains vascular permeability and regulates substance exchange. The endothelial cells also regulate inflammation, thrombosis, vasoconstriction and blood flow (He et al., 2015). Chronic inflammation is usually associated with endothelium dysfunction. Therefore, the function of vascular endothelial cells can reflect the functional status of blood vessels. It is generally believed that endothelial dysfunction is mainly ascribed to two principal factors which relate to the vascular endothelium oxidative stress. First, the reduction of endothelial function and formation of peroxynitrite (ONOO−) by enhancing the vascular generation of superoxide anion (O2·−) that bioinactivates endothelial-derived NO and decomposes endothelial nitric oxide synthase (eNOS). Second, the generation of asymmetric dimethylarginine (ADMA), which can inhibit the uptake of L-arginine by the cationic amino acid transporters (CATs) and inhibit nitric oxide synthase (Wang et al., 2009). The most common modification associated with endothelium functional impairment is alteration of leukocyte and lymphocyte adhesion proteins, whose expression is regulated by inflammatory cytokines. These molecules are cell surface proteins involved in the cell-to-cell communication that regulate the movement of leukocytes and lymphocytes between body compartments (Vannini et al., 2008). Biomarkers used to assess endothelial function mainly comprise E-selectin, intercellular adhesion molecule (ICAM-1) and vascular cell adhesion molecule (VCAM-1) (Dzikowska-Diduch et al., 2017).
Endothelial dysfunction is usually associated with oxidative stress. Oxidative stress was briefly defined as metabolic disturbances, usually accompanied by increased production of oxidants and inadequate decompensation of endogenous antioxidants, and result in cellular damage (Lushchak, 2014), DNA damage, increased lipid and protein oxidation (Giordano et al., 2013). There are two kinds of oxidants; one is reactive oxygen species (ROS). ROS are the main marker of oxidative stress, but ROS content was difficult to detect in vivo directly. Malondialdehyde (MDA), an end product of lipid hydroperoxide decomposition, is the most frequently measured index of lipid peroxidation, which can indirectly reflect the ROS content (Soni et al., 2018). The other one is reactive nitric species (RNs). RNs include oxidatively active free radicals and nitro compounds produced by the interaction of ROS with nitric oxide (NO), mainly including NO and peroxynitrite (ONOO-). The antioxidant system consists of two major parts, enzyme antioxidants (including superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH- Px)) and non-enzymatic antioxidants (Hussein et al., 2017).
In previous studies, renal immune injury was found in ODMLT patients and TCE-sensitized mice (Zhang et al., 2016; Zhao et al., 2012). Elevated oxidative stress was also found in TCE-sensitized guinea pig livers (Xu et al., 2010). In summary, it is speculated that TCE-sensitized mice renal immune injury may be associated with renal vascular endothelial cell injury, and oxidative stress may be involved. This experiment was based on the mature BALB/c mice sensitized model (Wang et al., 2015). It was injected intraperitoneally with 4-Hydroxy-TEMPO (Tempol) to improve the oxidative stress level in mice. The levels of adhesion molecules and relative molecules under different oxidative stress conditions were measured to investigate the mechanism of TCE-sensitized vascular endothelial cells impairment.
MATERIALS AND METHODS
Regents
TCE, Freund’s complete adjuvant (FCA) and 4-Hydroxy-TEMPO (Tempol) were bought from Sigma (St. Louis, MO, USA); olive oil and acetone were purchased from the Shanghai Chemical Reagent Company (Shanghai, China); anti-rabbit monoclonal VCAM-1 antibody, anti-mouse monoclonal ICAM-1 antibody and anti-rabbit polyclonal CD62E (E-selectin) antibody were purchased from Abcam (Cambridge, UK); rabbit anti-mouse polyclonal eNOS antibody was bought from Bioss (Beijing, China); goat anti-rabbit secondary antibody, rabbit anti-mouse secondary antibody and phenylmethylsulfonyl fluoride (PMSF) were bought from Cell Signaling Technology (Danvers, MA, USA); radio immuno-precipitation lysis buffer (RIPA) and BCA Kit was from Beyotime (Shanghai, China); Creatinine Assay Kit, Urea Assay Kit, Nitric Oxide (NO) assay kit (Nitrate reductase method), AdeNOSinedeaminase assay kit, Superoxide Dismutase (SOD) assay kit (WST-1 method) and Malondialdehyde (MDA) assay kit (TBA method) were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China); Immunohistochemistry kits, Histostain Plus and DAB substrate kits were purchased from ZSJQ-BIO (Beijing, China).
