2020 Volume 45 Issue 11 Pages 673-680
2020 Volume 45 Issue 11 Pages 673-680
The epidermal growth factor receptor tyrosine kinase inhibitors (EGFR TKIs) have been approved for non-small cell lung cancer. Although EGFR TKIs are less toxic than traditional cytotoxic therapies, they cause many severe idiosyncratic drug reactions. Reactive metabolites can cause cellular damage with the release of danger-associated molecular patterns (DAMPs), which is thought to be involved in immune activation. Inflammasomes can be activated by DAMPs, and this may be a common mechanism by which DAMPs initiate an immune response. We tested the ability of afatinib, dacomitinib, erlotinib, gefitinib, and osimertinib to induce the release of DAMPs that activate inflammasomes. Human hepatocarcinoma functional liver cell-4 (FLC-4) cells were used for bioactivation of drugs, and the detection of inflammasome activation was performed with the human macrophage cell line, THP-1 cells. Gefitinib is known to be oxidized to a reactive iminoquinone metabolite. We found that the supernatant from the incubation of gefitinib with FLC-4 cells for 7 days led to increased caspase-1 activity and production of IL-1ß by THP-1 cells. In the supernatant of FLC-4 cells with gefitinib, the heat shock protein (HSP) 40, 70 and 90 were significantly increased. In addition, activated THP-1 cells secreted high mobility group box 1 (HMGB1) protein. These results support the hypothesis that the reactive iminoquinone metabolite can cause the release of DAMPs from hepatocytes, which in turn, can activate inflammasomes. Inflammasome activation may be an important step in the activation of the immune system by gefitinib, which in some patients, can cause immune-related adverse events.
Recently, many molecular-targeting drugs have been developed for the treatment of cancer. Although they demonstrate lower degrees of toxicity than traditional cytotoxic drugs, severe adverse events, including immune-related adverse events, have been reported (Li et al., 2009). Selective and multiple kinase inhibitors are being actively researched and developed as chemotherapeutic agents because they target processes involved in signal transduction cascades related to tumor growth and maintenance (Grünwald et al., 2009; Müller, 2009). The epidermal growth factor receptor tyrosine kinase inhibitors (EGFR TKIs) have been approved for non-small cell lung cancer. Although EGFR TKIs are believed to be safer than traditional cytotoxic therapies, they have been associated with many severe idiosyncratic drug reactions (IDRs). Among EGFR TKIs, gefitinib was the first EGFR TKI and caused many IDRs such as interstitial lung disease (ILD, Cohen et al., 2003; Ieki et al., 2003; Inomata et al., 2004; Takano et al., 2004; Kitajima et al., 2006), skin rash (Pascual et al., 2005; Dudek et al., 2006) and liver injury (Yoshimoto et al., 2004; Carlini et al., 2006; Bunchorntavakul and Reddy, 2017). These severe IDRs are reported to be immune related.
There is a large amount of circumstantial evidence that many, if not most, IDRs are caused by reactive metabolites (Cho and Uetrecht, 2017). However, many drugs that form reactive metabolites are not associated with a high risk of IDRs (Cho and Uetrecht, 2017), which begs the question of what separates reactive metabolites that cause IDRs from those that do not. Virtually all reactive metabolites can act as haptens, but the danger hypothesis suggests that an agent must also cause some type of cell stress or damage in order to induce an immune response (Matzinger, 1994). This damage causes the release of danger-associated molecular pattern molecules (DAMPs) that can activate antigen-presenting cells. DAMPs are also known to activate inflammasomes, which may be a common pathway by which drugs or reactive metabolites can induce an immune response (Cho and Uetrecht, 2017). We have already demonstrated that several drugs that are associated with a relatively high incidence of IDRs activate inflammasomes (Kato and Uetrecht, 2017; Mak et al., 2018; Weston and Uetrecht, 2014). This may be a common theme that would make it possible to screen drug candidates for their ability to cause IDRs (Cho and Uetrecht, 2017). In addition, inhibition of EGFR leads to a proinflammatory environment involving activated macrophages, which may further increase the risk that they will cause IDRs (Mascia et al., 2013).
In this study, we tested the ability of the culture supernatant from functional liver cell (FLC)-4 cells (a human hepatocyte cell line with high drug metabolizing capacity) incubated with afatinib, dacomitinib, erlotinib, gefitinib and osimertinib to activate differentiated THP-1 cells, a human macrophage cell line.
