Abstract
Trichloroethylene (TCE) as a common organic solvent in industrial production can cause occupational medicamentosa-like dermatitis (OMDT) in some exposed workers. In addition to systemic skin damage, OMDT is also accompanied by severe kidney injury. Our previous studies show that complement (C) plays an important role in immune kidney injury caused by TCE. Specifically, C3 is mainly deposited on glomeruli. Recent studies have found that intracellular complement can be activated by cathepsin L (CTSL) and exert a series of biological effects. The purpose of this study was to explore where C3 on glomeruli comes from and what role it plays. A BALB/c mouse model of skin sensitization induced by TCE in the presence or absence of CTSL inhibitor (CTSLi,10 mg/kg). In TCE sensitization-positive mice, C3 was mainly expressed on podocytes and the expression of CTSL significantly increased in podocytes. Kidney function test and related indicators showed abnormal glomerular filtration and transmission electron microscopy revealed ultrastructure damage to podocytes. These lesions were alleviated in TCE/CTSLi positive mice. These results provide the first evidence that in TCE-induced immune kidney injury, intracellular complement in podocytes can be over-activated by CTSL and aggravates podocytes injury, thereby damaging glomerular filtration function. Intracellular complement activation and cathepsin L in podocytes may be a potential target for treating immune kidney injury induced by TCE.
INTRODUCTION
Trichloroethylene (TCE) is a volatile halogenated hydrocarbon organic solvent and has been widely used in industry for its good cleaning and defatting effects on metal parts (Friesen et al., 2015). Many occupational workers exposed to TCE can develop occupational medicamentosa-like dermatitis due to trichloroethylene (OMDT) (Liu et al., 2015). As OMDT progresses rapidly, the case fatality rate is high to 9-13%, which brings huge economic burden and health loss to the patients (Kamijima et al., 2007).
OMDT is a systemic allergic disease. Its clinical manifestations not only include severe skin damage, liver damage and fever, but also kidney injury – one of its important manifestations (Vermeulen et al., 2012). A study found that skin damage and kidney damage are positively correlated (Zhang et al., 2011). The International Agency for Research on Cancer classified TCE as Class I carcinogen based on a large amount of research evidence (Guha et al., 2012). In particular, there was a clear correlation between TCE exposure and kidney cancer (Alanee et al., 2015; Buhagen et al., 2016; Karami et al., 2012; Kim et al., 2014). A study found that creatinine (CRE) and blood urea nitrogen (BUN) levels were significantly elevated in OMDT patients; ultrasonography also revealed different morphological changes during kidney injury progression (Zhang et al., 2011). In our previous studies, CRE and BUN levels were also significantly increased in TCE-sensitized mice (Yu et al., 2012; Zhang et al., 2016). These findings indicate that there has been a significant impairment of glomerular filtration in OMDT.
Many studies found that delayed type IV allergy cannot fully explain the pathogenesis of OMDT (Huang et al., 2015; Yu et al., 2017; Zhang et al., 2017), and the complement system also played an important role in it (Yue et al., 2007; Zhao et al., 2012). Our earlier study showed that in TCE-sensitized mice, C3 was mainly deposited on glomeruli and C5a, C5b-9 were mainly deposited on renal tubules (Liu et al., 2016). Furthermore, pretreatment of TCE-sensitized mice with C5aR antagonists significantly improved renal tubular injury but not glomerular injury (Zhang et al., 2016). These studies further suggest that there were different mechanisms of complement activation mediating glomeruli and tubular injury. Complement can be activated via the classical, alternative and lectin pathways, which are independent and interact with each other. All three routes will cleave C3 into C3a and C3b, C3b further forms C5a and C5b under the action of C5 invertase, and C5b assembles together with C6, C7, C8, C9 on the target cell to form the membrane attack complex C5b-9 (Ricklin et al., 2016), thereby causing cell damage. The cleavage products C3a and C5a as anaphylatoxins also can mediate cell damage directly (Ricklin et al., 2010). We know that most of C3 is synthesized by the liver, but the latest studies show that there are also intracellular complement synthesis and activation in a variety of immune and non-immune cells (Arbore et al., 2017; Minton, 2014). Liszewski and colleagues found that C3 could be cleaved into biologically active C3a and C3b fragments in T lymphocytes through the cell’s internal cathepsin L (CTSL), resulting in intracellular complement activation (Liszewski et al., 2013). The activated products bind to corresponding complement receptors on lysosome, initiate the rapamycin toxin (mTOR) signaling pathway, and subsequently produce a series of biological effects.
The podocyte is a highly differentiated epithelial cell, which plays an important role in maintaining glomerular filtration function, but it is vulnerable to many factors such as inflammatory factors, immune complexes and complement (Pavenstädt et al., 2003; Saleem, 2015). A study found that the expression levels of many complement components in cultured podocytes were influenced by various factors (Li et al., 2016a). Another study showed that a large amount of cathepsin L was expressed in podocytes and was stimulated by growth factors (Asanuma et al., 2002). Taken together these findings suggested that podocytes can be a source for activation of intracellular complement.
Whether intracellular complement activation in podocytes contributes to TCE sensation-induced kidney damage and how this activated have never been examined. We hypothesize that podocyte intracellular complement is activated by the action of intracellular cathepsin L to cause podocyte injury, which in turn affects the glomerular filtration function. In this study, we set up a BALB/c mouse model of TCE sensitization and employed cathepsin L inhibitor in TCE-sensitized mice to investigate whether podocyte complement was activated through this pathway and influenced kidney function (Wang et al., 2015; Woo et al., 1996).