Establishment of TCE-induced skin sensitization modeling BALB/c mice
The TCE sensitization mouse model was established referring to Wang et al. (2015). In this study, totally 40 6-8-week-old BALB/c female mice, obtained from the Experimental Animal Center of Anhui (Anhui, China), were randomly divided into a blank control group (N = 5), a solvent control group (N = 5), a TCE-treatment group (N = 15), and a TCE+Tempol treatment group (N = 15) after 1 week of adaptive feeding. On the last day of adaptive feeding, an area of 2 × 2 cm hair on the dorsalis was removed with electric clipper. All BALB/c mice were housed at a specific pathogen-free laboratory on a manual 12-hr-light/12-hr-dark cycle every day. Animals are able to freely get standard mouse food and safe water. Twenty-four hours after dorsal hair removal (day 1), the TCE treatment group mice and the TCE+Tempol treatment group mice were treated with dorsal subcutaneous injection of 100 μL of a mixture of 50% TCE (TCE: olive oil: acetone = 5:2:3) and equal volume of FCA. Then 100 μL of 50% TCE was painted on the shaved area of the dorsal skin for continued sensitization on days 4, 7, 10. On days 17 and 19, 100 μL of 30% TCE (TCE:olive oil:acetone = 3:2:5) was painted on the same area for challenging. The area on each mouse was then immediately covered with filter paper and sealed with non-irritating tape for 24 hr after each painting. In the TCE+Tempol treatment group, an intraperitoneal injection (since Tempol is highly oxidizable when exposed to air and could not be applied by skin) of Tempol (100 mg/kg) was given to each mouse 30 min before TCE challenge on Day 19 (El-Sayed et al., 2011). In the solvent control group, the mice were treated with 100 μL mixture containing the same proportions of olive oil and acetone as when treating the TCE treatment group mice and with an intraperitoneal injection of 100 μL saline before challenge on Day 19. Blank control group mice only received an intraperitoneal injection of 100 μL saline on Day19.
Twenty-four hours after the second challenge, cutaneous reactions in the dorsal skin were evaluated and scored on a 4-point scale: 0 (no reaction), 1 (scattered mild redness), 2 (moderate and diffuse redness) and 3 (intensive erythema and swelling) (Wang et al., 2014). Cutaneous reaction scores ≥ 1 were judged as positive and classified into the sensitization positive group, and otherwise were divided into the sensitization negative group, so that the TCE treatment group mice could be divided into a TCE sensitization positive group (TCE+) and a TCE sensitization negative group (TCE-),. and so that the TCE+Tempol treatment group could be divided into a TCE + Tempol sensitization positive group (TCE + Tempol +) and a TCE + Tempol sensitization negative group (TCE + Tempol -). All mice were sacrificed on day 22 (3 days after the second challenge), then kidneys and blood were collected.
All animal experimental protocols used were conducted in accordance with National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by Anhui Medical University.
Evaluation of kidney injury
Eye venous blood was collected and placed under room temperature for two hours, and the serum was collected after centrifugation at 3000 rpm for 5 min. The serum was used for Cre and BUN analyzing by AU5800 automated biochemistry analyzer. Part of the renal cortex was removed and fixed in 4% neutral buffered formaldehyde for 24 hr, then embedded in paraffin. A 5 μm-thick pathological section was sliced and stained with hematoxylin and eosin for histological examination (HE).
Kidney biochemical analysis
Kidney homogenates need to be prepared firstly, then centrifuged at 2500 rpm for 15 min to generate supernate and testing total protein content by using BCA kit. Supernate levels of NO, NOS, SOD and MDA were measured using Nitric Oxide (NO) assay kit (Nitrate reductase method), AdeNOSinedeaminase assay kit, Superoxide Dismutase (SOD) assay kit (WST-1 method) and Malondialdehyde (MDA) assay kit (TBA method), respectively.
