2020 Volume 45 Issue 9 Pages 539-548
2020 Volume 45 Issue 9 Pages 539-548
We investigated the mechanism underlying intestinal cadmium (Cd) uptake based on the mediators (metal transporters) of essential elements, such as Fe, Zn, Cu, and Ca, under normal conditions in female rats. These elements interact with Cd uptake from the intestinal tract. Cd concentration at each site of the small intestine (duodenum, jejunum, and ileum) increased as Cd exposure increased. However, Cd concentration was the highest in the duodenum. The gene expression of ZIP14, DMT1, and ATP7A increased with increase in Cd concentration. Further, Cu concentration decreased as Cd concentration increased. In contrast, Fe concentration displayed a decreasing tendency with the increase in Cd concentration. The gene expression levels of ZIP14, DMT1, and ATP7A were positively correlated with Cd concentration. Immunohistochemical staining revealed the positive sites of ZIP14 and DMT1 scattered in the area adjacent to the goblet cells, resorbable epithelial cells, and lamina propria in the duodenum tissue, according to the increase in Cd concentration. Cd is induced to synthesize and bind to metallothionein (MT-I and -II) and accumulate in the intestinal tissues, mainly in the duodenum. Such findings suggest that Cd, a contaminant element, is taken up from the intestinal tract by multiple metal transporters such as Cu, Fe, and Zn, thereby involving in the intestinal Cd absorption.
Cadmium (Cd) is a ubiquitous toxic element present in our living environment. Cd uptake from the intestinal tract is the main route of Cd absorption upon exposure via the ingestion of Cd-contaminated food and water. Cd uptake via intestinal absorption was often found to be less than 5% of the ingested Cd, indicating a lower absorption efficiency. Once absorbed, Cd accumulates in the body with a half-life of 10-30 years (Järup et al., 1998). However, the precise mechanism of Cd uptake from the intestinal tract is difficult to clarify. Many studies, including in vitro, in vivo, and nutritional studies in humans, have reported that Cd uptake from the intestinal tract is affected by iron (Fe), zinc (Zn), and calcium (Ca). Several other factors have been suggested to influence intestinal Cd uptake. In general, women have a higher body burden of Cd than men (Nishijo et al., 2004; Vahter et al., 2007). For intestinal Cd uptake, the chemical form of Cd, such as Cd ion and Cd bound to metallothionein (Cd-MT) is reported to affect intestinal Cd uptake, Cd distribution in the liver and kidney, and its transport to the fetus via the placenta. Cd internalized as Cd-MT from the intestinal tract was selectively transported to the kidney in an in situ culture experiment; hence, the chemical form of Cd-MT is assumed to be incorporated by endocytosis into the intestinal tissue (Ohta and Cherian, 1991; Nakamura et al., 2012; Ohta et al., 1989).
In recent years, megalin and ubiquitin have been reported in the small intestine and kidneys as receptor proteins involved in membrane permeation of substances, such as cholesterol, sugar, and Cd-MT (Onodera et al., 2012). However, the amount of Cd-MT uptake from the intestinal tract compared to that of Cd uptake in its inorganic form, has been found to be small. In other words, the uptake of Cd-MT by receptors such as megalin in the intestinal tract is small. Hence, metal transporters such as Fe and Zn are suggested to increase Cd uptake (Christensen and Birn, 2002).
The increased gastrointestinal uptake of Cd at low Fe stores is mediated by the upregulation of divalent metal transporter 1 (DMT1) and ferroportin 1 (FPN1) (Kim et al., 2007: Ryu et al., 2004). In pregnant rats at gestational day 21, the DMT1 expression level in the duodenum was approximately 6-fold higher than that in non-pregnant rats. Further, Cd accumulation was significantly higher in the small intestine, liver, and kidneys (Leazer et al., 2002). Low dietary intake of Zn and Ca is reported to be associated with elevated Cd accumulation (Min et al., 2008b; Reeves and Chaney, 2008). The involvement of the bivalent metal transporter 1 (DMT1) (Leazer et al., 2002) and the two zinc transporters of ZIP8 and ZIP14 (Fujishiro et al., 2009, Girijashanker et al., 2008) has been reported. Several types of voltage-gated calcium (CaV) formation channels, such as L-type CaV and T-type CaV, have been reported to be related to metal transport (Friedman and Gesek, 1994; Souza et al., 1996).
Recent accumulating evidence suggests that intestinal Cd uptake is mediated via several different pathways that maintain essential elements in homeostasis, not only DMT1 but also transporters of Zn, copper(Cu), and Ca, such as ZIP4, ZIP8, ZIP14, ZnT1, ATP7A, and TRVP6 (Gunshin et al., 1997; Bressler et al., 2004; Suzuki et al., 2008; Min et al., 2008; Moreau et al., 2003; Kovacs et al., 2013; Reeves and Chaney, 2004; Kambe, 2013; Dalton et al., 2005; He et al., 2009; Girijashanker et al., 2008; Himeno et al., 2009; Fujishiro et al., 2009, 2013, 2019; Bouron et al., 2015; Kimura and Kambe, 2016; Reeves et al., 2005; Vesey, 2010).
Although many in vitro and in vivo studies have been conducted to date, they employed relatively high doses of Cd. As a result, only few studies have considered the low level of Cd, daily human intake, and the normal physiological condition with the coexisting matrix in vivo.
In the present study, a model for the oral administration of Cd was employed under normal physiological conditions to clarify the mechanism of intestinal Cd uptake by considering metal transporters for essential elements, such as Fe, Zn, and Cu.