MATERIALS AND METHODS
Animals
BALB/c mice (female, 6-8 weeks, 18-20 g, n = 120) were obtained from the Experimental Animal Center of Anhui Medical University. The mice were housed in specific pathogens-free conditions, and had free access to standard mouse food and safe water. The housing conditions were maintained at 22.5 ± 0.5°C, 50 ± 5% humidity, and a 12-hr light/dark circadian rhythm. The entire research process was reviewed and approved by Animal Ethics Committee of Anhui Medical University.
Reagents
TCE, Freund’s complete adjuvant (FCA) and Dimethyl sulfoxide (DMSO) were purchased from Sigma (St. Louis, MO, USA). Olive oil and acetone were from Shanghai Chemical Reagent Company (Shanghai, China). Cystatin C (Cys-C) and Podocalyxin (PCX) ELISA kits were from Elabscience Biotechnology Co., Ltd. (Wuhan, China). CTSL inhibitor, CTSL and Nephrin primary antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-C3, anti-β actin primary antibodies and corresponding secondary antibodies used for IF were bought from Abcam (Cambridge, UK). IHC kit was from ZSGB Biotechnology Co., Ltd. (Beijing, China). Collagenase A was from Roche (Mannheim, Germany). Dynabeads M-450 Tosylactivated and magnetic particle concentrator were bought from ThermoFisher Scientific (Waltham, MA, USA). 100 μm nylon cell strainer was from Falcon (Corning, NY, USA).
Treatments of animals
After 1 week of adaptive feeding, all mice were randomly assigned into blank control group (n = 15), solvent control group (n = 15), TCE treatment group (n = 45) and TCE+CTSL inhibitor pretreatment group (TCE/CTSLi, n = 45). The TCE sensitization model was established as shown in Fig. 1. In brief, 24 hr prior to treatments, the dorsal hair of each mouse was shaved with an area about 4 cm2 and kept free from hair throughout the entire experiment. In the TCE treatment group, mice were injected subcutaneously on the shaved area with 100 μL of a mixture of 50% TCE (TCE: olive oil: acetone = 5:2:3) and FCA in an equal volume on the first day. On day 4, 7 and 10, 100 μL 50% TCE was painted on the same area for sensitization. On day 17 and 19, 100 μL 30% TCE (TCE: olive oil: acetone = 3:2:5) was applied to the same area for primary and final challenge. After each application of TCE, the skin was covered with filter paper and sealed with non-irritating tape for 24 hr to reduce the evaporation of TCE. The mice in the blank control group received no treatment, while the mice in the solvent control group was treated with the same proportion of solvent at the above time points. For the mice in the TCE/CTSLi group, the basic protocol was the same as the TCE treatment group except that CTSL inhibitor (Z-FY-CHO, 10 mg/kg) was injected intraperitoneally 2 hr before each challenge. Z-FY-CHO is an effective and reversible inhibitor of cathepsin L and this inhibitor has been widely used in research on cathepsin L in vivo and in vitro (Li et al., 2016b; Markosyan et al., 2016; Woo et al., 1996). The 10 mg/kg dose has been proved to have a good inhibitory effect in in vivo experiments, and we therefore used this dose in our study.
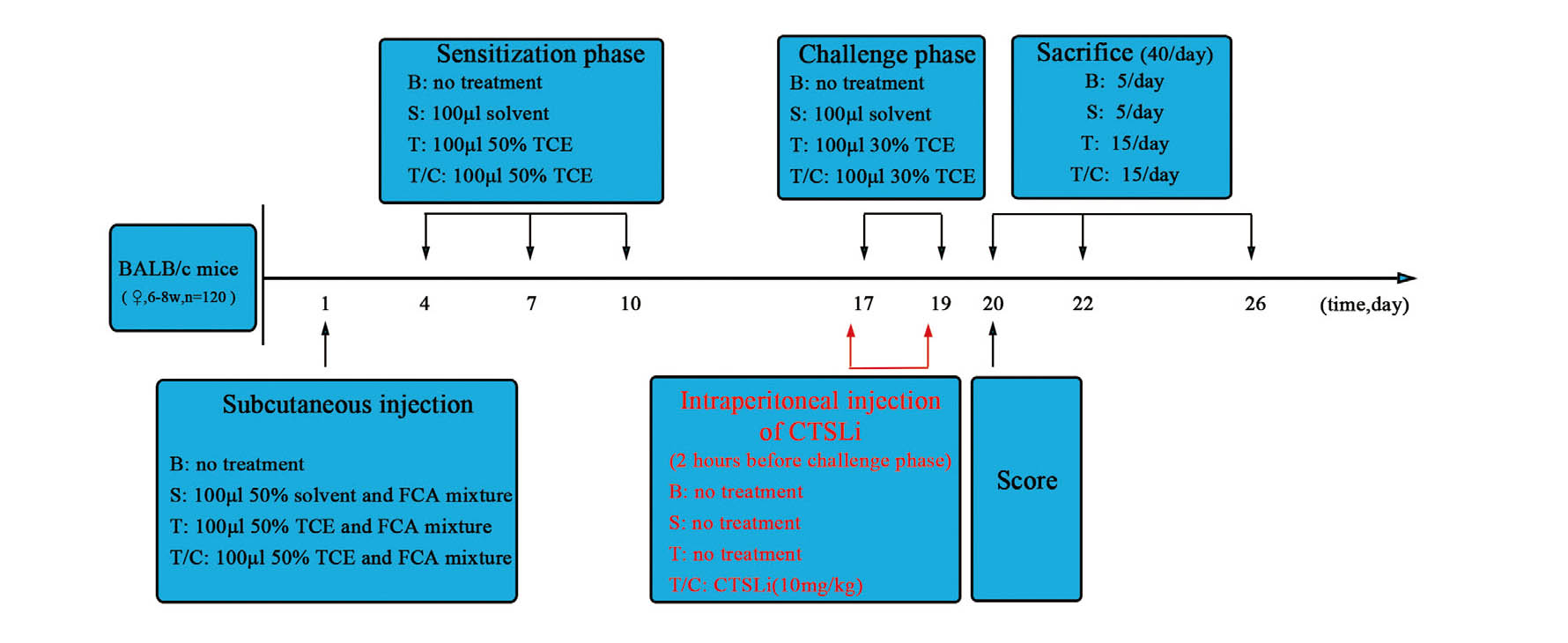
Twenty four hours after the last challenge, the skin sensitization was scored based on erythema and edema of the back skin. The scoring criteria was as follows: 0 = no reaction, 1 = scattered mild redness, 2 = moderate and diffuse redness, 3 = intensive erythema and swelling. Skin scores ≥ 1 were considered to be sensitization positive, otherwise negative. Mice were euthanized on day 1, 3, 7 after the last challenge, and the blood, urine and kidneys were collected for subsequent studies.
