2020 Volume 45 Issue 10 Pages 651-660
2020 Volume 45 Issue 10 Pages 651-660
Inhalation of silica particles leads to pulmonary inflammatory responses. Clara cell protein 16 (CC16) has been reported to played a protective role in inflammatory lung diseases. However, its role on silica particles-induced inflammation has not been fully clarified. In this study, THP-1 macrophages were exposed to 75 μg/cm2 silica particles with or without 2 μg/mL exogenous CC16 (recombinant CC16, rCC16) for 24 hr. The production of inflammatory cytokines, including interleukin (IL)-1β, tumor necrosis factor (TNF)-α and IL-6, in the cell supernatants of different groups was detected through ELISA kits and real-time RT-PCR, respectively. The nuclear translocation of nuclear factor (NF)-κB, protein levels of pro-IL-1β, the nucleotide-binding domain-like receptor protein 3 (NLRP3) and caspase-1 were evaluated via immunofluorescence or western blot. Results showed that, at 75 μg/cm2 silica particle concentration, the treatment of rCC16 significantly decreased IL-1β, TNF-α and IL-6 protein release and mRNA levels in THP-1 macrophages. Compared to those only exposed to silica particles, THP-1 macrophages exposed to both silica particles and rCC16 showed significantly lower nuclear levels and higher cytosol levels of NF-κB p65, as well as lower co-localization coefficients through immunofluorescence. Additionally, the administration of rCC16 significantly attenuated the increase of pro-IL-1β, NLRP3 and caspase-1 levels induced by silica particle exposure. Our results suggested that exogenous CC16 could inhibit silica particles-induced inflammation in THP-1 macrophages, mainly through suppressing NF-κB pathway and caspase-1 activation.
Crystalline silica (silica) is a common component of the earth’s crust, widely existing in living and work environments. Inhalation of silica particles directly leads to pulmonary inflammatory responses. Long-term exposure to silica particles can cause silicosis, a fatal and progressive pulmonary fibrotic disease characterized by the early accumulation of inflammatory cells in respiratory bronchus, alveolus and blood vessels in the lung (Leung et al., 2012; Chen et al., 2012). Alveolar macrophage is the primary immune cell that recognizes and engulfs silica particles (Leung et al., 2012; Mossman and Churg, 1998). Macrophages activated by silica particles demonstrate enhanced pro-inflammatory cytokine production, including interleukin (IL)-1β, tumor necrosis factor-α (TNF-α) and IL-6, which play central roles in the inflammatory response and the accumulation of lung collagen (Piguet et al., 1990; Tripathi et al., 2010; Liu et al., 2020). In animal models, suppressing pro-inflammatory cytokine production effectively inhibited silica-induced inflammation, even the fibrotic responses (Di Giuseppe et al., 2009; Guo et al., 2013; Sato et al., 2008). Silica particles-induced inflammatory releases mainly rely on the activation of NALP3 (NACHT-, LRR- and PYD domains-containing protein 3) inflammasome which appears to occur in two steps (Mezzasoma et al., 2017; Dostert et al., 2008; Leung et al., 2012; Barker et al., 2011; Bryant and Fitzgerald, 2009). The priming step mainly involves the activation of nuclear factor (NF)-κB pathway, which in return upregulates the transcription of NALP3 inflammasome related components including inactive nucleotide-binding domain-like receptor protein 3 (NLRP3) and pro-IL-1β (Porter et al., 2002).The second step is the subsequent assembly of NLRP3, apoptosis-associated speck-like protein (ASC) and pro-caspase-1 to NALP3 inflammasome and subsequent caspase-1 activate fragment production, which promotes the mature and secretion of IL-1β. Therefore, inhibition of cytokine releases via suppressing the NF-κB and caspase-1 activation might be a potential therapeutic strategy for silicosis.