Immunohistochemical detection
Sections 5 μm thick were sliced, dewaxed, rehydrated, endogenous peroxidase blocked, antigen retrieved and incubated in goat serum for 15 min at 37°C, then incubated with eNOS antibody, E-selectin antibody, VCAM-1 antibody and ICAM-1 antibody overnight at 4°C. The sections were then washed with PBS after reaching room temperature, and incubated with anti-rabbit IgG or anti-mouse IgG for 15 min and horseradish peroxidase-labeled avidin-biotin complex for 15 min at 37°C. Lastly, slides were processed with DAB kits and hematoxylin. The immuno-binding products were analyzed by using a light microscope.
Western blot analysis
A 30 mg kidney tissue was homogenized with RIPA and PMSF (100:1) mixture to extract proteins, then centrifuged at 15,000 rpm for 15 min. Supernatants were isolated and protein concentration was determined by using BCA Kit. Equal amounts (25 μg) of protein extracts were loaded and separated by SDS-PAGE by using 5% and 12.5% acrylamide gradients. Next, protein extracts were transferred to transfer membranes and blocked in PBS-5% nonfat milk for 2 hr at room temperature. The membranes were washed with ultrapure water and incubated in Phosphate Buffered Saline (PBS) mixed with E-selectin antibody or VCAM-1 antibody or ICAM-1 antibody at 4°C overnight, then washed with DPBS and incubated in PBS-5% nonfat milk containing goat anti-rabbit IgG or rabbit anti-mouse IgG for 2 hr at room temperature. The membranes were washed with Dulbecco's phosphate-buffered saline (DPBS) again, and the signal was developed by using enhanced chemiluminescence (ECL) detection kit.
Data analysis
All data are presented as mean ± standard deviation (M ± SD). A one-way analysis of variance (ANOVA) with post hoc least-significant (LSD) test was used to examine the difference between differents groups. P < 0.05 was considered to be statistically significant. Statistical analyses were performed with SPSS V.23.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Sensitization rates
Sensitization rates were calculated by observing cutaneous erythema and edema response. Cutaneous reaction scores ≥ 1 were regard as sensitization-positive. There were no sensitized mice in the blank control group and the solvent control group. There were 6 mice in the TCE-treatment group with scores ≥ 1, and 5 in the TCE+Tempol treatment group with scores ≥ 1; theie sensitization rate was 40% and 33.3%, respectively (see Table 1).
Table 1. Sensitization rates.
| Group |
n |
score |
Sensitization rate (%) |
| 0 |
1 |
2 |
3 |
| blank control |
5 |
0 |
0 |
0 |
0 |
0.0 |
| solvent control |
5 |
0 |
0 |
0 |
0 |
0.0 |
| TCE |
15 |
9 |
4 |
1 |
1 |
40.0 |
| TCE+Tempol |
15 |
10 |
4 |
1 |
0 |
33.3 |
TCE-induced kidney injury
To detect the kidney injury induced by TCE, we performed the renal HE and kidney function experiments. The results from renal HE showed that tubules and glomeruli had clear structure, renal tubular epithelial cells were neatly arranged, and no inflammatory cell infiltration or obvious pathological changes were found in the blank control group, solvent control group, TCE- group and TCE + Tempol- group. In the TCE+ group, a partial area of vacuolar degeneration and lysed epithelial cells were observed in renal tubules. In the TCE+TEMPOL+ group, vacuolar degeneration and dissolution was also found in some areas of the renal tubules, and the area was significantly reduced compared to the solvent control group (Fig. 1).

Kidney function analysis displayed similar results. Serum Cre and BUN levels significantly increased in the TCE+TEMPOL+ group and the TCE+ group compared to the solvent control group (P < 0.05). However, kidney function improved in the TCE+TEMPOL+ group compared to the TCE+ group (P < 0.05). No significant differences were found among the blank control group, solvent control group, TCE- group and TCE + Tempol- group (P > 0.05) (Fig. 2).