The present study was approved by the Animal Ethics Committee of Kitasato University, Japan. Five-week-old female Wistar rats were purchased from CLEA Japan, Inc., Tokyo, Japan. The animals were fed commercial pellets (CE-2, CLEA Japan, Inc.) and administered water ad libitum. The animals were kept in temperature- and humidity-controlled rooms on a 12-hr light/dark cycle during the experimental period. After preliminary breeding for 1 week, rats were used in the experiment from the age of 6 weeks.
The animals were divided into four experimental groups (n = 5 per group). Cd (0, 1, 2, and 5 mg Cd/kg/day) was orally administered to rats five days per week for five weeks and distilled water was administered to the control group. At 24 hr after the final administration of Cd, animals were killed by cardiac blood collection under somnopentyl anesthesia. The animals were perfused with physiological saline from the heart to remove residual blood from the organs, and the intestinal tissue was collected immediately. The small intestine was washed with physiological saline and divided into three equal parts from the pyloric region to the large intestine.
Starting at the pylorus, the first 15 cm were regarded as the duodenum; the subsequent 30 cm were regarded as the jejunum; and the final 30 cm were regarded as the ileum. If the intestine was markedly longer or shorter than 75 cm, the segments were divided into similar proportions.
A part of each intestinal tissue was used for determination of metallothionein of metal concentration, metallothionein assay, and gene expression of metal transporter.
Gene expression analysis of the metal transporter and metallothioneinTotal RNA was isolated from the small intestine using ISOGEN (Nippon Gene Co. Ltd., Tokyo, Japan), according to the manufacturer’s instructions. Complementary DNA (cDNA) was synthesized from 1 µg of total-RNA using a PrimeScript®II 1st strand cDNA Synthesis kit (TaKaRa Bio Inc., Shiga, Japan). Quantitative real-time PCR was performed using Fast SYBR® Green Master Mix (Applied Biosystems) and a thermocycler (StepOne Real-Time PCR Systems: Applied Biosystems, Foster City, CA, USA).
Real-time PCR was carried out with a Fast SYBR® Green Master Mix using 1 μL of cDNA as the template and 1 μL of each primer in a total volume of 20 µL. The thermal cycling conditions of conventional real-time PCR were 10 min at 95°C, followed by 40 cycles of 15 sec at 95°C and 30 sec at 60°C. The primer sequences used for PCR of MT-I, II, III, ZIP4, ZIP14, DMT1, ATP7A, ZnT1, TRVP6, and β-ACTIN (BC063166) cDNAs are presented in Table 1. Primer specificity was evaluated by 1.0% agarose gel electrophoresis and as well as with the melt curve analysis. A melt curve analysis was performed to observe melting characteristics of the amplicon to determine the presence of the specific product. The PCR products of each gene were cloned into the pCRII®-TOPO® Vector (Invitrogen, Carlsbad, CA, USA). The standard curves were generated from serially diluted solutions (102−108 copies/µL) of each plasmid clone. A standard curve was prepared using the plasmid clone, and the expression level of the target gene in each tissue was quantified. The copy number (the number of genes) was calculated from the concentration of the plasmid DNA.
 Measurement of metal concentrations
Measurement of metal concentrations
The concentrations of Cd, Zn, Cu, and Fe in each part of the small intestine, namely the duodenum, jejunum, and ileum, were measured using flame and flameless methods with an atomic absorption photometer (Hitachi Z-5010) following wet-ashing of the tissues with nitric acid.
Measurement of MT concentrationEach sample of intestinal tissue were homogenized in a 0.25 M sucrose solution and centrifuged at 13000 rpm for 15 min. A portion of the resulting supernatant was used for MT determination. MT was measured by ELISA using the iso-MT (I, II) antibody (Metallothionein ELISA kit, Frontier Laboratories, Hokkaido, Japan).
Immunohistochemical stainingThe collected intestinal tissues were fixed in formalin. The paraffin blocks were synthesized with the Sakura automatic packaging machine, VIP-5Jr (Sakura Finetech, Tokyo, Japan) and cut into 3 microns with a Yamato microtome to prepare three consecutive sections, which were stained with a Ventana automatic immunostaining apparatus (Roche, Switzerland). Primary antibodies against anti-MT-I, II (rabbit, polyclonal), and the anti-MTIII (mouse, monoclonal) antibodies were obtained from Frontier Laboratories, Hokkaido, Japan. Antibodies of ZIP14(PAB19383 Abnova Corporation, Taipei, Taiwan), DMT1(LS-A9281-50 LifeSpan BioSciences, Seattle, WA, USA), ATP7A(bs-1572R-A48 Bioss Inc), and ZnT1(bs-6440R-Cy3 Bioss Inc., Boston, MA, USA) for immunostaining were purchased from Funakoshi Co., Ltd. (Tokyo, Japan).
Statistical analysesThe results are presented as mean and standard deviation. Data were analyzed using one-way analysis of variance (ANOVA), with Scheffé and Fisher’s PLSD post hoc tests at a significance level of P < 0.05. To reveal the correlation, Pearson’s correlation test was performed at a significance level of p < 0.05.
The Cd concentration at each site of the small intestine increased with an increase in Cd exposure. In particular, Cd concentration in the duodenum was high (Fig. 1). Although the concentrations of Cd in the duodenum, jejunum, and ileum increased, Zn concentration in the tissue at each site of the small intestine remained unchanged (Fig. 1, Table 2). Nonetheless, the concentrations of Cu and Fe in each part of the small intestine, especially the duodenum and jejunum, decreased with an increase in Cd concentration (Table 2). Cu concentration in the duodenum and jejunum significantly decreased in the 5 mg Cd/kg group (Table 2). Similarly, Fe concentration in the small intestine tissue tended to decrease with an increase in Cd concentration, and was significantly decreased in the jejunum of the 2 mg Cd/kg group (Table 2).