Isolation of glomeruli
Glomeruli were isolated as described previously (Liu et al., 2013; Takemoto et al., 2002). Briefly, mice were anesthetized by intraperitoneal injection of 10% chloral hydrate (0.03 mL/10 g). The kidneys were perfused with Dynabeads through the distal abdominal aorta. Then the kidneys were removed, minced into 1 mm3 pieces and digested with collagenase A (1 mg/mL) at 37°C for 30 min with gentle agitation every 5 min. The digested tissue was passed through a 100 μm cell strainer and the cell strainer were rinsed with 5 mL ice-cold sterile PBS. The filtration and rinsing process was repeated as above, and the cell suspension then was centrifuged at 200 × g for 5 min. The supernatant was discarded, and the cell pellet was resuspended with 3 mL PBS. Finally, glomeruli with Dynabeads in cell suspension were isolated by a magnetic particle concentrator.
Assessment of kidney function
The collected blood samples were left at room temperature (RT) for 2 hr and then centrifuged at 3000 × g at 4°C for 15 min. The levels of serum CRE and BUN were detected by an automatic biochemical analyzer.
ELISA assay
The levels of serum Cys-C and urine PCX were measured by corresponding commercial ELISA kits, all operations were carried out in accordance with the manufacturer’s instructions.
Immunohistochemistry detection of C3, CTSL and Nephrin
The collected kidneys were made into 5 μm thick paraffin slices according to the routine protocol. The slices were placed in different concentrations of xylene and ethanol for dewaxing and hydration followed by antigen retrieval in a microwave oven. After the goat serum was blocked for 15 min at 37°C, the corresponding primary antibodies were added and incubated overnight at 4°C. The slices were then washed with PBS and incubated with biotin-labeled secondary antibody for 15 min at 37°C. The slices were washed again with PBS and incubated with horseradish peroxidase labeled streptavidin for 15 min at 37°C, then stained by diaminobenzidine and counter-stained by hematoxylin. Finally, photos were taken under an optical microscope and integral optical density (IOD) determined using Image-Pro plus software 6.0 (MediaCybernetics, Rockville, MD, USA) for semi-quantitative analysis.
Immunofluorescence detection of C3 and CTSL expression in podocytes
Fresh kidneys were immediately embedded in optimum cutting temperature compound and frozen at -80°C. The frozen kidneys were cut into 4 μm thick sections and then fixed in pre-chilled acetone for 5 min. After washing with PBS for 15 min, 0.3% Tritor-X 100 was added and the sections were incubated for 30 min at 37°C. The sections were washed again with PBS for 15 min and then blocked with goat serum for 2 hr at 37°C. The serum was discarded and appropriate concentration of primary antibody mixture added directly followed by incubation at 4°C overnight. On the second day, the sections were washed again with PBS and incubated with corresponding fluorescence-labeled secondary antibodies mixture for 2 hr at RT. The nucleus was stained with DAPI for 15 min at RT. Finally, the results were observed immediately with a fluorescence microscope and images taken for later analysis.
Western blot analysis of C3, CTSL and Nephrin proteins
Total protein in glomeruli was extracted with RIPA lysates followed by determination of protein concentration using bicinchoninic acid (BCA) assays. Each sample was loaded with 40 μg protein and separated by different concentrations of acrylamide gels. The proteins in separation gel were electro-transferred to polyvinylidene fluoride (PVDF) membrane and blocked in 5% skim milk for 2 hr at RT. The membranes were then incubated with different primary antibodies at 4°C overnight. After washing with PBS, corresponding secondary antibodies added, the membrane was incubated for 2 hr at RT. Finally, the membrane proteins were visualized using an enhanced chemiluminescence (ECL) detection kit. The gray value of protein strips was analyzed by Image J software.
Observation of podocytes injury by transmission electron microscope (TEM)
The fresh kidney cortex was fixed in 2.5% glutaraldehyde for 6-12 hr. After washing with PBS for 1-6 hr, the samples were fixed in 1% citric acid for 1-2 hr. Before transferring to 70% ethanol uranyl acetate for 2-12 hr, the samples were fixed in 30%, 50% ethanol for 10-15 min, respectively. After being immobilized in 80%, 95%, 100% ethanol for 10-45 min, samples were transferred into propylene oxide for 30 min. Then the samples were placed in propylene oxide: epoxy resin for 1-2 hr and then transferred to pure epoxy resin for 2-3 hr. After being embedded in pure epoxy resin, samples were placed in an oven at 40°C for 12 hr and then temperature adjusted to 60°C for 48 hr. The embedded blocks were removed and trimmed, and 70-nm thick slices were cut out on an LKB-NOVA microtome. The slices were stained with lead and uranium, and the damage of podocytes was observed with Nissan JEM-1230 TEM and photographs were taken.
