2020 Volume 45 Issue 1 Pages 1-14
2020 Volume 45 Issue 1 Pages 1-14
Butyl 2,3-epoxypropyl ether (CAS No. 2426-08-6, synonym: n-butylglycidyl ether, BGE) was exposed by whole body inhalation to F344 rats and BDF1 mice of both sexes (50 animals per group) 6 hours per day, 5 days per week for 104 weeks at targeted concentrations of 0, 10, 30 or 90 ppm (v/v) for rats and 0, 5, 15 or 45 ppm for mice. In rats, 90 ppm of BGE increased the incidences of nasal squamous cell carcinomas in both sexes. Nasal adenomas and splenic mononuclear cell leukemia were increased in male rats exposed to 30 ppm. Splenic mononuclear cell leukemia was increased in female rats by trend test. Non-neoplastic nasal lesions, such as squamous cell hyperplasia with atypia, squamous cell metaplasia and the inflammation of the respiratory region and atrophy of the olfactory epithelium were increased in both sexes in a dose-dependent manner. In mice, the incidences of histiocytic sarcomas of the uterus in female mice were increased in a dose-dependent manner and the incidences of nasal hemangiomas in both sexes were increased in a dose-dependent manner. Nasal squamous cell carcinoma, a rare tumor, was observed, although not statistically significant, in both sexes. Non-neoplastic lesions such as nodular hyperplasia of the transitional epithelium and cuboidal changes of the respiratory epithelium in the nasal cavity, were increased both in males and females in a dose-dependent manner. The present study demonstrated clear evidence of carcinogenicity of BGE in both rats and mice by the 2-year whole body inhalation exposure.
Butyl 2,3-epoxypropyl ether (CAS No. 2426-08-6, synonym: n-butylglycidyl ether, BGE) is used primarily as a reactive diluent for epoxy resins, serving as a viscosity-reducing agent, an acid acceptor for stabilizing chlorinated solvents, and as a chemical intermediate. The annual production and import of BGE in Japan was estimated between 100 and 1000 tons in 2016 (Japan Ministry of the Economy Trade and Industry, 2018). And the Pollutants Release and Transfer Register in Japan was 10.2 tons in 2017 (Japan Ministry of the Environment, 2019).
Subchronic inhalation studies up to 10 weeks conducted by private companies are cited in reviews (Gardiner et al., 1992; National Toxicology Program (NTP), 2004), and NTP conducted a short-term inhalation study but a report was not prepared (NTP Management Status Report, 2019). There are no reports on chronic toxicity or carcinogenicity.
The majority of in vitro and in vivo genotoxicity studies on BGE are positive for genotoxicity (Canter et al., 1986; Connor et al., 1980; Frost and Legator, 1982; Thompson et al., 1981; von der Hude et al., 1991; Wade et al., 1979). The International Agency for Research on Cancer (IARC) and the American Conference of Governmental Industrial Hygienists (ACGIH) have not evaluated the carcinogenicity of BGE while the German Research Foundation (DFG) listed BGE as a substance that is justifiably suspected of having carcinogenic potential (Carcinogen category IIIB) (DFG, 1992) and the Japan Society for Occupational Health (JSOH) classified BGE as a group 2B carcinogen in 2016 (JSOH, 2016) based on the JBRC report (in Japanese) of the present studies to the Japanese Government (JBRC, 2005). ACGIH recommended the TLV-TWA value of 3 ppm for BGE, based on a NOAEL of 38 ppm in the subchronic study of rats described above (ACGIH, 2014). The US Occupational Safety and Health Administration (OSHA) established the Permissible Exposure Limit (PEL) value of 50 ppm for BGE. The NIOSH designated the Recommended Exposure Limit (REL) value of 5.6 ppm (ceiling, 15-min) for BGE, based on skin and mucous membrane effects, sensitization potential, and possible hematopoietic effects (NIOSH, 1992).
As mentioned above, a report of the present study in Japanese to the Japanese government has been available (JBRC, 2005). This paper provides the data with further discussion on the carcinogenic effect of BGE to the rodent test animals.
The study was conducted at the Japan Bioassay Research Center (JBRC) from 2001 through 2003 with reference to the Organisation for Economic Co-operation and Development (OECD) Guideline for Testing of Chemicals 451 “Carcinogenicity Studies” (OECD, 1981), and in accordance with the OECD Principles of Good Laboratory Practice (OECD, 1998). The animals were cared for in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996). The present study was approved by the Ethics Committee of the JBRC.