Cadmium concentration in the intestine of female rats after the oral Cd administration. Data are expressed as mean ± SD for five animals. *: Significant difference relative to the control group at p < 0.05 by ANOVA-Scheff or Fisher-PLSD.

Table 3 shows the gene expression of the metal transporter at each site of the small intestine. Based on the metal transporter gene expression, the gene expression of ZIP14, DMT1, ATP7A, and TRPV6 increased with a corresponding increase in the concentration of Cd in the duodenum. However, Zn concentration did not show any change in tendency (Table 2). Additionally, the gene expression of MTI and II significantly increased with an increase in Cd concentration (Fig. 2). In the jejunum and ileum, an increase in the tendency of MT gene expression was observed (results not shown). However, no change was observed in the expression of the MTIII gene (Fig. 2). Based on ELISA, the duodenal MT concentration (with antibody MTI, II) showed a tendency to increase as Cd concentration increased. Further, it significantly increased in the 2 mg Cd/kg and 5 mg Cd/kg administration groups (Fig. 3).


Gene expression of metallothione in the duodenum of female rats after the oral Cd administration. Data are expressed as mean ± SD for five animals. MTI: metallothionein I MTII: metallothionein II MTIII: metallothionein III. Data represent the relative gene expression to β-actin (x103). *: Significant difference relative to the control group at p < 0.05 by ANOVA-Scheff or Fisher-PLSD.