Statistical analysis
All measurement data in this study were normal distributed after normality test and expressed as mean ± SD. One-way analysis of variance (ANOVA) was used to compare the mean of multiple groups, and the mean between groups was compared with least significant difference (LSD) method. P value < 0.05 was considered statistically significant.
RESULTS
Sensitization rate
There were no erythema and edema on the skin of mice in the blank control group and solvent control group. Based on skin scores, the sensitization rate was 33.3% (15/45) in the TCE treatment group and 31.3% (14/45) in the TCE/CTSLi treatment group. After statistical analysis, there was no statistically significant difference between the two sensitization rates (P > 0.05), suggesting that the use of CTSli will not affect the sensitization rate.
Kidney function detection results
There was no significant difference in the levels of CRE, BUN, Cys-C and PCX between the solvent control group and blank control group (P > 0.05) (Fig. 2). Compared with the solvent control group, the expression levels of Cys-C and PCX increased in the TCE 1d positive group, all four indexes increased in the TCE 3d positive group and CRE, Cys-C, PCX increased in the TCE 7d positive group (P < 0.05). Compared with solvent control group, some of the above indexes in the TCE/CTSLi positive groups at each time point also increased, but the extent of increase was less than that in the TCE positive groups at the corresponding time points (P < 0.05). Compared with the solvent control group, expression levels of all four indexes in negative groups at each time point in each treatment group showed no significant difference (P > 0.05). By testing four indexes of kidney function, we found that all indexes reached the peak at 3d after last challenge and then gradually recovered or decreased. Therefore, in the follow-up study, we mainly focused on changes of the relevant indicators in 3d groups.

Immunohistochemistry results showed that C3 was mainly deposited on glomeruli in the TCE 3d positive group and moderately expressed in the TCE/CTSLi 3d positive group; there was no detectable expression in all other four groups (Fig. 3). The results of immunohistochemistry scoring showed that the score in the TCE 3d positive group was significantly higher than in the five other groups (P < 0.05) (Table 1). Immunofluorescence showed that C3 was mainly expressed on podocytes in the TCE 3d positive group and no significant expression in the five other groups (Fig. 4). The expression of C3 in glomeruli was detected by Western blot (Fig. 5A). There was no significant difference between solvent control group, blank control group and two corresponding negative groups (P > 0.05). Compared with solvent group, C3 in the TCE 3d positive group was significantly increased (P < 0.05). Compared with the TCE 3d positive group, the C3 expression in the TCE/CTSLi 3d positive group was significantly decreased (P < 0.05) (Fig. 5B).

Table 1. The average optical density of C3, CTSL, Nephrin in different groups.
| Group |
N |
C3 |
CTSL |
Nephrin |
| Blank control |
5 |
0.013 |
± |
0.001 |
0.023 |
± |
0.004 |
0.228 |
± |
0.012 |
| Solvent control |
5 |
0.015 |
± |
0.002 |
0.022 |
± |
0.003 |
0.226 |
± |
0.009 |
| TCE 3d positive |
5 |
0.074 |
± |
0.009abc |
0.134 |
± |
0.007abc |
0.107 |
± |
0.006abc |
| TCE 3d negative |
10 |
0.014 |
± |
0.002 |
0.022 |
± |
0.004 |
0.220 |
± |
0.008 |
| TCE/CTSLi 3d positive |
5 |
0.026 |
± |
0.003ab |
0.035 |
± |
0.005ab |
0.217 |
± |
0.011 |
| TCE/CTSLi 3d negative |
10 |
0.015 |
± |
0.003 |
0.022 |
± |
0.004 |
0.211 |
± |
0.010 |
Note: compared with solvent control group: aP < 0.05; compared with the corresponding negative group: bP < 0.05; compared with the corresponding inhibitor positive group: cP < 0.05
Expression of CTSL in kidney
The results of immunohistochemistry showed that CTSL was expressed in all treatment groups, but the expression on glomeruli in the TCE 3d positive group was significantly increased (P < 0.05) (Fig. 6). Immunohistochemistry scoring showed that the score in the TCE 3d positive group was significantly higher than in the five other groups (P < 0.05) (Table 1). Immunofluorescence assay also found that CTSL was mainly expressed on podocytes in the TCE 3d positive group (Fig. 7). The results detected by Western blot were consistent with the results of IHC (Fig. 5A, 5C).
Expression of Nephrin in kidney
Immunohistochemistry showed that the expression of Nephrin was significantly down regulated in the TCE 3d positive group (P < 0.05) and there was no significant difference in the five other groups (P > 0.05) (Fig. 8). Immunohistochemistry scoring showed that the score in the TCE 3d positive group was significantly lower than in the five other groups (P < 0.05) (Table 1). The results detected by Western blot were consistent with the results of immunohistochemistry (Fig. 5A, 5D).
Ultrastructural damage of podocytes
TEM observation results showed that in the blank control group, solvent control group and two corresponding negative groups, podocytes’ foot processes were intact, the basement membrane thickness were uniform, and there was no obvious organelle damage. In the TCE 3d positive group, we observed significant podocyte foot process effacement, inhomogeneous thickening of the basement membrane, mitochondria vacuolar degeneration and crest rupture. In the TCE/CTSLi 3d positive group, there was only some foot process effacement but no other obvious abnormalities; the degree of injury was significantly less than that in the TCE 3d positive group (Fig. 9).