Renal biochemical results
To reflect renal oxidative stress status in the mice, we detected renal NO/NOS levels and renal SOD/MDA levels.
Renal NO/NOS levels
Compared to the solvent control group, the levels of NO and NOS were significantly decreased in the TCE + Tempol+group and TCE+ group (P < 0.05). The levels of the TCE + Tempol+ group mildly increased compared to the TCE+ group (P < 0.05). There were no significant differences among the blank control group, solvent control group, TCE- group and TCE + Tempol- group (P > 0.05) (Fig. 3).
Renal SOD/MDA levels
No significant differences were found among the blank control group, solvent control group, TCE- group and TCE + Tempol- group (P > 0.05) for renal SOD and MDA levels. Renal SOD level decreased and MDA level increased in the TCE + group and TCE + Tempol+ group compared to the solvent control group (P < 0.05). Compared to the TCE+ group, renal SOD level increased and MDA level decreased significantly (P < 0.05) (Fig. 4) in the TCE + Tempol+ group.
Renal eNOS, E-selectin, VCAM-1 and ICAM-1 expression
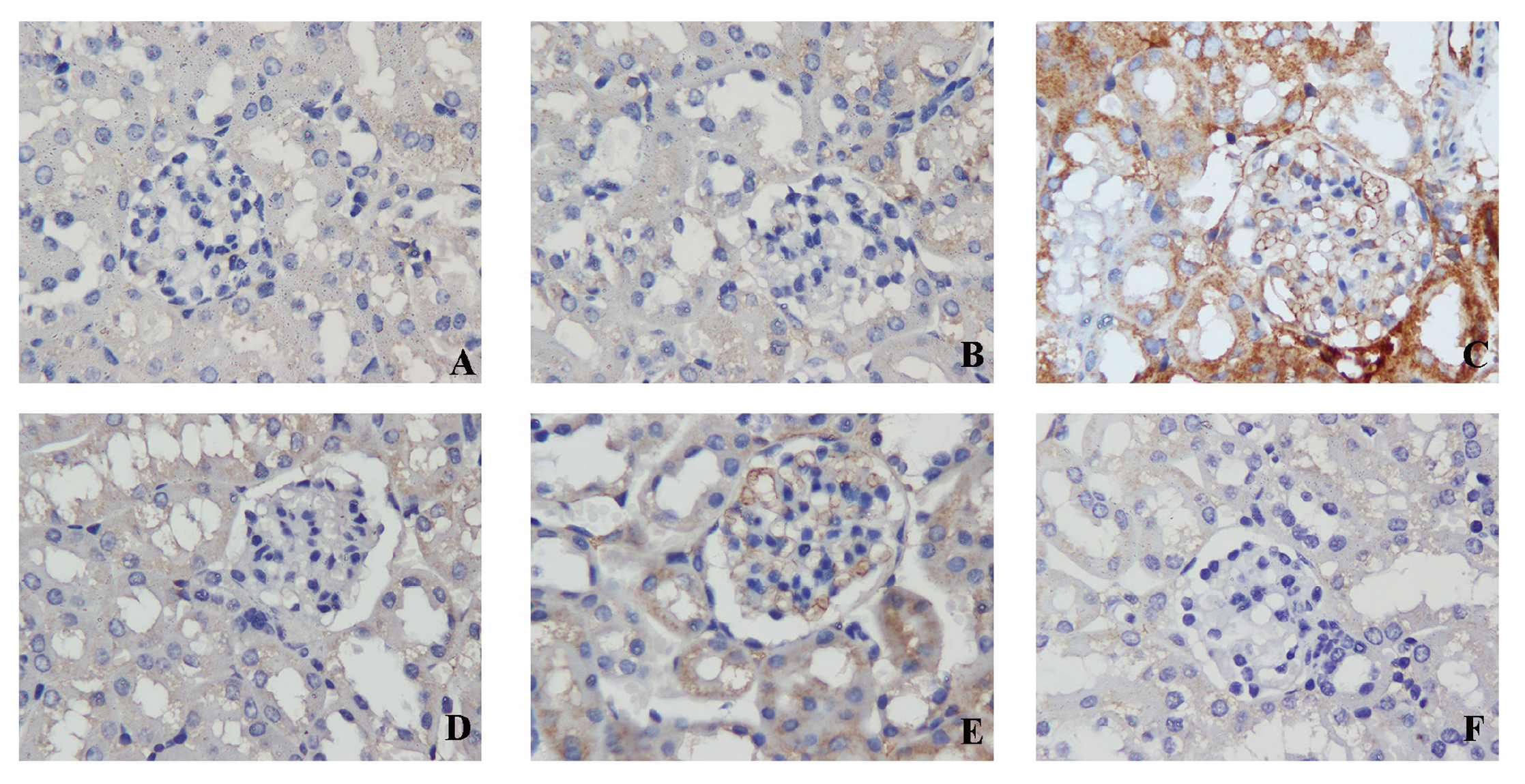
To reflect renal oxidative stress status, we performed immunohistochemical (IHC) staining of eNOS. The results showed that plenty of eNOS expressed in tubules and glomeruli in the blank control group, solvent control group, TCE- group and TCE + Tempol- group. There were little immunoreactant deposits on tubules for the TCE+ group and deposits on tubules and glomeruli for the TCE + Tempol+ group (Fig. 5).

The results of local expression of adhesion molecules, which include E-selectin, VCAM-1 and ICAM-1, are shown in Figs. 6, 7, and 8, respectively. There were no visible immunoreactant depositions found in the blank control group, solvent control group, TCE- group and TCE + Tempol- group, but salient deposits were found on tubules and glomeruli for the TCE+ group mice and little depositions were found for the TCE + Tempol+ group. The main deposition sites for E-selectin, VCAM-1 and ICAM-1were found on tubules. Western blot analysis found that E-selectin, VCAM-1 and ICAM-1 levels were significantly increased in samples from the TCE+ group and TCE + Tempol+ group compared to the solvent control group, but mildly decreased in the TCE + Tempol+ group compared to the TCE+ group (Fig. 9).