Clara cell protein-16 (CC16) is a 16-17 kDa protein secreted by Clara cells in the bronchiolar lining fluid of the lung (Halatek et al., 1998). Epidemiology studies found that serum CC16 level was negatively associated with lung function of patients with respiratory diseases, such as chronic obstructive pulmonary disease (COPD) and asthma (Tsoumakidou et al., 2010; Laucho-Contreras et al., 2016; Emmanouil et al., 2015). In a murine model of COPD, exogenous recombinant CC16 (rCC16) was found to have therapeutic effects by downregulating pro-inflammatory factors via the NF-κB pathway, including the production of TNF-α, IL-6 and IL-8 (Pang et al., 2018). Another in vitro study also found that rCC16 inhibited lipopolysaccharide (LPS)-induced cytokine production in a concentration-dependent manner via inhibiting the nuclear translocation of NF-κB (Pang et al., 2017). In recent years, significant reduction of serum CC16 in workers exposed to silica has been reported, suggesting that Clara cells might be damaged or reduced in number following chronic exposure to silica particles (Broeckaert et al., 2000; Bernard et al., 1994; Liu et al., 2019a, 2019b). However, the effects of exogenous rCC16 on silica particles-induced inflammatory effects have not been investigated in vitro.
The aims of the present study were to test the hypothesis that exogenous CC16 could suppress silica particles-induced inflammation and to identify the underlying mechanisms via an in vitro human macrophage model. The effects of exogenous CC16 on silica particles-induced cytotoxicity, production of pro-inflammatory cytokines, NF-κB nuclear translocation and caspase-1 activation in THP-1 macrophages were all evaluated.
The macrophage model was established by incubating THP-1 cells, a human monocyte cell line which can be differentiated into macrophages by phorbol 12-myristate 13-acetate (PMA). THP-1 cells were purchased from the Cell Bank of Type Culture Collection of Chinese Academy of Science (Shanghai, China). Cells were cultured as regularly reported (Cui et al., 2019). Cultured THP-1 cells were seeded in flat-bottom 96-well, 6-well plates, or in 4-well chamber slides chamber sliders (Fig. 1 A), and were differentiated into a mature macrophage-like state by incubation with 10 ng/mL PMA for 48 hr (Daigneault et al., 2010). After incubation, the adherent cells were cultured in serum-free medium overnight, followed by adding silica particles suspensions with/without rCC16 for 24 hr. We chose to add rCC16 at the same time as, but not 2-24 hr before, the addition of silica particles, because this adding time produced stronger anti-inflammatory effects through our preliminary experiments (data not shown). Figure 1A shows the workflow of the entire experiment.

Workflow of the entire experiment and the cell viability and cytotoxicity assessment of the dose escalation experiment. THP-1 macrophages were incubated with different concentrations of silica particles (0-100 μg/cm2) with or without rCC16 (2 μg/mL) in 96-well plates for 24 hr (n = 6). (A) Workflow of the entire experiment. (B) Influence of different concentrations of silica particles with or without rCC16 on the viability of THP-1 macrophages by CCK8 test. (C) Influence of different concentrations of silica particles with or without rCC16 on the leakage of LDH from THP-1 macrophages. Results are expressed as mean ± SD for six independent experiments. *P < 0.05 vs. cells at 0 μg/cm2 silica particle suspension and 0 μg/mL rCC16; **P < 0.01 vs. cells at 0 μg/cm2 silica particle suspension and 0 μg/mL rCC16; † P < 0.05 vs. cells at 0 μg/cm2 silica particle suspension and 2 μg/mL rCC16; †† P < 0.01 vs. cells at 0 μg/cm2 silica particle suspension and 2 μg/mL rCC16.
To examine the cytotoxic effect of silica particles and define an appropriate dose for achieving approximately 75% control cell viability, we conducted a dose escalation experiment (Henderson et al., 1998). THP-1 macrophages were incubated in 96-well plates (1.0 × 105 cells/well of 1 mL medium) and then treated with increasing doses of silica particles (0, 25, 50, 75 and 100 μg /cm2) with or without rCC16 (2 μg/mL) for 24 hr. The silica particles were purchased from US Silica Company (MIN-U-SIL 5; Mill Creek, OK, USA) with an average particle diameter of 1.40 μm and a SiO2 content of 99.30%. The exogenous CC16 (rCC16), was purchased from ProSpec company (CYT-746, Rehovot, Israel) and its concentration was defined as 2 μg/mL according to previous in vitro studies (Pang et al., 2017, 2015).