DISCUSSION
Patients with TCE-caused OMDT not only have severe skin damage, but also kidney injury as one of the important clinical manifestations. Increasing studies have found that the pathogenesis of OMDT cannot be fully explained by delayed type IV allergy (Huang et al., 2015; Yu et al., 2017; Zhang et al., 2017), and complement system played an important role (Yue et al., 2007; Zhao et al., 2012). To investigate the role of complement in this process, our previous studies found that there was a large amount of complement deposition in kidneys of TCE positive mice. Specifically, C3 was mainly deposited on podocytes. In recent years, there is increasing evidence for intracellular complement. Studies have found that in the interior of T lymphocytes, C3 can be cut into C3a and C3b by CTSL to produce a series of biological effects (Liszewski et al., 2013). In view of the fact that podocytes have intracellular complement activation similar to T lymphocytes, the current study tested the hypothesis that intracellular complement activation in podocytes was involved in the impairment of kidney filtration function and used CTSL inhibitor to examine whether this CTSL was involved in intracellular C3 activation and if so whether the inhibitor could reduce glomerular injury.
CRE and BUN are commonly used indicators reflecting glomerular filtration function. In this study, CRE and BUN were increased to different degrees in TCE positive groups and peaked on the third day after the last challenge. Although CRE and BUN in TCE/CTSLi positive groups also increased at some time points, the extent was significantly smaller than that in corresponding time points in the TCE positive groups. In order to assess more accurately glomerular filtration functional damage, we selected a more specific indicator Cys-C. Cys-C is a small molecule with a 13 kDa and can be produced in all nucleated cells in the body. Its metabolic feature is that it does not bind to other molecules and is only cleared by glomeruli. Unlike serum CRE, the level of Cys-C in serum is not related to sex, age and muscle content of the study subjects. Therefore, many studies have suggested that Cys-C was a highly sensitive and specific index for glomerular filtration function (Ferguson et al., 2015; Kwon et al., 2017; Odutayo and Cherney, 2012; Onopiuk et al., 2015). Our results showed that the pattern of change in Cys-C in all groups was similar to that in CRE. The above consistent results clearly demonstrate that abnormal glomerular filtration did indeed occur in TCE positive mice and was most severe on the third day after the last challenge, whereas the degree of damage in TCE/CTSLi positive group was reduced.
Podocytes play a key role in maintaining glomerular filtration function and it has been found that podocyte injury was an important part of pathogenesis of glomerular diseases (Mallipattu and He, 2016). Once podocytes are injured, the glomerular filtration function will inevitably be abnormal. Many studies have found that PCX as a marker protein of podocyte apical membrane area, compared with other marker proteins, it was the most stable marker molecule for diagnosis and could well reflect podocyte injury (Hara et al., 2012; Hayashi et al., 2017). PCX increased in the urine of all positive groups and peaked in the 3d groups. Compared with the TCE 3d positive group, PCX in TCE/CTSLi 3d positive group was significantly decreased. The result of PCX showed that podocyte in TCE positive mice had obvious damage, and CTSL inhibitor could effectively reduce the damage of podocytes caused by TCE.
Since the purpose of this study was to investigate the role of podocyte intracellular complement activation in immune kidney injury induced by TCE, we examined the expression of C3 in kidney. The results of immunohistochemistry showed that C3 was mainly expressed on glomeruli in the TCE 3d positive group, and was slightly expressed in the TCE/CTSLi 3d positive group. No obvious expressions were observed in the four other groups. Furthermore, we used immunofluorescence double-labeling method and found a large number of C3 deposited on podocytes in the TCE 3d positive group and no obvious C3 was seen on podocytes in the five other groups. Combined with the above test results we found that C3 in podocytes was significantly elevated in the TCE 3d positive group and CTSL inhibitor could reduce the expression of C3. These results indicated that there was a significant complement activation in podocytes in TCE positive mice and CTSL may play an important role in podocytes intracellular complement activation.
CTSL is a cysteine proteolytic enzyme in the papain family and is usually in the lysosome of cells in the form of a zymogen, which plays a role in activation of prohormones, antigen presentation and development of tissues and organs. Many studies have found that CTSL was involved in the pathogenesis of a variety of glomerular diseases, cancer, and cardiovascular diseases, and the high expression of CTSL was related to autoimmune diseases such as rheumatoid arthritis and systemic sclerosis. (Cao et al., 2017; Feldreich et al., 2016; Garsen et al., 2016; Sever et al., 2007; Sudhan and Siemann, 2015; Weitoft et al., 2015; Yamashita et al., 2016). Given that CTSL plays a key role in intracellular complement activation, the above data suggest that CTSL may play an important role in immune kidney damage caused by TCE. Our test results showed that CTSL was expressed in all six groups. Among them, the expression of CTSL was significantly increased on podocytes in the TCE 3d positive group. Compared with the TCE 3d positive group, CTSL in the TCE/CTSLi 3d positive group was significantly reduced. These observation results suggested that CTSL was involved in immune kidney injury induced by TCE and that the CTSL inhibitor we used in this study was effective.
Considering the importance of podocyte injury in this study, we not only detected PCX in urine, but also detected podocyte injury by immunohistochemistry and TEM. Nephrin, a marker protein of podocyte, plays an important role in maintaining the integrity of the podocyte septum and related signaling pathways. It has been found that Nephrin was down-regulated in a variety of human glomerular diseases and animal models, and there was a certain correlation between changes in Nephrin expression levels and disease progression (Benigni et al., 2004; Fukusumi et al., 2014; Welsh and Saleem, 2010). Immunohistochemistry results showed that the expression of Nephrin in the TCE 3d positive group was significantly decreased, while no significant difference was observed in the five other groups. We know that under normal conditions, Nephrin is expressed abundantly. Nephrin expression is reduced only when podocyte is damaged or the number is reduced. The above immunohistochemistry results suggest that podocyte injury occurred in TCE positive mice. TEM revealed that foot processes were extensively fused, basement membrane appeared to have an inhomogeneous increase and the mitochondria vacuolization and coercion rupture in the TCE 3d positive group. However, only part of foot process fusion was observed and no obvious other abnormalities were seen in the TCE/CTSLi 3d positive group, the injury extent was significantly less than TCE 3d positive group. Combined with immunohistochemistry and TEM observation results, we found that severe damage occurred in podocytes in TCE positive mice and CTSL inhibitor significantly reduced podocyte injury.