Afatinib, dacomitinib and gefitinib was obtained from Tokyo Chemical Industry Co., Ltd (Tokyo, Japan). Erlotinib and osimertinib were obtained from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan) and Toronto Research Chemicals (Tronto, ON, Canada), respectively. 1-Aminobenzotriazole (ABT) and Ac-YVAD-cmk (YVAD) were obtained from Tokyo Chemical Industry Co., Ltd. and Promega Corporation (Madison, WI, USA), respectively. Other reagents and solvents were commercially available extra-pure grade chemicals.
Cell cultureFLC-4 cells [previous names, JHH-4 (JCRB0435, Hasumura et al., 1988)] were obtained from Health Science Research Resources Bank (Osaka, Japan). THP-1 cells (JCRB0112) were obtained from Japanese Collection of Research Bioresources Cell Bank (Osaka, Japan). FLC-4 cells (passage 20-60) were grown and maintained in Dulbecco’s Modified Eagle Medium (DMEM, 4.5 g glucose/L, Nacalai Tesque, Inc., Kyoto, Japan) and THP-1 cells (passage 20-60) were grown and maintained in Roswell Park Memorial Institute Medium (RPMI, Nacalai Tesque, Inc.), containing 10% fetal bovine serum (BioSource International Inc., Camarillo, CA, USA) at 37ºC with 5% CO2 in 25-cm2 tissue culture flasks (Becton, Dickinson and Company, Franklin Lakes, NJ, USA).
A FLC-4 cell suspension (100 µL, 1.5 × 104 cells/mL) and the same volume of medium were applied to a Prime Surface 96U plate (Sumitomo Bakelite Co., Ltd., Tokyo, Japan) for 3D culture. The cells in the 96U plate were cultured at 37°C with 5% CO2 in the presence of drugs and/or inhibitor for 7 days.
THP-1 cells (4 × 105 cells/mL) were differentiated in medium containing phorbol 12-myristate 13-acetate (50 ng/mL, Merck KGaA, Darmstadt, Germany) for three days in a 24-well multiplate. On the fourth day, each well was washed with Ca2+ and Mg2+ free phosphate-buffered saline, medium (500 µL) was added to each well, and the cells were incubated at 37°C with 5% CO2 for 24 hr. Then the medium was aspirated, and culture medium including drugs and/or inhibitors was added and incubated at 37°C with 5% CO2 for 24 hr.
The drug concentrations used in this study were similar to their therapeutic concentrations (afatinib, 0.03-0.3 μM; dacomitinib, 0.03-0.3 μM; erlotinib, 1-10 μM; gefitinib, 0.3-3 μM; osimertinib, 0.3-3 μM) (Takahashi et al., 2012; Kobayashi et al., 2016; Planchard et al., 2016; Wind et al., 2017; Endo-Tsukude et al., 2018). ABT (1 mM) was used to inhibit cytochrome P450 activities (Sharma et al., 2013; Parrish et al., 2016), and YVAD (1 μM) was used to inhibit caspase-1 activity.
Western blottingSupernatants from FLC-4 cells incubated with gefitinib or from THP-1 cells incubated with the supernatants from FLC-4 cells incubated with gefitinib (20 μL) were loaded onto a 12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel. The resolved proteins were then electro-transferred onto a polyvinylidene difluoride membrane (0.45 µm, Merck KGaA). High mobility group box (HMGB) 1 rabbit polyclonal antibody (10829-1-AP, Proteintech Group, Inc., Rosemont, IL, USA), HSP32 rabbit polyclonal antibody (10701-1-AP, Proteintech Group, Inc.), HSP40 rabbit polyclonal antibody (13174-1-AP, Proteintech Group, Inc.), HSP60 rabbit polyclonal antibody (15282-1-AP, Proteintech Group, Inc.), HSP70 rabbit polyclonal antibody (10995-1-AP, Proteintech Group, Inc.), HSP90 rabbit polyclonal antibody (13171-1-AP, Proteintech Group, Inc.), S100 calcium-binding protein (S100) A8 rabbit polyclonal antibody (GTX54721, GeneTex, Inc., Irvine, CA, USA) and S100A9 rabbit polyclonal antibody (GTX129575, GeneTex, Inc.) were used as the primary antibody and were detected by goat anti-rabbit IgG-peroxidase (Santa Cruz Biotechnology, Dallas, TX, USA). Bound peroxidase was visualized by using Luminata™ Classico Western HRP Substrates (Merck KGaA).
Measurement of IL-1β concentration in culture mediumThe culture medium from differentiated THP-1 cells was collected and stored at -80ºC until analysis. IL-1β was measured in each culture medium sample using an ELISA kit (BioLegend, Inc., San Diego, CA, USA).