DISCUSSION
TCE is one of the most common organic solvents. Some workers’ exposure to TCE can cause severe skin damage, including toxic epidermal necrolysis, exfoliative dermatitis, erythema multiforme, and Stevens-Johnson syndrome. These skin disorders are defined as “occupational dermatitis medicamentosa-like of TCE” (ODMLT) (Xia et al., 2006). There were more than 441 cases in Guangdong, China from the first report of a patient with severe TCE-exposure exfoliative dermatitis until 2015 (Huang and Huang, 2010; Li et al., 2017). In the past three years, there have been more than a dozen new cases in Guangdong Province every year. The number of patients suffering from TCE-related severe skin disorders increased in Asia (Kamijima et al., 2007). Additionally, the severe substantial organ damage usually accompanying the skin disorders is the main reason for the high mortality. Although the treatment of this disease have been improved continually over the years, due to its long course, patients are devastatingly subject to relapse and a high mortality rate, and thus this disease still threatens sufferers' health heavily. Therefore, the mechanism of ODMLT induced by TCE is of great significance for clinical treatment.
Patients with ODMLT may also experience severe kidney damage. It has been reported that ultrasound examination of ODMLT patients showed an increase in the size of both kidneys, an increase and thickening of the cortical echo, thinning of the renal cortex and a decrease in the volume of the severe renal damage. BUN and Cre levels were significantly elevated (Zhang et al., 2011). In this study, no significant differences in serum Cre and BUN levels were found among the blank control group, solvent control group and TCE- group, but the levels were significantly increased in TCE+ group mice. The results from renal HE showed that tubules and glomeruli had clear structure, renal tubular epithelial cells were neatly arranged, and no inflammatory cell infiltration or obvious pathological changes were found in the blank control group, solvent control group, and TCE- group. In the TCE+ group, a partial area of vacuolar degeneration and lysed epithelial cells were observed in renal tubules.