Test substanceBGE (purity greater than 99.7%) was obtained from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). The identity of BGE was confirmed by mass spectrometry and infrared spectrometry, and the purity and stability of each lot of the BGE used in the present studies were monitored by gas chromatography before and after use. No gas chromatographic peak other than BGE itself was detected from the inhalation exposure chambers throughout the study.
Animals and husbandryFour-week-old F344/DuCrj (SPF) rats and Crj:BDF1 (SPF) mice of both sexes were obtained from Charles River Japan, Inc. (Kanagawa, Japan). The animals were quarantined and acclimated for 2 weeks, and divided by stratified randomization into 4 body-weight-matched groups with 50 rats and 50 mice of both sexes. The animals were housed individually in stainless-steel wire cages (150 mm [W] x 270 mm [D] x 176 mm [H] for rats, and 100 mm [W] x 116 mm [D] x 120 mm [H] for mice), 50 males and 50 females each in stainless steel inhalation exposure chambers (8.5m3 for rats and 3.9 m3 for mice in capacity) maintained at a temperature of 22.9~23.1°C and at a relative humidity of 53.7~56.8% with a flow of 12 air changes per hour. Due to the relatively low vapor pressure of BGE, the air change rate was reduced to 6 times per hour during the 6-hr exposure period in order to gain the targeted concentration. Fluorescent lamp lighting was controlled by a timer to give a 12-hr light/dark cycle. All rats and mice had free access to sterilized water and a γ-irradiation-sterilized commercial pellet diet (CRF-1, Oriental Yeast Co., Ltd., Tokyo, Japan).
Experimental designFifty rats and mice of both sexes per group were exposed to airflow containing BGE vapor at a target concentration of 0, 10, 30, or 90 ppm and 0, 5, 15 or 45 ppm respectively for 6 hr per day, 5 days per week for 104 weeks (2 years). Respective controls, i.e., 0 ppm groups, were exposed to clean air in the inhalation exposure chambers for the same time period. The top concentrations were chosen not to exceed the maximum tolerated dose (MTD) consulting the results of 2-week and 13-week dose-finding studies conducted prior to this study (c.f. https://anzeninfo.mhlw.go.jp/user/anzen/kag/carcino_report.htm, in Japanese).
Inhalation conditionsThe saturated vapor-air mixture was generated by bubbling clean air through the BGE liquid in a temperature-regulated glass flask (26°C), followed by cooling through a coiled-type glass condenser at 20°C, diluted with clean air, and warmed up to 26°C by another glass condenser. The vapor-air mixtures were further diluted with humidity- and temperature-controlled clean air by line mixers immediately before introducing to the inhalation exposure chambers. Concentration of BGE of each chamber was monitored by gas chromatography every 15 min, and maintained at 10.1 ± 0.1 (mean ± SD), 30.1 ± 0.2 and 90.1 ± 0.6 ppm for rats, and at 5.0 ± 0.0, 15.0 ± 0.1 and 45.0 ± 0.3 ppm for mice, throughout the 2-year exposure period.
Clinical observations and pathological examinationsThe animals were observed daily for clinical signs. Body weights and food consumption were measured once a week for the first 14 weeks, and every 4 weeks thereafter. Moribund animals were euthanized under anesthesia. All animals received complete necropsy. Urinary parameters were measured with Ames Reagent Strips (Multistix for rats and Uro-Labstix for mice, Bayer-Sankyo Co., Tokyo, Japan) at the last week of the 2-year exposure period. Hematology and blood chemistry was conducted for all animals at terminal necropsy after overnight fasting. The blood samples were analyzed using an Automatic Blood Cell Analyzer (ADVIA120, Bayer Corp., New York, NY, USA) for hematology, and an Automatic Analyzer (HITACHI 7070, Ibaraki, Japan) for blood chemistry. Organs were weighed, and examined for macroscopic lesions at the necropsy. All organs and tissues indicated in the OECD test guideline (OECD, 1981) and the entire respiratory tract, including three levels of nasal cavity, pharynx, and larynx, were examined for histopathology. The nasal cavity was decalcified in formic acid-formalin solution and frontally sectioned at three levels as described previously (Nagano et al., 1997), i.e., at the level of the posterior edge of the upper incisor teeth (Level 1), incisive papilla (Level 2) and anterior edge of the upper molar teeth (Level 3). The organs and tissues were fixed in 10% neutral buffered formalin and embedded in paraffin. Tissue sections 5-μm thick were stained with hematoxylin and eosin. Diagnosis was made with reference to the criteria of the International Classification of Rodent Tumours (IARC, 1992) and the International Harmonization of Nomenclature and Diagnostic Criteria for Lesions in Rats and Mice (Schwarz et al., 1994).