Cell viability was detected using the Cell Counting Kit-8 (CCK-8; Dojindo Laboratories, Tokyo, Japan) assay in accordance with the manufacturer’s instructions. Cytotoxicity was assessed by lactate dehydrogenase (LDH) leakage from damaged cells using a LDH assay kit (Jiancheng Bio-Engineering, Nanjing, China).
Treatment of THP-1 macrophages with silica particles and/or rCC16According to results of the dose escalation experiment, the dose of 75 μg/cm2 silica particles (corresponding to approximately 75% control cell viability) was selected for the rCC16 interference experiment. THP-1 macrophages seeded in the 6-well plates (2.5 × 106 cells/well of 2.5 mL medium) or chamber slides (1 × 104 cells) were incubated for 24 hr with culture medium (blank), culture medium containing 2.0 μg/mL rCC16, culture medium containing 75 μg/cm2 silica particles, or culture medium containing 75 μg/cm2 silica particles and 2.0 μg/mL rCC16, respectively. Cells and cell-free supernatants were taken for further examination.
Measurement of IL-1β, TNF-α and IL-6 by ELISAIL-1β, TNF-α and IL-6 in the cell-free supernatants were detected by commercially available ELISA kits (Xinbosheng Biological Technology, Shenzhen, China) according to the manufacturer’s instructions.
Gene expression profiling by real-time RT-PCRTotal RNA was isolated from THP-1 macrophages seed in 6-well plates after 24 hr of treatment using Trizol reagent (Invitrogen, Carlsbad, CA, USA). For the synthesis of cDNA, 2.0 ug of total RNA was reversely transcribed using a ReverTra Ace qPCR RT Kit (Toyobo Co, Osaka, Japan) following the supplier’s instructions. Real-time RT-PCR was performed using the SYBR Green Realtime PCR Master Mix (Toyobo Co.) and analyzed through an ABI Prism 7900 real-time cycler (Applied Biosystems, Foster City, CA, USA). The expression levels of target genes were standardized to GAPDH. Primers are listed below: IL-1β, 5’-GGCTTATTACAGTGGCAATGAGG-3’ and 5’-GTAGTGGTGGTCGGAGATTCGT-3’; TNF- α, 5’-TCTACTCCCAGGTCCTCTTCAAG-3’ and 5’- GGAAGACCCCTCCCAGATAGA-3’; IL-6, 5’- GCCACTCACCTCTTCAGAACGA-3’ and 5’-CAGTGCCTCTTTGCTGCTTTC-3’; GAPDH, 5’-ACTTTGGTATCGTGGAAGGACTCAT-3’, and 5’-GTTTTTCTAGACGGCAGGTCAGG-3’. All PCRs were performed in duplicate and the quantification of the transcripts was performed by the 2-ΔΔCT method.
Immunofluorescence assay for nuclear translocation of NF-κB p65THP-1 cells were seeded on 4-well chamber slides (Lab-Tek, Nunc, Roskilde, Denmark) and stimulated to differentiate for 48 hr with PMA (Fig. 1A). After the exposure treatment, THP-1 macrophages were then fixed in 4% paraformaldehyde for 20 min at room temperature, washed three times with phosphate-buffered saline (PBS), and then exposed to the blocking solution (PBS containing 5% phosphate-buffered saline) for 30 min at 37°C. Then the slides were incubated with rabbit anti-NF-κB p65 polyclonal antibody (Servicebio, Wuhan, China) at 4°C overnight, washed three times with PBS, and incubated with CY3 labeled goat anti-rabbit IgG (Servicebio) in darkness at temperature for 1.5 hr. Slides were then washed three times with PBS, stained with 4,6-diamidino-2-phenylindole (DAPI, 0.2 μg/mL; Sigma, St Louis, MO, USA). The fluorescent images were taken using an AX70 widefield microscope (Olympus, Tokyo, Japan), and all morphometric measurements were observed by at least three independent individuals in a blinded manner. Red fluorescence indicated NF-κB p65 and blue fluorescence indicated cell nucleus; red and blue overlapping in the cells represented NF-κB nuclear translocation. To analyze the extent of the nuclear translocation of the NF-κB p65, the co-localization of NF-κB p65 with nuclear was analyzed by Image J, and the co-localization coefficient (for NF-κB p65 co-localization in the nuclei) was evaluated by Pearson’s correlation coefficient (Chkourko et al., 2012).