Kidney function is inseparable from the function of the kidney vasculature since the renal unit is rich in vascular network. The vascular endothelium is an indispensable component of blood vessels. It is generally recognized that NO and eNOS are generated by endothelial cells and can reflect the function of endothelium. NO plays a central role in endothelial dysfunction and injury (Cheng and Harris, 2014). Fukumura et al. found that eNOS played a predominant role in VEGF-induced angiogenesis and vascular permeability (Fukumura et al., 2001), and eNOS insufficiency may accelerate nephropathy in mouse models of both type 1 and type 2 diabetes (Zhao et al., 2006). Furthermore, some adhesion molecules are also closely related to endothelial cells and can be used as biomarkers for endothelial dysfunction, including VCAM-1, ICAM-1 and E-selectin. E-selectin is produced exclusively by endothelial cells and therefore is considered to be a superior biomarker of endothelial dysfunction compared to other cell adhesion molecules. E-selectin mediates the transient rolling of leukocytes along the endothelium while VCAM-1 and ICAM-1 mediate stronger attachment of leukocytes to the endothelium (Kunutsor et al., 2017). VCAM-1 is mainly expressed on endothelial cells, but is also expressed on leukocytes. VCAM-1 is important for mediating the adhesion of leukocytes and endothelial cells and can reflect endothelial cell disorders, but is not as specific as E-selectin. The expression of ICAM-1 is more extensive, not only in endothelial cells, but also in hematopoietic cells and fibroblasts (Kunutsor et al., 2017). In this study, E-selectin, VCAM-1 and ICAM-1 levels increased in the TCE sensitized group; eNOS, NO and NOS decreased in the TCE sensitized group, indicating that renal endothelial cells were damaged in the TCE sensitized group.
In this experiment, we speculate that oxidative stress occurred in trichloroethylene-sensitized mice. A TCE+Tempol group was added to the experimental model to determine whether renal endothelial cell damage was associated with oxidative stress. The results showed that there was no significant difference in the sensitization rate between the TCE+Tempol group and TCE group. The oxidative stress index from kidney biochemical analysis showed that MDA level increased in the TCE+ group and TCE + Tempol+ group compared to the solvent control group, but decreased in the TCE + Tempol+ group compared to the TCE+ group. The levels of eNOS, NO, NOS and SOD were significantly decreased in the TCE + Tempol+ group and the TCE+ group compared to the solvent control group. However, they were mildly increased in the TCE + Tempol+ group compared to the TCE+ group. There were no significant differences among the blank control group, solvent control group, TCE- group and TCE + Tempol- group. All the oxidative stress results showed that the lipid peroxidation level in the kidneys of the TCE+ group was significantly increased, while the activity of antioxidant enzymes was decreased. As a result, compared to the TCE+ group, the levels of E-selectin, VCAM-1, ICAM-1 in TCE+Tempol+ group were decreased. The pretreament with Tempol did not restore renal oxidative stress in TCE-sensitized mice to normal levels, but it attenuated renal oxidative stress to some extent. It is possible that oxidative stress, although involved in TCE-sensitized mice renal injury, can not completely explain the renal injury in TCE-sensitized mice. Another possibility is that the dose of antioxidants is insufficient, so that kidney damage can not be restored to a normal state. Combined with the results of the sensitization rate and the results of renal function, it was found that the use of antioxidants can reduce the renal injury of TCE-sensitized mice to a certain extent, accompanied by a decrease in the level of oxidative stress in the kidney. The parallel relationship between the two suggests that the level of oxidative stress plays an important role in TCE sensitized mice renal injury, but antioxidants could not change the sensitization rate.
In conclusions, in this study, E-selectin, VCAM-1 and ICAM-1 levels increased and eNOS, NO and NOS decreased in TCE sensitized group mice compared to solvent control group mice, which indicates that TCE sensitized mouse kidney renal injury is associated with endothelial cell damage. The MDA level increased and NO, NOS and SOD levels significantly decreased in the TCE + group and TCE + Tempol+ group compared to the solvent control group, while the MDA level decreased and the NO, NOS and SOD levels increased in the TCE + Tempol+ group compared to the TCE+ group, suggesting that the use of antioxidants can reduce endothelial cell damage in TCE-sensitized mice. In summary, trichloroethylene-sensitized mice renal injury is associated with renal endothelial cells’ oxidative stress state.