Caspase-1 activity of differentiated THP-1 cellsDifferentiated THP-1 cells (4 × 105 cells/mL) were cultured with FLC-4 spheroid culture medium for 24 hr in a 24-well plate. Caspase-1 activity was measured using the Caspase-Glo® 1 Inflammasome Assay (Promega Corporation). Caspase-Glo reagent was added to each well and then incubated for 1 hr at room temperature. Supernatant from the THP-1 cells (200 µL) was transferred to a 96-well white plate and the luminescence was measured with a plate reader.
Data analysisResults are expressed as means ± SD. Statistical analyses were performed using the Tukey multiple comparison tests (StatMate III, ATMS Co., Ltd., Tokyo, Japan). P < 0.05 was considered statistically significant.
Incubation of THP-1 cells with afatinib, dacomitinib, erlotinib, gefitinib and osimertinib did not lead to an increase in IL-1ß production (Fig. 1).
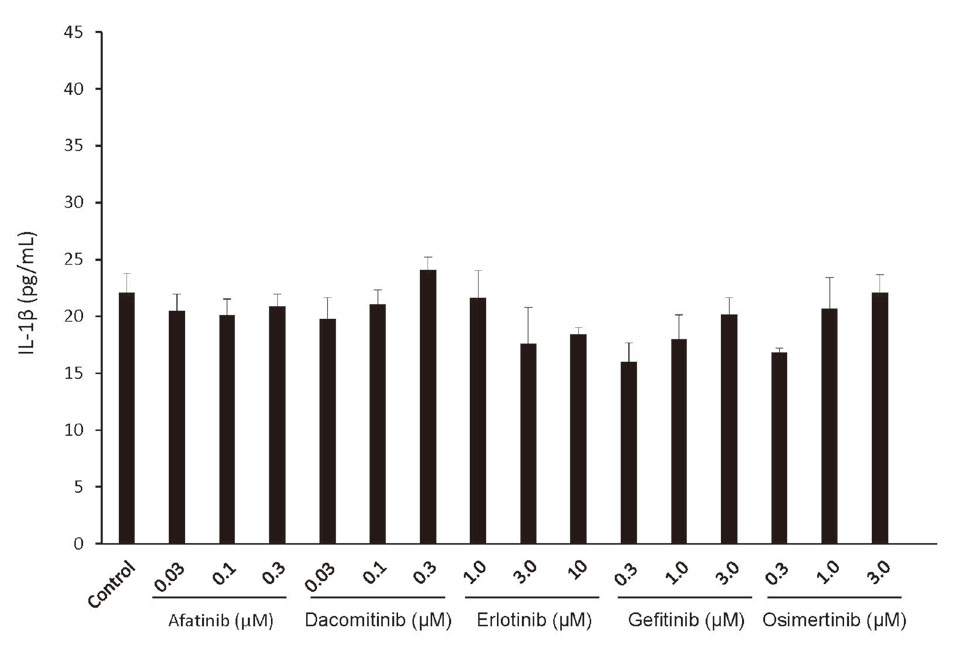
Incubation of THP-1 cells with afatinib, dacomitinib, erlotinib, gefitinib, and osimertinib did not lead to release of IL-1ß. Levels of IL-1β secreted by THP-1-derived macrophages incubated for 24 hr with afatinib, dacomitinib, erlotinib, gefitinib, and osimertinib itself, n = 3.
Incubation of THP-1 cells with the supernatant from an incubation of FLC-4 cells with gefitinib led to an increase in IL-1ß production, and the production of IL-1ß was inhibited by adding the cytochrome P450 inhibitor ABT to the FLC-4 cell culture or caspase-1 inhibitor YVAD to THP-1 cells (Fig. 2). However, incubation with the supernatants from FLC-4 cells with afatinib, dacomitinib, erlotinib and osimertinib did not lead to an increase in the production of IL-1ß (Fig. 2).