Protein extraction and western blot analysisTHP-1 cells were seeded in 6-well plates, induced to a mature macrophage-like state by PMA. After treated with silica particles and/or rCC16, the macrophages were washed by PBS and then scraped into 1.5-mL EP tubes. Total cell lysates, cytosolic or nuclear extracts were separated by sodium dodecyl sulfate-polyacrylamide gels (SDS–PAGE) and then transferred onto polyvinylidene difluoride (PVDF) membranes. Nuclear and cytosolic extracts was extracted with a Nuclear and Cytoplasmic Protein Extraction kit (P0028, Beyotime Biotechnology, Shanghai, China) in accordance with the manufacturer’s instructions. After blockade with 5% BSA, PVDF membranes were incubated overnight at 4°C with corresponding primary antibodies and followed by incubation with appropriate secondary antibodies. The primary antibodies used in this study included: rabbit anti-NF-κB p65, rabbit anti-NLRP3 (both from Cell Signaling Technology, Danvers, MA, USA), mouse anti-IL-1β (R&D Systems, Minneapolis, MN, USA), rabbit anti-caspase-1 p10, rabbit anti-β-actin and rabbit anti-Histone H3 antibodies (all from Santa Cruz Biotechnology, Santa Cruz, CA, USA). Secondary antibodies were horseradish peroxidase-conjugated anti-mouse and Santi-rabbit IgG (both from Santa Cruz Biotechnology).
Statistical analysisAll values were presented as mean ± standard deviation (SD). Multiple group comparisons were performed using Tukey HSD (Tukey high significant difference) one-way ANOVA test. Statistical analyses were performed using R version 4.0.0. All tests were two sided and P < 0.05 was considered statistically significant.
Assessing of the effects of different silica particle concentrations on the survival of THP-1 macrophages through CCK8 test, we found that the concentration of 25 μg/cm2 did not significantly affect the survival of THP-1 macrophages (Fig. 1B). Higher silica particle concentrations caused a gradual decrease in cell viability, either in the presence or absence of rCC16 (2 μg/mL). At a silica particle concentration of 75 μg/cm2 (with or without the presence of rCC16), the cell viabilities decreased to about 75% of the control. No significant difference was observed between cells at the same silica particle concentration with or without rCC16 (P > 0.05). With the cell viabilities approximating 75%, the concentration of 75 µg/cm2 of silica particles was chosen for further experiment (Henderson et al., 1998; Fotakis and Timbrell, 2006).
Exogenous rCC16 suppresses silica-induced pro-inflammatory cytokine production in THP-1 macrophagesTo investigate whether rCC16 regulates silica-induced inflammatory effects, we assessed three key pro-inflammatory cytokines in THP-1 macrophages: IL-1β, TNF-α and IL-6 (Fig. 2). Compared to the blank groups, THP-1 macrophages incubated with 75 μg/cm2 silica particles produced significant higher IL-1β, TNF-α and IL-6 protein release and mRNA levels. However, significant decreases in both protein release and mRNA levels of IL-1β, TNF-α and IL-6 were observed in cells exposed to silica particles with rCC16 (P < 0.01 or P < 0.05). No obvious significance was found between cells of the blank groups, with or without rCC16 (P > 0.05).

Exogenous rCC16 reduces pro-inflammatory cytokine production from THP-1 macrophages. THP-1 macrophages were incubated with culture medium (blank) or silica particle suspension (silica, 75 μg/cm2) with or without rCC16 (2 μg/mL) for 24 hr. (A-C) Concentrations of IL-1β, TNF-α and IL-6 in the culture supernatant of THP-1 macrophages detected by ELISA kits. (D-F) Relative mRNA levels of IL-1β, TNF-α and IL-6 in THP-1 macrophages detected by real-time RT-PCR. Results are expressed as mean ± SD of six independent experiments. ** P < 0.01;* P < 0.05.
The nuclear translocation of the NF-κB p65 subunit in THP-1 macrophages, a marker of NF-κB pathway activation, was determined by western blot and immunofluorescence staining (Fig. 3 and 4).