MT concentration in the duodenum of female rats after the oral Cd administration. Data are expressed as mean ± SD for five animals. MT was measured by ELISA using the iso-MT (I, II) antibody. *: Significant difference relative to the control group at p < 0.05 by ANOVA-Scheff or Fisher-PLSD.
The correlation between Cd concentration in the duodenum and the gene expression of each transporter is presented in Figs. 4, 5, 6, and 7. At 5 weeks after the oral Cd administration, the gene expression levels of ZIP14, DMT1, and ATP7A were positively correlated with Cd concentration in the duodenum. TRPV6, a Ca transporter, also displayed a positive correlation trend (Fig. 7). Despite the decrease in Cu concentration with increase in Cd concentration, ATP7A of the Cu transporter showed a tendency to increase with an increase in Cd concentration (Fig. 6, results not shown). The expression of the DMT1 gene increased with an increase in Cd concentration, and the Fe concentration showed a decreasing tendency (Figs. 5 and 8). As Cd concentration increased, the concentration of Cu in the duodenum decreased. Further, Fe concentration displayed a decreasing trend; however, Zn concentration remained unchanged (Fig. 8).

Correlation between Cd concentration and the relative gene expression of ZIP14 in the duodenum of female rats after the oral Cd administration. Gene expression is represented as relative gene expression to β-actin (x103). Correlation coefficient and p value are represented in graph. Pearson’s correlation test was performed at a significance level of p < 0.05.

Correlation between Cd concentration and the gene expression of DMT1 in the duodenum of female rats after the oral Cd administration. Gene expression is represented as relative gene expression to β-actin (x103). Correlation coefficient and p value are represented in graph. Pearson’s correlation test was performed at a significance level of p < 0.05.

Correlation between Cd concentration and the gene expression of ATP7A in the duodenum of female rats after the oral Cd administration. Gene expression is represented as relative gene expression to β-actin (x103). Correlation coefficient and p value are represented in graph. Pearson’s correlation test was performed at a significance level of p < 0.05.

Correlation between Cd concentration and the gene expression of TRVP6 in the duodenum of female rats after the Cd oral administration. Gene expression is represented as relative gene expression to β-actin (x103). Correlation coefficient and p value are represented in graph. Pearson’s correlation test was performed at a significance level of p < 0.05.
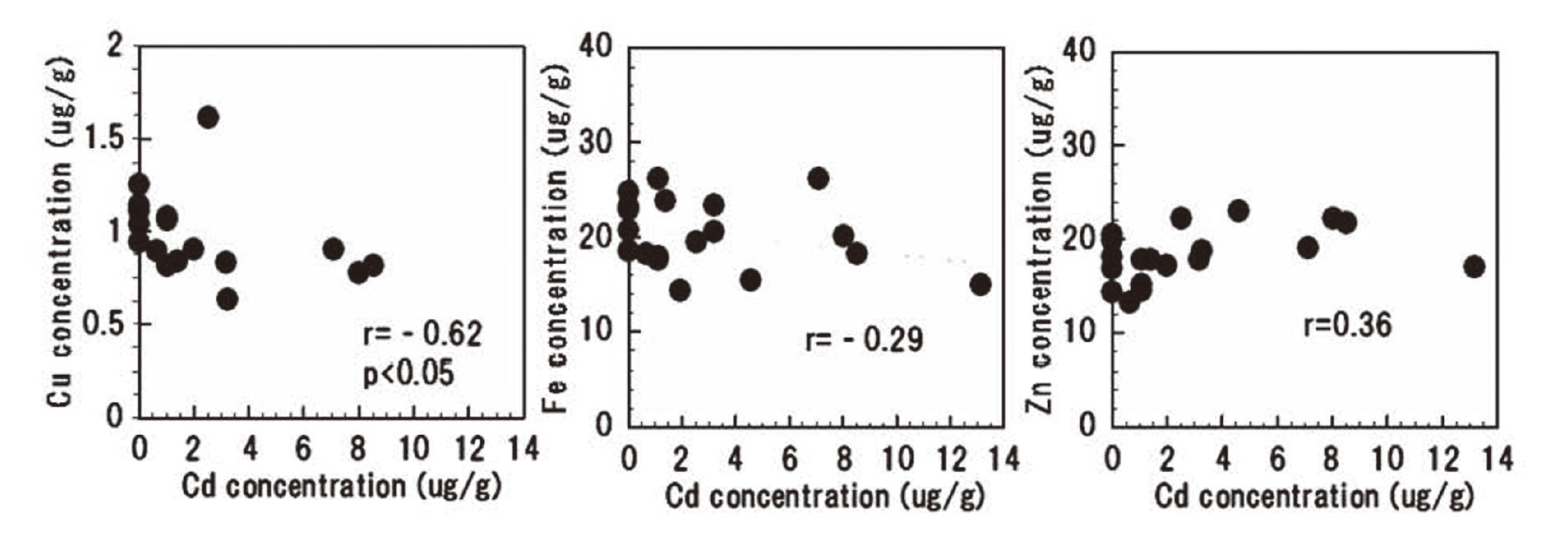
Relationship between cadmium and copper, iron, and zinc in the intestine of female rats after the oral Cd administration. Correlation coefficient (r) is represented in each graph. Pearson’s correlation test was performed at a significance level of p < 0.05.
The immunostaining results of ZIP14, DMT1, and ATP7A in the duodenal tissue at 5 weeks of Cd administration are shown in Fig. 9. Immunostaining revealed positive sites of ZIP14, DMT1, and ATP7A scattered in the area adjacent to the goblet cells, resorbable epithelial cells, and the lamina propria in the small intestine tissue according to the increase in Cd concentration (Fig. 9).