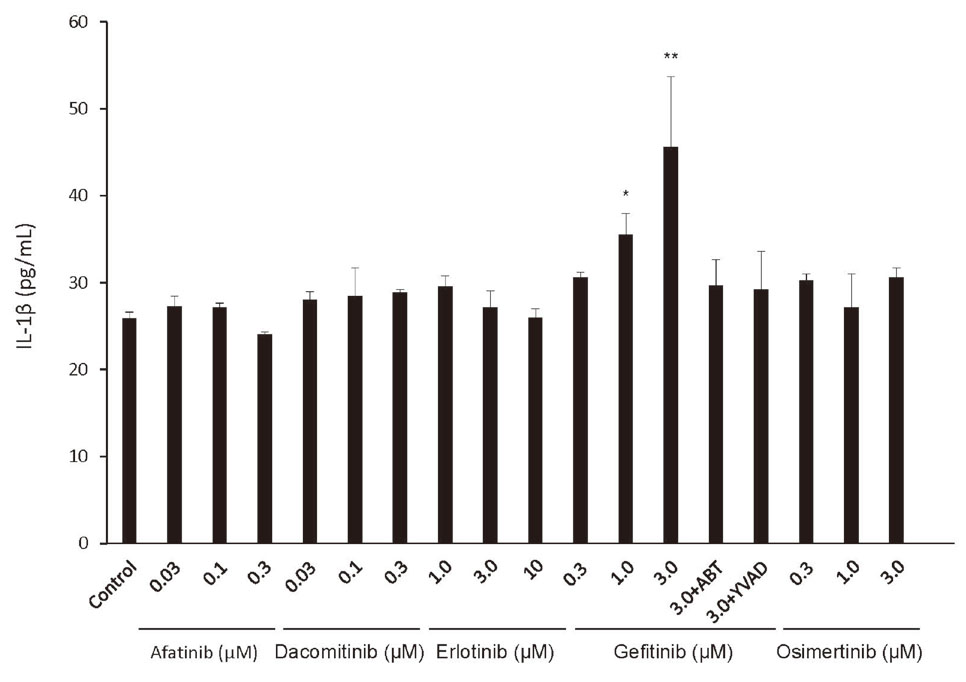
Incubation of THP-1 cells with the supernatant from hepatocytes incubated with gefitinib lead to release of IL-1ß. Levels of IL-1β secreted by THP-1-derived macrophages incubated for 24 hr with the supernatant from an incubation of hepatocytes with afatinib, dacomitinib, erlotinib, gefitinib, and osimertinib for 7 days, with and without, a cytochromes P450 inhibitor (1-aminobenzotriazole, ABT) or a caspase-1 inhibitor (Ac-YVAD-cmk, YVAD). Statistical significance was determined using the Tukey multiple comparison tests, where *, p < 0.05, **, p < 0.01, n = 3.
Incubation of THP-1 cells with the supernatant from an incubation of FLC-4 cells with gefitinib led to caspase-1 activation. This did not occur if ABT was added to the FLC-4 cell culture or YVAD to THP-1 cells (Fig. 3).
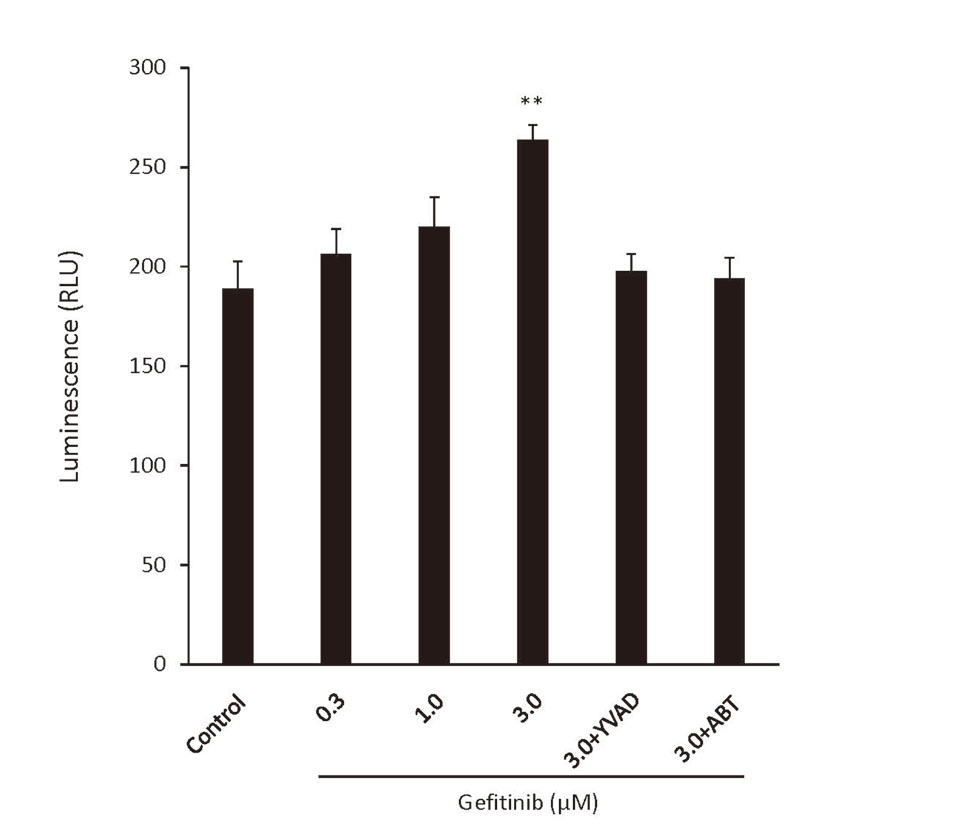
An increase in caspase-1 activity correlates with the release of IL-1ß. Caspase-1 activity of THP-1-derived macrophages in response to incubation for 24 hr with the supernatant from incubation of hepatocytes with gefitinib, with or without, a cytochromes P450 inhibitor (1-aminobenzotriazole, ABT) or a caspase-1 inhibitor (Ac-YVAD-cmk, YVAD). Statistical significance was determined using the Tukey multiple comparison tests, where **, p < 0.01, n = 3.