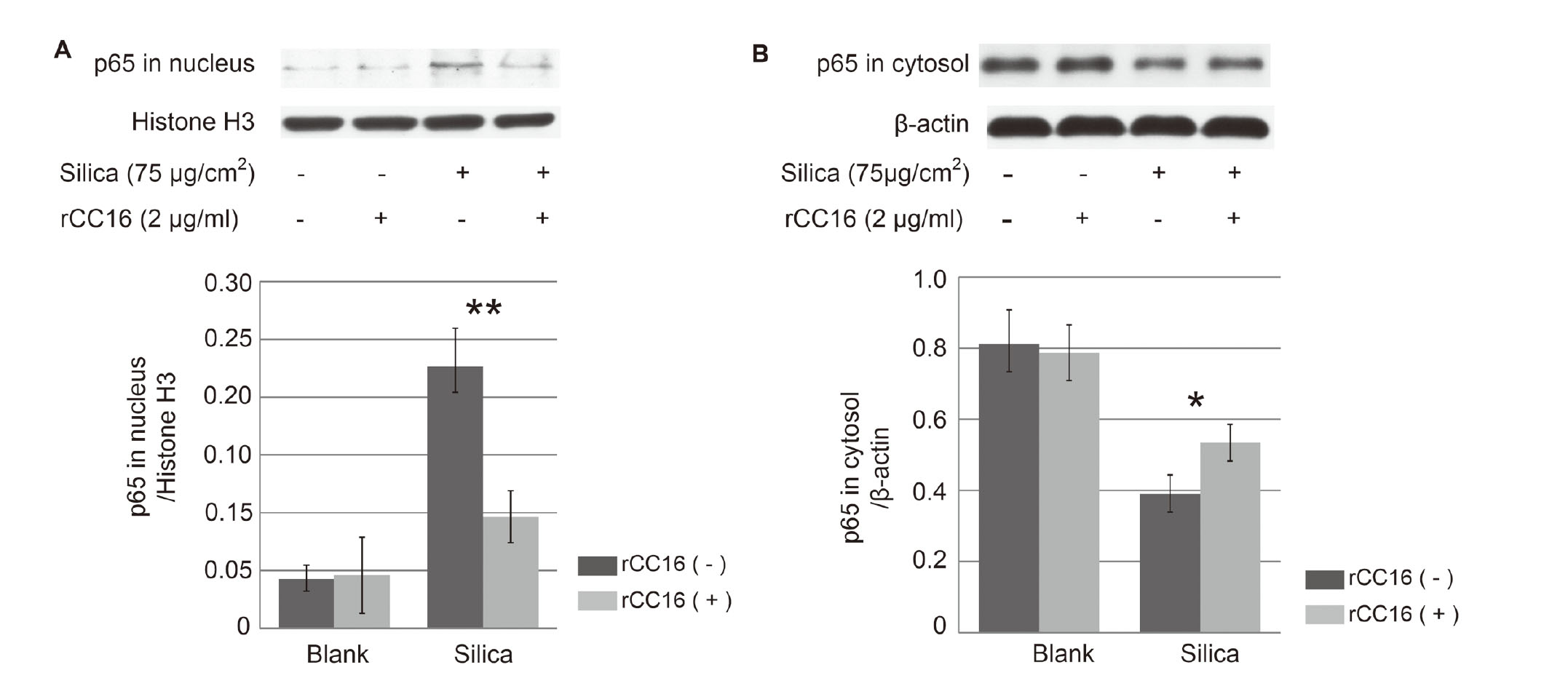
Exogenous rCC16 suppresses silica-induced NF-κB nuclear translocation in THP-1 macrophages. THP-1 macrophages were incubated with culture medium (blank) or silica particle suspension (silica, 75 μg/cm2) with or without rCC16 (2 μg/mL) for 24 hr. Nuclear and cytosolic extracts were separated and detected for NF-κB p65 by western blot. The intensity of the bands was quantified by densitometric analysis through Image J. (A) Representative western blot and corresponding densitometric analyses (normalized with Histone H3, a nuclear marker) of NF-κB p65 in nucleus. (B) Representative western blot and corresponding densitometric analyses of NF-κB p65 (normalized with β-actin) in cytosol. Results are expressed as mean ± SD of three independent experiments. * P < 0.05; ** P < 0.01.