In summary, we found that a large amount of C3 and CTSL were deposited on podocytes in TCE positive mice, and that podocytes showed severe damage and affected glomerular filtration function. After pretreatment with CTSL inhibitor, these changes were significantly attenuated. These results suggest that in TCE-induced immune kidney injury, podocyte intracellular complement is indeed activated by CTSL. Intracellular complement activation in podocyte is involved in podocytes damage in TCE sensitization-induced immune kidney injury, causing foot process effacement, inhomogeneous thickening of the basement membrane, mitochondria vacuolar degeneration and crest rupture, further compromising the glomerular filtration function. This is mediated by CTSL up-regulation. As CTSL inhibitor can effectively reduce podocyte injury induced by TCE, CTSL may serve as a possible target for the treatment of OMDT. There were some limitations in this study. In view of difficulties in separation of podocyte, we used glomeruli protein instead of podocyte protein in Western blot test to reflect the trend of changes, although the groups are comparable as they are normalized to the same reference base. In addition, the reason for the high expression of CTSL, the upstream events of CTSL, and the precise mechanisms are unclear. Recent studies have found that asparagine-based endopeptidase is an upstream event that activates CTSL in intracellular complement activation (Freeley et al., 2018). This new discovery provides new ideas and directions for our next in-depth research.
ACKNOWLEDGMENTS
This project was supported by the National Natural Science Foundation of China (grant numbers 81673141,81874259). CW gratefully acknowledges support from Biotechnology and Biological Sciences Research Council (BBSRC) (BB/P004695/1) and National Institute of Aging (NIA, 1R01AG049321-01A1). YY acknowledges support from Anhui university natural science research project (KJ2018A0178).
Conflict of interest
The authors declare that there is no conflict of interest.
REFERENCES
- Alanee, S., Clemons, J., Zahnd, W., Sadowski, D. and Dynda, D. (2015): Trichloroethylene Is Associated with Kidney Cancer Mortality: A Population-based Analysis. Anticancer Res., 35, 4009-4013.
- Arbore, G., Kemper, C. and Kolev, M. (2017): Intracellular complement - the complosome - in immune cell regulation. Mol. Immunol., 89, 2-9.
- Asanuma, K., Shirato, I., Ishidoh, K., Kominami, E. and Tomino, Y. (2002): Selective modulation of the secretion of proteinases and their inhibitors by growth factors in cultured differentiated podocytes. Kidney Int., 62, 822-831.
- Benigni, A., Gagliardini, E., Tomasoni, S., Abbate, M., Ruggenenti, P., Kalluri, R. and Remuzzi, G. (2004): Selective impairment of gene expression and assembly of nephrin in human diabetic nephropathy. Kidney Int., 65, 2193-2200.
- Buhagen, M., Grønskag, A., Ragde, S.F. and Hilt, B. (2016): Association Between Kidney Cancer and Occupational Exposure to Trichloroethylene. J. Occup. Environ. Med., 58, 957-959.
- Cao, Y., Liu, X., Li, Y., Lu, Y., Zhong, H., Jiang, W., Chen, A.F., Billiar, T.R., Yuan, H. and Cai, J. (2017): Cathepsin L activity correlates with proteinuria in chronic kidney disease in humans. Int. Urol. Nephrol., 49, 1409-1417.
- Feldreich, T., Carlsson, A.C., Risérus, U., Larsson, A., Lind, L. and Ärnlöv, J. (2016): The association between serum cathepsin L and mortality in older adults. Atherosclerosis, 254, 109-116.
- Ferguson, T.W., Komenda, P. and Tangri, N. (2015): Cystatin C as a biomarker for estimating glomerular filtration rate. Curr. Opin. Nephrol. Hypertens., 24, 295-300.
- Freeley, S., Cardone, J., Günther, S.C., West, E.E., Reinheckel, T., Watts, C., Kemper, C. and Kolev, M.V. (2018): Asparaginyl Endopeptidase (Legumain) Supports Human Th1 Induction via Cathepsin L-Mediated Intracellular C3 Activation. Front. Immunol., 9, 2449.
- Friesen, M.C., Locke, S.J., Chen, Y.C., Coble, J.B., Stewart, P.A., Ji, B.T., Bassig, B., Lu, W., Xue, S., Chow, W.H., Lan, Q., Purdue, M.P., Rothman, N. and Vermeulen, R. (2015): Historical occupational trichloroethylene air concentrations based on inspection measurements from Shanghai, China. Ann. Occup. Hyg., 59, 62-78.
- Fukusumi, Y., Miyauchi, N., Hashimoto, T., Saito, A. and Kawachi, H. (2014): Therapeutic target for nephrotic syndrome: identification of novel slit diaphragm associated molecules. World J. Nephrol., 3, 77-84.
- Garsen, M., Rops, A.L., Dijkman, H., Willemsen, B., van Kuppevelt, T.H., Russel, F.G., Rabelink, T.J., Berden, J.H., Reinheckel, T. and van der Vlag, J. (2016): Cathepsin L is crucial for the development of early experimental diabetic nephropathy. Kidney Int., 90, 1012-1022.