In this study, we sought candidate DAMPs in the supernatants from FLC-4 cells incubated with gefitinib. As seen in Fig. 4A, there was no signal of HMGB1, HSP32 or HSP60 proteins. There was no change in S100A8 and S100A9 proteins compared with control (Figs. 4E and 4F). However, HSP40, HSP70, and HSP90 proteins were significantly increased in the supernatants from FLC-4 cells incubated with gefitinib, and it was decreased by adding ABT to the FLC-4 cell culture (Figs. 4B, 4C and 4D).
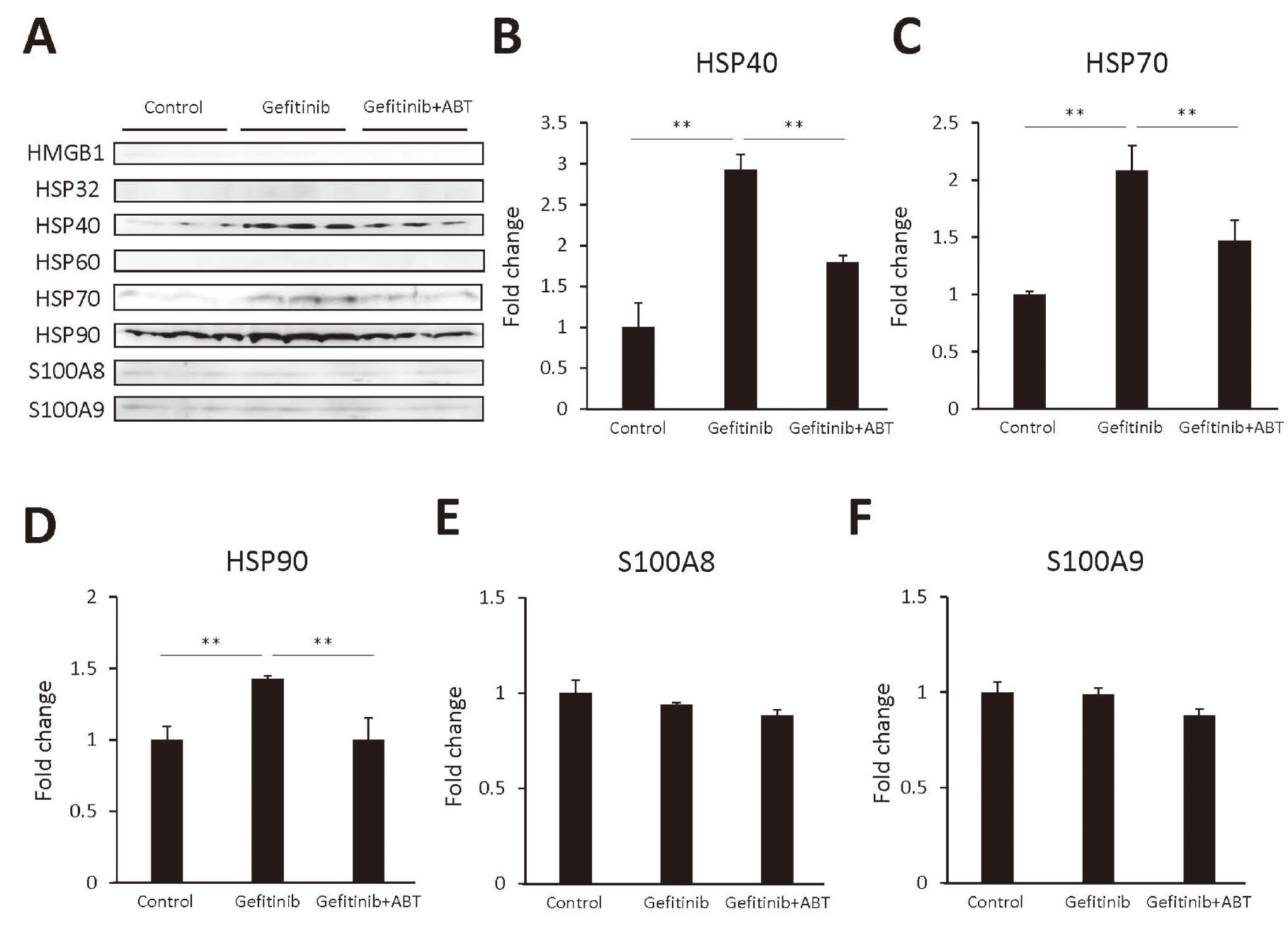
Heat shock protein (HSP) 40, 70, and 90 were released from hepatocytes as danger-associated molecular pattern molecules (DAMPs). (A) Western blot analysis of proteins [high mobility group box 1 (HMGB1), HSP32, HSP40, HSP60, HSP70, HSP90, S100 calcium-binding protein (S100) A8 and S100A9] that were released from hepatocytes incubated for 7 days with gefitinib (3 μM), with and without, a cytochromes P450 inhibitor (1-aminobenzotriazole, ABT), and their quantitative analysis of HSP40 (B), HSP70 (C), HSP90 (D), S100A8 (E), and S100A9 (F). The volume of culture medium that was loaded was 20 μL per lane for all blots; the primary antibodies (HMGB1, HSP32, HSP40, HSP90, S100A8 and S100A9 antibody) were diluted 1: 1000, and the anti-HSP60 and anti-HSP70 antibodies were