Statistics and data analysisA positive trend for neoplastic incidence was analyzed by Peto’s test (Peto et al., 1980). The incidences of neoplastic and non-neoplastic lesions among groups were analyzed by Fisher’s exact test. Body weight, organ weights, and hematological and blood biochemical parameters were analyzed by an algorithm (Hamada et al., 1998) as follows; Bartlett’s test was used to test whether the variance was homogeneous or not. When the variance was homogeneous, one-way ANOVA was applied. When the variance was not homogeneous, the Kruskal-Wallis rank sum test was performed. Statistical differences in the means among the groups were analyzed by Dunnett’s multiple comparison tests when the variance was homogeneous and Dunnett’s multiple comparison tests by rank when the variance was not homogeneous.
Survival curves were plotted according to the Kaplan-Meier method, (Kaplan and Meier, 1958), and the log-rank test (Peto et al., 1977) was used to test a statistically significant difference.
When a rarely occurring tumor was observed with no statistical significance compared with the control group by either Fisher’s exact test or Peto’s test, an increase in the tumor incidence was evaluated in light of incidences of the same tumor type in the historical control data of JBRC; 37 studies of male rats, 34 studies of female rats and 32 studies of male mice and female mice corresponding to 1,849 male and 1,697 female F344Crj (SPF) rats and 1,596 male and female BDF1 (SPF) mice. The incidences of rarely occurring tumors were in line with the incidences described by Haseman et al. (1984 and 1995) for NTP studies.
The nomenclature, diagnostic criteria, and final diagnosis were peer reviewed by invited certified pathologists with minimal discrepancy with that of JBRC pathologists.
The survival rates at the end of 2-year exposure period of the 0 (clean air control), 10, 30, and 90 ppm groups were 76%, 76%, 76%, and 22% for males and 80%, 90%, 82%, and 30% for females (Table 1, Fig. 1A, B). The decrease in survival rate of 90 ppm groups was attributed to an increase in lethal nasal tumors. The body weights of the 10, 30, and 90 ppm groups at the end of the 2-year exposure period were 100%, 99%, and 70% for males and 101%, 100% and 77% for females of the controls (Table 1, Fig. 1C, D).


Survival (A and B) and growth curves (C and D) of male and female rats exposed by inhalation to BGE for 2 years.
In hematological examination, hemoglobin concentration and hematocrit, MCH, and MCHC were significantly decreased in the 90 ppm males. In blood chemistry, AST, ALP, CK, potassium and inorganic phosphorus levels were significantly increased and albumin, total-cholesterol, triglyceride, phospholipid and calcium levels were significantly decreased in the 90 ppm males. ALP and CK levels were significantly increased and albumin and sodium levels were significantly decreased in the 90 ppm females. Total-cholesterol, triglyceride, phospholipid and calcium levels were significantly increased in 30 ppm females (Table 2, 3).

 Histopathology
Histopathology
The incidences of nasal lesions of the rats are summarized in Table 4. The incidences of squamous cell carcinomas (SCCs) in both male and female rats exposed to 90 ppm were significantly increased and also significant in positive trend by Peto’s test. SCC is a rare tumor in rats and has not been observed in the JBRC historical control data (0/1,849 male and 0/1,697 female controls). The nasal SCCs, well-differentiated keratinizing with cancer pearls, showed exophytic growth into the nasal cavity lumen in sizes up to 1 cm in diameter, destroying and invading the cavity bone structure (Fig. 2). In some case, the carcinoma directly invaded the olfactory bulbs and Harderian glands. Distant metastases to the lung and local lymph nodes were also found in a few rats (Fig. 3). In addition, one esthesioneuroepithelioma was observed in the 90 ppm male group, and one adenosquamous carcinoma, two esthesioneuroepitheliomas, and one sarcoma NOS in the 90 ppm female group. The incidences of all nasal cavity malignancy were significant for the 90 ppm groups and Peto’s test. The incidences of splenic mononuclear cell leukemia were increased in males exposed to 30 ppm, and were increased in females by a significant positive trend by Peto’s test (Table 5).