ACKNOWLEDGMENTS
Author contributions: Bodong Li contributed to conception and design, contributed to acquisition, analysis, and interpretation. Haibo Xie contributed to analysis, interpretation, contributed to drafted manuscript, and critically revised manuscript; Xian Wang and Xiaodong Yang contributed to conception and acquisition; Ling Yang contributed to conception and design; Jiaxiang Zhang contributed to acquisition, analysis, and interpretation; Feng Wang contributed to interpretation, drafted manuscript, and critically revised manuscript; Tong Shen contributed to conception and design, contributed to acquisition, analysis, and interpretation, drafted manuscript, and critically revised manuscript; Qi-xing Zhu, contributed to conception and design, contributed to acquisition, analysis, and interpretation, drafted manuscript, and critically revised manuscript. All authors gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
Funding information: This work was supported by the [National Natural Science Foundation of China#1] under Grant [number 81874259]; [National Natural Science Foundation of China #2] under Grant [number 81673141].
Conflict of interest
The authors declare that there is no conflict of interest.
REFERENCES
- Cheng, H. and Harris, R.C. (2014): Renal endothelial dysfunction in diabetic nephropathy. Cardiovasc. Hematol. Disord. Drug Targets, 14, 22-33.
- Dzikowska-Diduch, O., Domienik-Karłowicz, J., Górska, E., Demkow, U., Pruszczyk, P. and Kostrubiec, M. (2017): E-selectin and sICAM-1, biomarkers of endothelial function, predict recurrence of venous thromboembolism. Thromb. Res., 157, 173-180.
- El-Sayed, N.S., Mahran, L.G. and Khattab, M.M. (2011): Tempol, a membrane-permeable radical scavenger, ameliorates lipopolysaccharide-induced acute lung injury in mice: a key role for superoxide anion. Eur. J. Pharmacol., 663, 68-73.
- Fukumura, D., Gohongi, T., Kadambi, A., Izumi, Y., Ang, J., Yun, C.O., Buerk, D.G., Huang, P.L. and Jain, R.K. (2001): Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc. Natl. Acad. Sci. USA, 98, 2604-2609.
- Giordano, S., Darley-Usmar, V. and Zhang, J. (2013): Autophagy as an essential cellular antioxidant pathway in neurodegenerative disease. Redox Biol., 2, 82-90.
- He, M., Zuo, X. and Xie, W. (2015): Endoplasmic reticulum stress and endothelial dysfunction. China Med. Herald, 12, 30-34.
- Huang, Y. and Huang, H. (2010): Research progress on immune injury resulted from occupational medicamentose-like dermatitis induced by trichloroethylene. China Occup. Med., 37, 157-159.
- Hussein, M.A., El-Gizawy, H.A., Gobba, N.A. and Mosaad, Y.O. (2017): Synthesis of Cinnamyl and Caffeoyl Derivatives of Cucurbitacin-Eglycoside Isolated from Citrullus colocynthis Fruits and their Structures Antioxidant and Anti-inflammatory Activities Relationship. Curr. Pharm. Biotechnol., 18, 677-693.
- Jollow, D.J., Bruckner, J.V., McMillan, D.C., Fisher, J.W., Hoel, D.G. and Mohr, L.C. (2009): Trichloroethylene risk assessment: a review and commentary. Crit. Rev. Toxicol., 39, 782-797.
- Kunutsor, S.K., Bakker, S.J. and Dullaart, R.P. (2017): Soluble Vascular Cell Adhesion Molecules May be Protective of Future Cardiovascular Disease Risk: Findings from the PREVEND Prospective Cohort Study. J. Atheroscler. Thromb., 24, 804-818.
- Li, R.W., Zhang, Z.J. and Zhou, S.Y. (2017): Analysis of serum non-enzymatic antioxidant concentration in occupational dermatitis medicamentosa-like of TCE patients. Pract. Prev. Med., 11, 1366-1368.
- Kamijima, M., Hisanaga, N., Wang, H. and Nakajima, T. (2007): Occupational trichloroethylene exposure as a cause of idiosyncratic generalized skin disorders and accompanying hepatitis similar to drug hypersensitivities. Int. Arch. Occup. Environ. Health, 80, 357-370.