diluted 1: 3000 and were detected by goat anti-rabbit IgG-peroxidase. Bound peroxidase was visualized by using Luminata™ Classico Western HRP Substrates. Secondary antibody was diluted 1:1000. Statistical significance was determined using the Tukey multiple comparison tests, where **, p < 0.01, n = 3.
Incubation of THP-1 cells with the supernatants from FLC-4 cells incubated with gefitinib led to an increase of HMGB1 secretion, and it was decreased by adding ABT to the FLC-4 cell culture (Fig. 5).
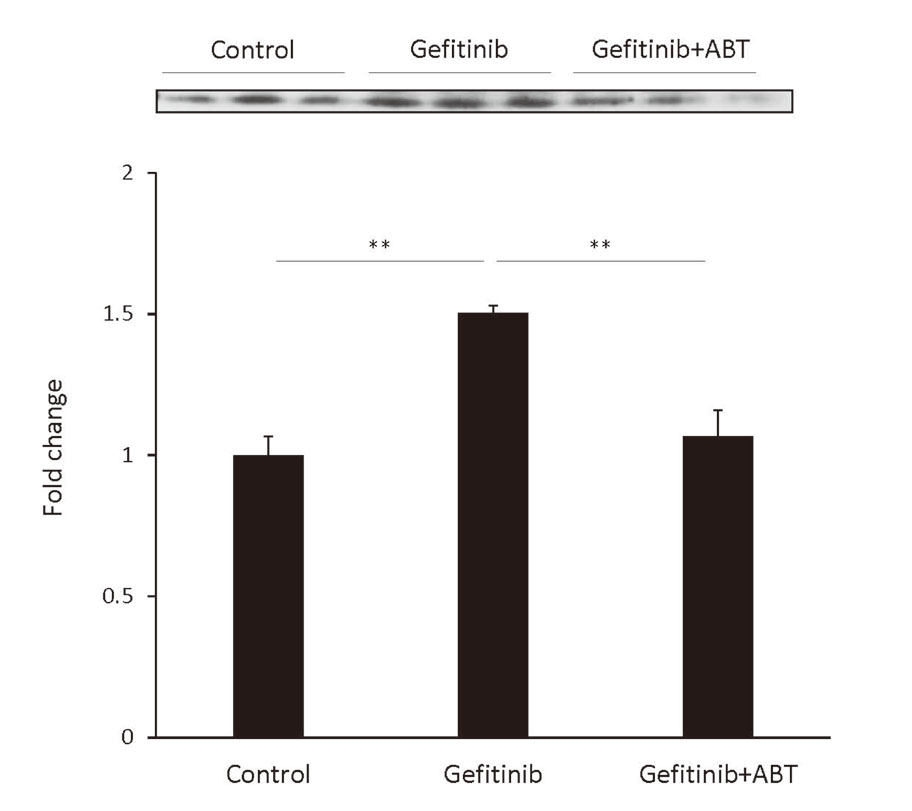
High mobility group box 1 (HMGB1) was released from THP-1-derived macrophages. Western blot analysis of HMGB1 proteins that were released into the culture medium from THP-1-derived macrophages incubated for 24 hr with the supernatant from hepatocytes incubated with gefitinib (3 μM) with and without, a cytochromes P450 inhibitor (1-aminobenzotriazole, ABT) for 7 days. The volume of culture medium that was loaded was 20 μL per lane for all blots; the primary antibodies (HMGB1 antibody) were diluted 1: 1000 and were detected by goat anti-rabbit IgG-peroxidase. Bound peroxidase was visualized by using Luminata™ Classico Western HRP Substrates. Statistical significance was determined using the Tukey multiple comparison tests, where **, p < 0.01, n = 3.