Nasal tumor of a male rat exposed to 90 ppm of butyl 2,3-epoxypropyl ether. A: Low power view. Squamous cell carcinoma shows exophytic growth with abundant horny substances into the nasal cavity and its cellular component destroying the nasal bone and bulging dorsally to the subcutis of the nose skin. In this case, carcinoma reaches the other side of the nasal cavity. B: High power view of the dorsal end of the deviated nasal septum. Well-differentiated squamous cell carcinoma with cancer pearls destroys and invades the bone and cartilage of the nasal septum. C: Cellular component of the carcinoma destroys the nasal bone and reaches the subcutis of the skin that covers the nose. Level 1 section of the nasal cavity, H & E stain.

Metastasis and invasion of the squamous cell carcinoma of the nasal cavity of a rat exposed to 90 ppm butyl 2,3-epoxypropyl ether. A: Lung metastasis. B: Metastasis to a mandibular lymph node. C: Direct invasion to the olfactory bulb. D: Direct invasion to the Harderian glands. H & E stain.

One nasal squamous cell papilloma was observed in the 90 ppm male group and adenomas were observed in 5 males and 2 females exposed to 30 ppm and one female exposed to 10 ppm. The incidences of these tumors were not statistically significant, but not observed in historical control data of JBRC (0/1,849 male and 0/1,697 female).
As for the non-neoplastic nasal cavity lesions, various changes were observed (Table 4). The incidences of squamous cell hyperplasia with atypia in the area of respiratory epithelium (respiratory region) was increased in both males and females exposed to 90 ppm. Hyperplasia with atypia in respiratory epithelium was observed in one 30 ppm male. The incidence of transitional epithelial hyperplasia was increased in 30 ppm males and females. Hyperplasia in submucosal gland was observed in 3 males exposed to 90 ppm. The incidence of squamous cell metaplasia of the respiratory epithelium was increased in both males and females exposed to 30 ppm and above. Respiratory metaplasia and squamous cell metaplasia of olfactory epithelium were increased in both males and females exposed to 90 ppm. In addition, atrophy of the olfactory epithelium was increased in males exposed to 30 ppm and above and females exposed to 90 ppm. The incidences of the inflammation in the respiratory region were increased in both males and females exposed to 90 ppm.
In the lung, foreign body inflammation was observed in males and females exposed to 90 ppm. The incidences of keratitis in eye, increased hematopoiesis in bone marrow, was increased both in males and females exposed to 90 ppm (data not shown). There was no other exposure-related lesion in other organs.
Mouse Study Survival, body weight, food consumption and clinical observations and analysesAt the end of 2-year exposure period, the survival rates of the 0 (clean air control), 5, 15, 45 ppm groups were 70%, 71%, 64%, and 73% for male and 66%, 62%, 54%, and 44% for female (Table 6, Fig. 4. A, B). The significant decrease in survival rate of 45 ppm females was attributed to the increase in the lethal uterine tumors. The body weights of the 5, 15, and 45 ppm groups at the end of the 2-year exposure period compared with the control were 100%, 93%, and 76% for males and 108%, 107% and 89% for females (Table 6, Fig. 4. C, D). Food consumption was significantly decreased in the 45 ppm male and female groups (data not shown).


Survival (A and B) and growth curves (C and D) of male and female mice exposed by inhalation to BGE vapor for 2 years.
In hematological examination, hematocrit was significantly increased and white blood cell counts were significantly decreased in the males exposed to 45 ppm (Table 7, 8). In blood chemistry, albumin-globulin ratio and ALP levels were significantly increased and total-cholesterol and phospholipid levels were significantly decreased in the males exposed to 45 ppm. Albumin-globulin ratio, total-bilirubin, ALP, CK and potassium levels were significantly increased and, ALT and calcium levels were significantly decreased in the females exposed to 45 ppm.