- Liu, W., Hong, W.X., Zhang, Y., Huang, P., Yang, X., Ren, X., Huang, H. and Liu, J. (2015): Proteomic profiling of occupational medicamentosa-like dermatitis induced by trichloroethylene in serum based on MALDI-TOF MS. Clin. Exp. Med., 15, 519-526.
- Lushchak, V.I. (2014): Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact., 224, 164-175.
- Soni, K.K., Zhang, L.T., Choi, B.R., Karna, K.K., You, J.H., Shin, Y.S., Lee, S.W., Kim, C.Y., Zhao, C., Chae, H.J., Kim, H.K. and Park, J.K. (2018): Protective effect of MOTILIPERM in varicocele-induced oxidative injury in rat testis by activating phosphorylated inositol requiring kinase 1α (p-IRE1α) and phosphorylated c-Jun N-terminal kinase (p-JNK) pathways. Pharm. Biol., 56, 94-103.
- Vannini, N., Pfeffer, U., Lorusso, G., Noonan, D.M. and Albini, A. (2008): Endothelial cell aging and apoptosis in prevention and disease: e-selectin expression and modulation as a model. Curr. Pharm. Des., 14, 221-225.
- Wang, D., Strandgaard, S., Iversen, J. and Wilcox, C.S. (2009): Asymmetric dimethylarginine, oxidative stress, and vascular nitric oxide synthase in essential hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol., 296, R195-R200.
- Wang, F., Zha, W.S., Zhang, J.X., Li, S.L., Wang, H., Ye, L.P., Shen, T., Wu, C.H. and Zhu, Q.X. (2014): Complement C3a binding to its receptor as a negative modulator of Th2 response in liver injury in trichloroethylene-sensitized mice. Toxicol. Lett., 229, 229-239.
- Wang, H., Zhang, J.X., Li, S.L., Wang, F., Zha, W.S., Shen, T., Wu, C. and Zhu, Q.X. (2015): An Animal Model of Trichloroethylene-Induced Skin Sensitization in BALB/c Mice. Int. J. Toxicol., 34, 442-453.
- Xu, H., Leng, J., Shen, T., Wang, H. and Zhu, Q.X. (2010): Determination of MDA level and SOD activity in liver of TCE sensitized guinea pig. China J. Ind. Med., 6, 406-408.
- Xia, L., Qiu, C., Li, L., Liu, H., Kong, L., Chen, B., Tang, X., Hang, W., Zhang, Y., Wu, B. and Huang, H. (2006): Editorial explanation for diagnostic criteria of occupational medicamentosa-like dermatitis induced by trichloroethylene. China Occup. Med., 34, 383-386.
- Zhao, H.J., Wang, S., Cheng, H., Zhang, M.Z., Takahashi, T., Fogo, A.B., Breyer, M.D. and Harris, R.C. (2006): Endothelial nitric oxide synthase deficiency produces accelerated nephropathy in diabetic mice. J. Am. Soc. Nephrol., 17, 2664-2669.
- Zhao, N., Wang, H.L., Yue, F., Zeng, Z.M., Li, H.L., Huang, Y.S. and Chen, R.T. (2012): [Studying the changes of the related serum complement immune indexes in patients with occupational medicamentosa-like dermatitis induced by trichloroethylene and workers occupationally exposed to trichloroethylene]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi, 30, 284-288.
- Zhang, J.X., Zha, W.S., Ye, L.P., Wang, F., Wang, H., Shen, T., Wu, C.H. and Zhu, Q.X. (2016): Complement C5a-C5aR interaction enhances MAPK signaling pathway activities to mediate renal injury in trichloroethylene sensitized BALB/c mice. J. Appl. Toxicol., 36, 271-284.
- Zhang, L., She, X., Li, M., Huang, L. and Liu, J. (2011): Dynamic observation on kidneys, livers and spleens by brightness mode ultrasonic imaging in patients of occupational medicamentosa-liked dermatitis induced by trichloroethylene. Chinese J. Ind. Med., 3, 185-187.