The mechanisms of gefitinib-induced idiosyncratic reactions are poorly understood. Gefitinib has been shown to be bio-activated to reactive metabolites by human hepatic, intestinal, and pulmonary microsomes (Li et al., 2009). Li et al. (2009) reported that gefitinib is oxidized to a reactive iminoquinone. This iminoquinone is plausibly responsible for the idiosyncratic drug reactions associated with gefitinib. In this study, only the supernatant from the incubation of gefitinib with FLC-4 cells for 7 days led to increased caspase-1 activity and production of IL-1ß by THP-1 cells.
As mentioned, reactive metabolites may activate the inflammasome by inducing the release of DAMPs. The activated inflammasome induces caspase-1 conversion of pro-IL-1β and pro-IL-18 into their bioactive mature forms. We evaluated inflammasome activation by measuring IL-1β concentration in the culture medium, and caspase-1 activity in differentiated THP-1 cells. IL-1β is a strong pro-inflammatory molecule and promotes an immune response (Guo et al., 2015; Pinheiro et al., 2017). Therefore, inflammasome activation may be an important bridge between reactive metabolite formation and the induction of an immune response. In this study, we tested the hypothesis that EGFR-TKIs that can trigger an idiosyncratic drug reaction activate the inflammasome at therapeutic concentrations.
Afatinib and dacomitinib have Michael acceptors in their structure; therefore, they can covalently bind to THP-1 cells. In our previous study, amodiaquine covalently binds to THP-1 cells directly, which causes activating inflammasomes (Kato and Uetrecht, 2017). Afatinib and dacomitinib could also activate inflammasomes, and we confirmed that they activated inflammasomes of THP-1 cells directly. However, the concentration required was well above their therapeutic concentrations (> 1 μM, data not shown); therefore, activation of inflammasomes is unlikely to contribute to the mechanism by which afatinib and dacomitinib cause idiosyncratic reactions.
We found that gefitinib itself did not activate inflammasomes in THP-1 cells, but the culture supernatant from FLC-4 cells incubated with gefitinib did. Although immortalized cell lines often lack liver-specific functions including the major P450s, we utilized 3D cultures of a FLC-4 cell line, which synthesize and secrete albumin, alpha-fetoprotein, and other liver-specific proteins (Kato et al., 2014). These cells also have significant drug-metabolizing capacity, and they were used in this study to produce reactive metabolites of gefitinib. Production of IL-1β was inhibited with the addition of the cytochrome P450 inhibitor, ABT, to the FLC-4 cell culture. This suggests that the iminoquinone is responsible for gefitinib-induced idiosyncratic reactions (Fig. 6). As mentioned in the Introduction, although inhibition of EGFR leads to an increase the risk that a drug will cause IDRs (Mascia et al., 2013), the results in this study demonstrate an additional mechanism that is likely to contribute gefitinib-induced idiosyncratic reactions.