Location of NF-κB p65 in THP-1 macrophages of different groups by immunofluorescence staining. THP-1 macrophages were incubated with culture medium (blank) or silica particle suspension (silica, 75 μg/cm2) with or without rCC16 (2 μg/mL) for 24 hr. (A) Representative images from different groups. NF-κB p65 (red) and nuclear (blue) are presented separately and in merged images (including enlarged images). Scale bars, 50 μm. (B) Co-localization coefficients (for NF-κB p65 co-localization in the nuclei) in THP-1 macrophages. Co-localization was analyzed by Image J, and the co-localization coefficients were represented by Pearson’s correlation coefficient. Results are expressed as mean ± SD of three independent experiments. * P < 0.05.
The western blot results showed that nuclear translocation of NF-κB p65 occurred in THP-1 macrophages after exposure to silica particles, as shown by higher levels of p65 in nuclear and lower levels of p65 in cytosol (Fig. 3). Compared with those only treated with silica particles, THP-1 macrophages treated with both silica particles and rCC16 had lower levels of p65 in the nucleus and higher levels of p65 in the cytosol (P < 0.01 or P < 0.05). These results confirmed that the addition of rCC16 significantly impaired the nuclear translocation of NF-κB p65 induced by silica particles. No significant differences were observed between cells of blank group (with or without rCC16).
To further confirm the results, we performed immunofluorescent analysis to measure NF-κB nuclear localization (Fig. 4). The staining macrophages showed that silica particle exposure significantly induced the nuclear accumulation of NF-κB p65. Silica particle exposure stimulated a 2-fold increase in NF-κB nuclear localization in THP-1 macrophages, with higher co-localization coefficients. However, the addition of rCC16 significantly decreased the co-localization extent induced by silica particles (Fig. 4b, P < 0.05). No significant differences were observed between cells of blank group (with or without rCC16).
Effects of rCC16 on silica-induced NALP3 inflammasome activation and pro-IL-1β productionTo further assess the effects of rCC16 on downstream signaling of NF-κB, we measured the expression of pro-IL-1β, NLRP3 and caspase-1 in THP-1 macrophages through western blot (Fig. 5). The results showed that silica particles significantly increased the levels of pro-IL-1β, NLRP3 and caspase-1, while the administration of rCC16 significantly suppressed these increases (P < 0.05).

Effects of rCC16 on silica-induced NALP3 inflammasome activation and pro-IL-1β production. THP-1 macrophages were incubated with culture medium (blank) or silica particle suspension (silica, 75 μg/cm2) with or without rCC16 (2 μg/mL) for 24 hr. Total cell lysates were separated and detected by western blot. The intensity of the bands was quantified by densitometric analysis through Image J. (A) Representative western blot of pro-IL-1β, NLRP3 and caspase-1 of THP-1 macrophages. (B-D) Densitometric analyses of pro-IL-1β, NLRP3 and caspase-1. Results are expressed as mean ± SD of three independent experiments. * P < 0.05.
Previous studies identified the anti-inflammatory function of exogenous rCC16 in LPS or cigarette smoke-induced inflammatory effects in mice or in vitro cells (Pang et al., 2017, 2018; Zhou et al., 2019). In this study, we confirmed that exogenous CC16 inhibited silica particles-induced inflammatory responses in THP-1 macrophages, mechanistically via suppressing the activation of NF-κB pathway and caspase-1 (Fig. 6).

Underlying mechanisms of rCC16 attenuating silica-induced pro-inflammatory cytokine production in macrophages.