- Guha, N., Loomis, D., Grosse, Y., Lauby-Secretan, B., El Ghissassi, F., Bouvard, V., Benbrahim-Tallaa, L., Baan, R., Mattock, H. and Straif, K.; International Agency for Research on Cancer Monograph Working Group. (2012): Carcinogenicity of trichloroethylene, tetrachloroethylene, some other chlorinated solvents, and their metabolites. Lancet Oncol., 13, 1192-1193.
- Hara, M., Yamagata, K., Tomino, Y., Saito, A., Hirayama, Y., Ogasawara, S., Kurosawa, H., Sekine, S. and Yan, K. (2012): Urinary podocalyxin is an early marker for podocyte injury in patients with diabetes: establishment of a highly sensitive ELISA to detect urinary podocalyxin. Diabetologia, 55, 2913-2919.
- Hayashi, T., Tokuriki, S., Okuno, T., Ohta, G., Igarashi, A. and Ohshima, Y. (2017): Urinary podocalyxin as a possible novel marker of intrauterine nephrogenesis and extrauterine podocyte injury. Pediatr. Nephrol., 32, 1891-1896.
- Huang, Y., Xia, L., Wu, Q., Zeng, Z., Huang, Z., Zhou, S., Jin, J. and Huang, H. (2015): Trichloroethylene Hypersensitivity Syndrome Is Potentially Mediated through Its Metabolite Chloral Hydrate. PLoS One, 10, e0127101.
- Kamijima, M., Hisanaga, N., Wang, H. and Nakajima, T. (2007): Occupational trichloroethylene exposure as a cause of idiosyncratic generalized skin disorders and accompanying hepatitis similar to drug hypersensitivities. Int. Arch. Occup. Environ. Health, 80, 357-370.
- Karami, S., Lan, Q., Rothman, N., Stewart, P.A., Lee, K.M., Vermeulen, R. and Moore, L.E. (2012): Occupational trichloroethylene exposure and kidney cancer risk: a meta-analysis. Occup. Environ. Med., 69, 858-867.
- Kim, I., Ha, J., Lee, J.H., Yoo, K.M. and Rho, J. (2014): The Relationship between the Occupational Exposure of Trichloroethylene and Kidney Cancer. Ann. Occup. Environ. Med., 26, 12.
- Kwon, Y.E., Lee, M.J., Park, K.S., Han, S.H., Yoo, T.H., Oh, K.H., Lee, J., Lee, K.B., Chung, W., Kim, Y.H., Ahn, C. and Choi, K.H. (2017): Cystatin C is Better than Serum Creatinine for Estimating Glomerular Filtration Rate to Detect Osteopenia in Chronic Kidney Disease Patients. Yonsei Med. J., 58, 380-387.
- Li, X., Ding, F., Zhang, X., Li, B. and Ding, J. (2016a): The Expression Profile of Complement Components in Podocytes. Int. J. Mol. Sci., 17, 471.
- Li, W., van Kuppeveld, F.J., He, Q., Rottier, P.J. and Bosch, B.J. (2016b): Cellular entry of the porcine epidemic diarrhea virus. Virus Res., 226, 117-127.
- Liszewski, M.K., Kolev, M., Le Friec, G., Leung, M., Bertram, P.G., Fara, A.F., Subias, M., Pickering, M.C., Drouet, C., Meri, S., Arstila, T.P., Pekkarinen, P.T., Ma, M., Cope, A., Reinheckel, T., Rodriguez de Cordoba, S., Afzali, B., Atkinson, J.P. and Kemper, C. (2013): Intracellular complement activation sustains T cell homeostasis and mediates effector differentiation. Immunity, 39, 1143-1157.
- Liu, M., Zhang, C., Yang, P., Huang, J., Zang, D.D., Zhang, J.X. and Zhu, Q.X. (2016): [Role of kallikrein kinin system activation in kidney injury induced by trichloroethylene sensitized mice]. Chin. J. Ind. Hyg. Occup. Dis., 34, 184-188.
- Liu, W., Hong, W.X., Zhang, Y., Huang, P., Yang, X., Ren, X., Huang, H. and Liu, J. (2015): Proteomic profiling of occupational medicamentosa-like dermatitis induced by trichloroethylene in serum based on MALDI-TOF MS. Clin. Exp. Med., 15, 519-526.
- Liu, X., Fan, Q., Yang, G., Liu, N., Chen, D., Jiang, Y. and Wang, L. (2013): Isolating glomeruli from mice: A practical approach for beginners. Exp. Ther. Med., 5, 1322-1326.
- Mallipattu, S.K. and He, J.C. (2016): The podocyte as a direct target for treatment of glomerular disease? Am. J. Physiol. Renal Physiol., 311, F46-F51.
- Markosyan, R.M., Miao, C., Zheng, Y.M., Melikyan, G.B., Liu, S.L. and Cohen, F.S. (2016): Induction of Cell-Cell Fusion by Ebola Virus Glycoprotein: Low pH Is Not a Trigger. PLoS Pathog., 12, e1005373.
- Minton, K. (2014): Innate immunity: the inside story on complement activation. Nat. Rev. Immunol., 14, 61.
- Odutayo, A. and Cherney, D. (2012): Cystatin C and acute changes in glomerular filtration rate. Clin. Nephrol., 78, 64-75.
- Onopiuk, A., Tokarzewicz, A. and Gorodkiewicz, E. (2015): Cystatin C: a kidney function biomarker. Adv. Clin. Chem., 68, 57-69.
- Pavenstädt, H., Kriz, W. and Kretzler, M. (2003): Cell biology of the glomerular podocyte. Physiol. Rev., 83, 253-307.