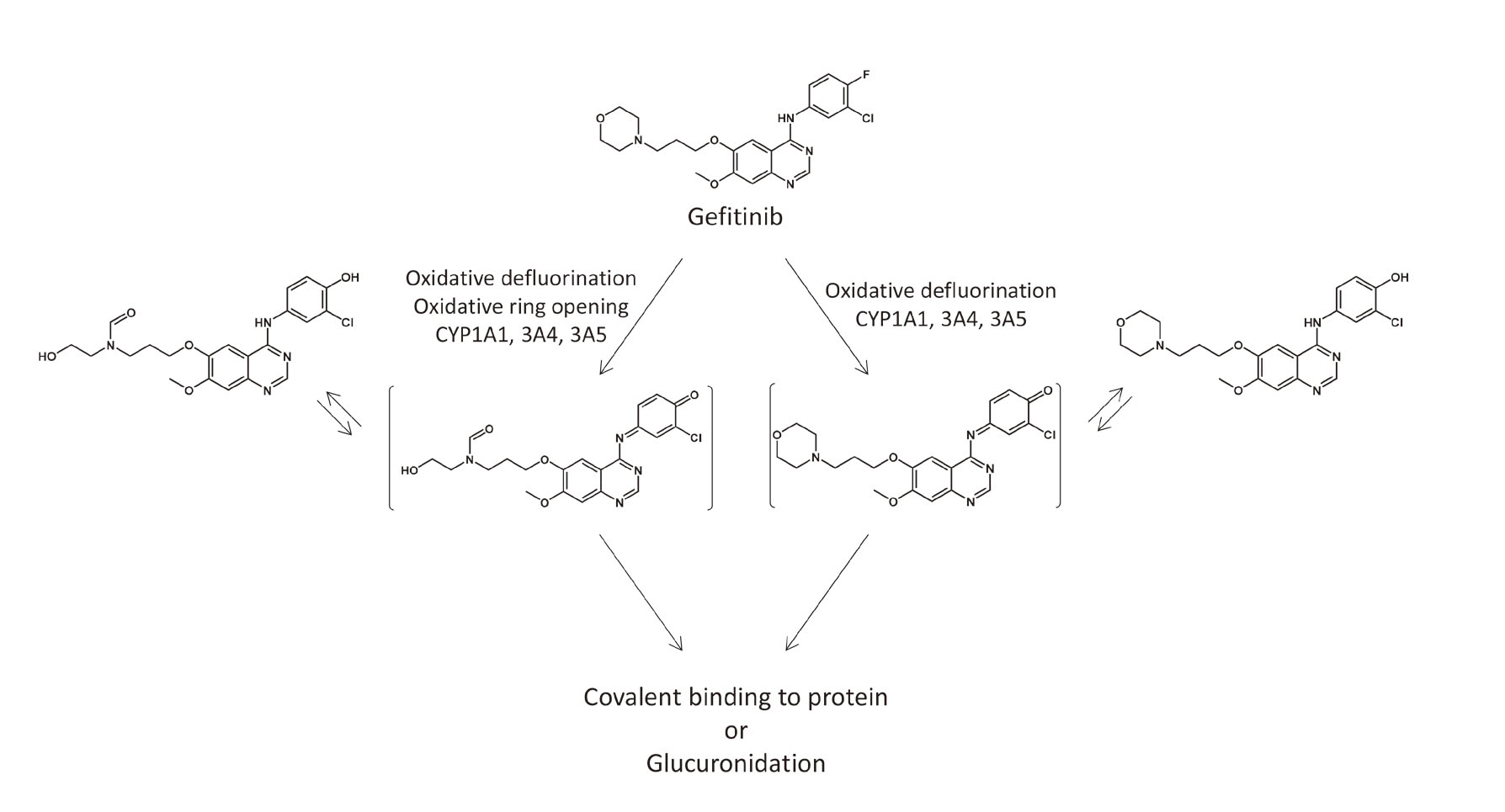
Proposed bioactivation pathways of gefitinib, including the pathway that leads to the formation of iminoquinone reactive intermediates.
In this study, DAMPs were identified in the culture supernatant of a hepatocyte cell line. HSP40, HSP70, and HSP90 were significantly increased in the culture supernatant of FLC-4 cells treated with gefitinib. The cytochrome P450 inhibitor, ABT, prevented this increase in HSP40, HSP70, and HSP90. HSP40, HSP70 and HSP90 are reported to be DAMPs that can activate inflammasomes (Mayor et al., 2007; Roh and Sohn, 2018; Zininga et al., 2018). Therefore, the release of these HSPs from the hepatocyte cell line caused by reactive metabolites of gefitinib may be responsible for inflammasome activation in THP-1 cells. In addition, activated THP-1 cells by reactive metabolites of gefitinib released HMGB1 to the culture medium. HMGB1 is a known DAMPs, which activates inflammasomes in APCs (Bianchi, 2007). The secreted HMGB1 from APCs leads to activation of other APCs with exacerbation of inflammation.
In conclusion, our results support the hypothesis that idiosyncratic reactions associated with gefitinib involve oxidation of gefitinib into reactive metabolites in hepatocytes, the most likely being the iminoquinone. This results in the production of DAMPs that activate inflammasomes thereby resulting in an immune response that causes immune related adverse event. The ability of a drug or its reactive metabolites to release DAMPs and activate inflammasomes may differentiate reactive metabolites that can cause a relatively high risk of IDRs from those that will not be associated with such a risk, and this, in turn, may provide a mechanism to screen drug candidates for their potential to cause serious IDRs.
This work was supported in part by a Grant-in-Aid for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science and Technology (Kakenhi, 18K06767).
Conflict of interestThe authors declare that there is no conflict of interest.