Significant reduction of serum CC16 in workers exposed to crystalline silica has been reported previously, suggesting close relationship between CC16 and silica-induced inflammatory responses (Bernard et al., 1994; Liu et al., 2019a, 2019b; Broeckaert et al., 2000). However, the effects of exogenous CC16 on silica-induced inflammatory responses have not been fully investigated. In this study, we found a dose-response relationship between silica particles concentrations and THP-1 macrophage activity, as well as the release of LDH. Extensive evidence showed that the phagocytosis of crystalline silica could initiate apoptotic and necrotic death of macrophages. The process might be related to the lysosomal destabilization and subsequent rupture releasing proteolytic enzymes caused by silica particles (Hornung et al., 2008a; Persson, 2005). In this study, we confirmed that the addition of rCC16 significantly suppressed silica particles-induced IL-1β, TNF-α and IL-6 production in THP-1 macrophages (in both protein release and mRNA levels). These was in coincidence with our previous in vivo study, in which rCC16 were applied to a silica particle exposure murine model and reduced IL-1β and TNF-α expression levels were found in the bronchoalveolar lavage fluids (Ma et al., 2017). Other in vitro or in vivo studies partly supported our results, in which rCC16 were proved to have anti-inflammatory effects on LPS or cigarette smoke-induced inflammatory responses (Pang et al., 2018, 2017). Therefore, we confirmed the negatively regulative role of exogenous rCC16 on silica particles-induced inflammation in THP-1 macrophages. It is also interesting to explore the potential mechanisms in further.
NF-κB plays a central role in inflammatory responses in macrophages. After silica particles exposure, NF-κB can transfer to nucleus, bind to specific sequences in promoter regions of target genes (e.g. IL-1β, IL-6, TNF-α and NLRP3), and induce their transcription (Brasier, 2006; Di Giuseppe et al., 2009; Porter et al., 2002). Inhibiting the activity of NF-κB could significantly suppress the production of both cytokine precursors and NLRP3 (Mezzasoma et al., 2017). In macrophages, the levels of cytokine precursors (e.g. pro-IL-1β) largely affected the production of inflammatory cytokines, and the synthesis of NLRP3 finally affected the NALP3 inflammasome levels. Furthermore, toll-like receptor (TLR4), a key upstream factor regulating the nuclear translocation of NF-κB, was reported to be negatively affected by the expression of CC16 in macrophages (Snyder et al., 2010). Thus, we hypothesized that rCC16 mediates anti-inflammatory effects through its suppression of NF-κB activation. Our results showed that rCC16 treatment significantly suppressed NF-κB nuclear translocation caused by silica particles exposure, followed by the decreased mRNA levels of pro-inflammatory cytokines (IL-1β, TNF-α and IL-6). Moreover, both the levels of pro-IL-1β and NLRP3 were significantly decreased after the administration of rCC16. Therefore, we inferred that rCC16 suppressed silica particles-induced inflammation via NF-κB inhibition (Fig. 6).
We also observed the effects of exogenous CC16 on silica particles-induced caspase-1 activation, a crucial protein promoting cytokine precursor mature and secretion in macrophages after silica particle exposure (Franchi et al., 2009). After the assembly of NALP3 inflammasome by NLRP3, ASC and pro-caspase-1, pro-caspase-1 dimerisation can be self-cleaved to become active caspase-1 and further turn the cytokine precursors into their mature forms (Martinon et al., 2002; Hornung et al., 2008b). In this study, the cleaved caspase-1 levels were found increased after silica particle exposure, and then significantly decreased after the administration of rCC16. These results suggested us that rCC16 might suppress the mature and release of cytokines through affecting caspase-1 levels. The decreased NLRP3 levels induced by suppressed NF-κB pathway might be in responsible for the down-regulation of caspase-1 levels. Previous study conducted by Zhou et al. (2019) was in coincidence with our results, in which both NLRP3 and caspase-1 levels were decreased after the administration of rCC16 in septic rat models. It was considered that the p38 MAPK and ERK signaling pathways might be involved in the process of rCC16 suppressing caspase-1 activation (Zhou et al., 2019), though investigations need to be conducted.
In summary, we identify exogenous CC16 as a negative regulator of silica particles-induced inflammation in THP-1 macrophages. Exogenous CC16 exerts anti-inflammatory effects by down-regulating NF-κB and caspase-1 activation, with the suppression of NLRP3 and pro-IL-1β. These findings provided evidence that exogenous CC16 has the potential to be an inflammatory inhibitor for the prevention or treatment of silica particles-induced inflammatory diseases.
This work was supported by the National Natural Science Foundation of China [grant numbers: 81402659, 81903292] and the Natural Science Foundation of Hubei Province [grant numbers 2016CFB520 and 2017CFB330].
Conflict of interestThe authors declare that there is no conflict of interest.