 Histopathology
Histopathology
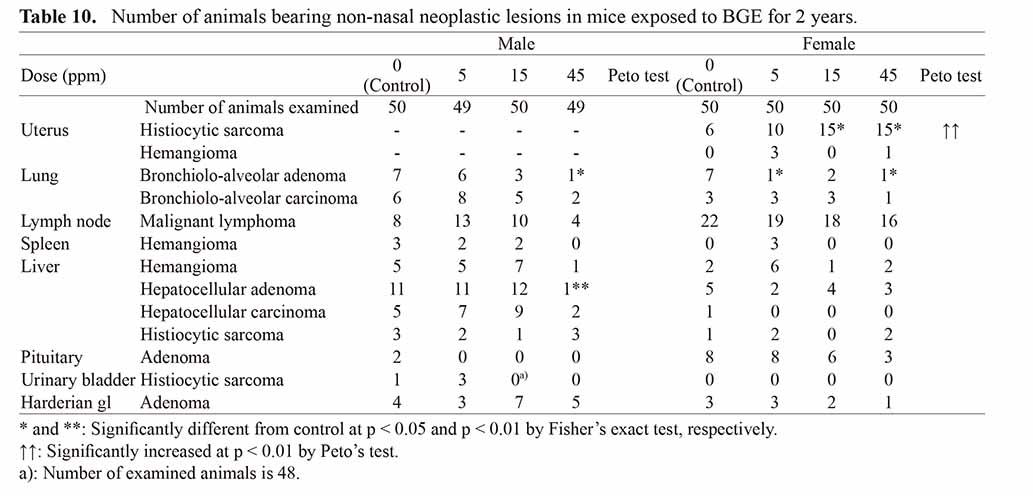
The incidences of nasal lesions in mice are summarized in Table 9. SCCs were observed in 2 males (101 weeks and terminal sacrifice) and 1 female (terminal sacrifice) in the 45 ppm groups. This type of tumor was also rare in the historical control data of JBRC in both sexes (0/1596). Histiocytic sarcomas of the uterus were increased in female mice exposed to 15 ppm and above, and significant by Peto’s test (Table 10, Fig. 5 A, B). Hemangiomas of the nasal cavity were increased in groups exposed to 15 ppm and above in male and 45 ppm in female, and were significantly positive by Peto’s test in both sexes. The nasal hemangioma is a rare tumor which was observed in no males (0/1596) and in one female (1/1596) in the JBRC historical control data. Hemangioma was found in the respiratory region of the anterior nasal cavity as a confined lobulated small mass up to 2 mm in diameter (Fig. 5. C, D). Histologically, a proliferation of slit-like to irregularly dilated vascular lumens lined by endothelial cells with minimal atypia accompanying various amount of hyalinous stroma was observed.


Uterine and nasal tumors in mouse exposed to 45 ppm butyl 2,3-epoxypropyl ether. A: Histiocytic sarcoma of the uterus. B: Higher magnification. C: Nasal hemangioma located at the respiratory region of the anterior nasal cavity (Level 1). D: Higher magnification. H & E stain.
Non-neoplastic lesions were observed mainly in the nasal cavity. The incidence of nodular hyperplasia of the transitional epithelium was increased in 45 ppm males and females. The incidence of respiratory metaplasia of the olfactory epithelium was increased in males exposed to 15 ppm and above and all exposed females, and the incidence of respiratory metaplasia of olfactory glands was increased in both male and female exposed to 15 ppm and above. Cuboidal change of the respiratory epithelium was increased in all exposed males and females exposed to 15 ppm and above. The incidence of angiectasis of the lamina propria of respiratory region was increased in males exposed to 15 ppm and above and females exposed to 45 ppm. The incidence of exudate in the posterior of the nasal cavity was increased in males and females exposed to 45 ppm. There was no other exposure-related lesion in other organs.
The 2-year whole body inhalation carcinogenicity study of BGE was conducted using rats and mice under Good Laboratory Practice. In rats, BGE significantly induced SCC of the nasal cavity at the highest dose in both sexes. All nasal cavity malignancy was also induced significantly at the highest dose in both sexes. As supporting evidence, squamous cell hyperplasia with atypia, metaplasia, and hyperplasia were increased in a dose-dependent manner from the middle dose.