- Ricklin, D., Hajishengallis, G., Yang, K. and Lambris, J.D. (2010): Complement: a key system for immune surveillance and homeostasis. Nat. Immunol., 11, 785-797.
- Ricklin, D., Reis, E.S. and Lambris, J.D. (2016): Complement in disease: a defence system turning offensive. Nat. Rev. Nephrol., 12, 383-401.
- Saleem, M.A. (2015): One hundred ways to kill a podocyte. Nephrol. Dial. Transplant., 30, 1266-1271.
- Sever, S., Altintas, M.M., Nankoe, S.R., Möller, C.C., Ko, D., Wei, C., Henderson, J., del Re, E.C., Hsing, L., Erickson, A., Cohen, C.D., Kretzler, M., Kerjaschki, D., Rudensky, A., Nikolic, B. and Reiser, J. (2007): Proteolytic processing of dynamin by cytoplasmic cathepsin L is a mechanism for proteinuric kidney disease. J. Clin. Invest., 117, 2095-2104.
- Sudhan, D.R. and Siemann, D.W. (2015): Cathepsin L targeting in cancer treatment. Pharmacol. Ther., 155, 105-116.
- Takemoto, M., Asker, N., Gerhardt, H., Lundkvist, A., Johansson, B.R., Saito, Y. and Betsholtz, C. (2002): A new method for large scale isolation of kidney glomeruli from mice. Am. J. Pathol., 161, 799-805.
- Vermeulen, R., Zhang, L., Spierenburg, A., Tang, X., Bonventre, J.V., Reiss, B., Shen, M., Smith, M.T., Qiu, C., Ge, Y., Ji, Z., Xiong, J., He, J., Hao, Z., Liu, S., Xie, Y., Yue, F., Guo, W., Purdue, M., Beane Freeman, L.E., Sabbisetti, V., Li, L., Huang, H., Rothman, N. and Lan, Q. (2012): Elevated urinary levels of kidney injury molecule-1 among Chinese factory workers exposed to trichloroethylene. Carcinogenesis, 33, 1538-1541.
- Wang, H., Zhang, J.X., Li, S.L., Wang, F., Zha, W.S., Shen, T., Wu, C. and Zhu, Q.X. (2015): An Animal Model of Trichloroethylene-Induced Skin Sensitization in BALB/c Mice. Int. J. Toxicol., 34, 442-453.
- Weitoft, T., Larsson, A., Manivel, V.A., Lysholm, J., Knight, A. and Rönnelid, J. (2015): Cathepsin S and cathepsin L in serum and synovial fluid in rheumatoid arthritis with and without autoantibodies. Rheumatology (Oxford), 54, 1923-1928.
- Welsh, G.I. and Saleem, M.A. (2010): Nephrin-signature molecule of the glomerular podocyte? J. Pathol., 220, 328-337.
- Woo, J.T., Yamaguchi, K., Hayama, T., Kobori, T., Sigeizumi, S., Sugimoto, K., Kondo, K., Tsuji, T., Ohba, Y., Tagami, K. and Sumitani, K. (1996): Suppressive effect of N- (benzyloxycarbonyl)-L-phenylalanyl-L-tyrosinal on bone resorption in vitro and in vivo. Eur. J. Pharmacol., 300, 131-135.
- Yamashita, T., Asano, Y., Taniguchi, T., Nakamura, K., Saigusa, R., Takahashi, T., Ichimura, Y., Toyama, T., Yoshizaki, A., Miyagaki, T., Sugaya, M. and Sato, S. (2016): A potential contribution of altered cathepsin L expression to the development of dermal fibrosis and vasculopathy in systemic sclerosis. Exp. Dermatol., 25, 287-292.
- Yu, J.F., Feng, Y.Y. and Shen, X.F. (2017): Potential immunotoxic effects of trichloroethylene-induced IV allergic reaction in renal impairment. Cent. Eur. J. Immunol., 42, 140-149.
- Yu, J.F., Leng, J., Shen, T., Zhou, C.F., Xu, H., Jiang, T., Xu, S.H. and Zhu, Q.X. (2012): Possible role of complement activation in renal impairment in trichloroethylene-sensitized guinea pigs. Toxicology, 302, 172-178.
- Yue, F., Zeng, Z.M., Chen, R.T., Li, S.H. and Huang, H.L. (2007): Research on the changes of complements in patients of dermatitis medicamentosa-like induced by trichloroethylene. Chin. Occup. Med., 34, 448-449.
- Zhang, J.X., Li, N., Wang, H., Shen, T. and Zhu, Q.X. (2017): The immune response in trichloroethylene hypersensitivity syndrome: A review. Toxicol. Ind. Health, 33, 876-883.
- Zhang, J.X., Zha, W.S., Ye, L.P., Wang, F., Wang, H., Shen, T., Wu, C.H. and Zhu, Q.X. (2016): Complement C5a-C5aR interaction enhances MAPK signaling pathway activities to mediate renal injury in trichloroethylene sensitized BALB/c mice. J. Appl. Toxicol., 36, 271-284.
- Zhang, L.H., She, X.J., Li, M., Huang, L.R. and Liu, J.H. (2011): Dynamic observation on kidneys, livers and spleens by brightness mode ultrasonic imaging in patients of occupational medicamentosa-like dermatitis induced by trichloroethylene. Chin. J. Ind. Med., 24, 185-187.
- Zhao, N., Wang, H.L., Yue, F., Zeng, Z.M., Li, H.L., Huang, Y.S. and Chen, R.T. (2012): Studing the changes of the releated serum complement immune indexes in patients with occupational medicamentosa-like dermatitis induced by trichloroethylene and workers occupationally exposed to trichloroethylene. Chin. J. Ind. Hyg. Occup. Dis., 30, 284-288.