In mice, SCC of the nasal cavity was induced in the highest dose group in both sexes in a small number and therefore was not statistically significant. However, SCC is a rare tumor type (0/1597 male and 0/1597 female mice in JBRC historical control) and therefore considered biologically significant in increase caused by BGE. As supporting evidence, hyperplasia and metaplasia of the nasal cavity mucosa were increased in a dose-dependent manner. In addition, although benign in nature, a statistically significant increase in the hemangiomas was observed in a dose-dependent manner, along with an increase in angiectasis in a dose-dependent manner. Outside of the nasal cavity, histiocytic sarcomas of the uterus were significantly increased in a dose-dependent manner by the incidences of 15/50 (30%) each in 15 and 45 ppm females and significant trend with p < 0.01 by Peto’s test. Although the nominal incidence was within the JBRC historical control range of 10%~32%, considering the lower survival in 15 ppm and 45 ppm groups, the increase in uterine histiocytic sarcomas was considered as a significant result of BGE treatment.
Figure 6, summarizes the outcome of carcinogenesis studies performed on glycidol and some glycidyl ethers, i.e. phenyl glycidyl ether (Lee et al., 1983), allyl glycidyl ether (NTP, 1990b; Renne et al., 1992) and diglycidyl ether (Kotin and Falk, 1963). Glycidol was positive in genotoxicity (Thompson et al., 1981; NTP 1990a; JETOC, 1996, 2005) and in 2-year rodent carcinogenesis assays (NTP 1990a, JBRC, 2003a, 2003b) with multiple target organs. Phenyl glycidyl was exposed to CD rats of male and female at concentrations of 1 or 12 ppm for 24 months, and nasal tumors, mostly SCCs (epidermoid carcinomas) were increased in 12 ppm exposed groups (Lee et al., 1983). Allyl glycidyl was exposed to Osborne-Mendel rats and B6C1F1 mice at concentrations of 5 or 10 ppm for 24-months. In male rats, SCC, poorly differentiated adenocarcinoma, and papillary adenoma of the nasal cavity were observed, and in male and female mice, papillary adenomas of the nasal cavity were observed (NTP, 1990b). Diglycidyl ether was repeatedly dosed (route was not specified) to C57 black mice at total doses of 0.25 or 0.75 mM. Skin tumors were observed in one of 20 in 0.25 mM, and 4 of 20 in 0.75 mM mice (Kotin and Falk, 1963). And the current study adds BGE to the series of carcinogenic glycidyl esters of which the mechanism is postulated as genotoxicity of the epoxide residue in the molecules, and a common target of nasal cavity when exposed by inhalation. Histiocytic sarcomas of the uterus of mice were induced by BGE in this study and in the glycidol inhalation study (JBRC, 2003b), and the carcinomas or adenocarcinomas of the uterus of mice were observed by glycidol gavage study (NTP, 1990a), supporting that some low molecular weight epoxy compounds might share extra-respiratory target as uterus as a common target.

Summary of the rodent carcinogenicity studies on glycidol and glycidyl ethers.
In the rat study, the body weight of the 90 ppm groups was about 20% less than the concurrent controls throughout the study and up to about 30% or more less at the terminal sacrifice. The increase in lethal nasal tumors after 70 weeks was responsible for the loss of body weight towards terminal sacrifice. The general status of the 90 ppm group rats without nasal tumor was within normal range.
In the mouse study, the body weight of 45 ppm males and females was about 30% and 20% less, respectively, than the concurrent control throughout the study. The general status of the mice was within normal range and the survival rate of 45 ppm male mice was not different. The lower survival rate in female mice was attributed to lethal uterine histiocytic sarcoma. As the general status of all dose groups was within normal range, and the low survival rates towards the terminal sacrifice seen in high dose groups were explained by the induction of lethal tumors, and the histopathological spectrum of the background non-neoplastic lesions, induced in a dose-dependent manner, were in line with the induced neoplastic lesions in higher dose groups, the authors consider that the present 2-year studies were conducted within the range of maximum tolerated dose (MTD) and, therefore present reliable data indicating that BGE is carcinogenic in two rodent species of both sexes by inhalation. This consideration is in accordance with the argument by Haseman (1985) that the recommendation regarding the 10% decrement in body weight is empirical and if exceeded in practice does not necessarily invalidate the 2-year carcinogenicity bioassay results.
In conclusion, the present 2-year inhalation studies demonstrated clear carcinogenicity of BGE in F344 rats and BDF1 mice of both sexes.
The present study was contracted and supported by the Ministry of Labour, Japan (the present Ministry of Health, Labour and Welfare).
Conflict of interestThe authors declare that there is no conflict of interest.