Immunohistochemical staining of the metal transporters in the duodenum of female rats after the oral Cd administration. Immunostaining revealed the positive sites of Zip14, DMT1, and ATP7A scattered in the Area of resorbable epithelial cells (x200). Arrows show strong positive immunochemical staining of the metal transporters.
There was no increase in the gene expression of ZIP4 and ZnT1, which correspond to the increase in Cd concentration (data not shown).
In the past, many studies have been revealed the metal–metal interactions on intestinal absorption and Cd distribution under conditions of excess and deficient Zn, Fe, Cu, and Ca (Foulkes, 1986). However, studies on the detailed mechanism of intestinal absorption of the contaminant Cd have not been sufficiently presented.
In recent years, the metal transport protein, DMT1, has been reported to be a membrane protein that transports Fe, Zn, Cu, Cd, Mn, and Pb (Gunshin et al., 1997). Many studies have also reported the existence and function of transport proteins related to the transport of Zn, Cu, Fe, and Ca. However, few studies have examined the intestinal absorption of Cd, a contaminant element, in relation to different metal transporters.
In the present study, the intestinal tissue concentrations of Cd, especially in the duodenum, significantly increased as the oral administration of Cd increased (Fig. 1). By analyzing MT gene expression in the intestinal tissues, we found that the expression of the MTI and MTII genes increased with increase in duodenal Cd exposure. However, MTIII gene expression level remained unchanged by the increase in Cd concentration. It was thought that Cd incorporated into the intestinal tissue bound to MT and accumulated in the intestinal tissue (Figs. 1, 2 and 3). According to the relationship between Cd concentration in the intestinal tissue and the Cu and Fe concentrations, both Cu and Fe tended to decrease as the Cd concentration increased. Further, a negative correlation was found between the Cd and Cu concentrations (Table 2, Fig. 8).
With the increase in Cd concentration, DMT1, ZIP14, ATP7A, and TRPV6 expression significantly increased in the duodenum. Additionally, a significant positive correlation was found between Cd concentration and the gene expression of ZIP14, DMT1, ATP7A, and TRPV6. Further, in the immunostaining, positive staining was observed in the epithelium (absorbing epithelial cells) of the duodenal tissue in the 5 mg Cd/kg administration group. In particular, a remarkable positive staining was observed, which was scattered at sites near the pannet cells, goblet cells, and duodenal glands. Such findings suggested the involvement of DMT1, ZIP14, and ATP7A, mainly in the Cd absorption in the duodenum. Additionally, the increased gene expression of TRVP6, the Ca-related transporter genes, suggested that it is possibly related to the intestinal Cd absorption (Fig. 7, Table 3). However, no correlation was found between Cu, Zn, and Fe concentrations and these metal transporters (results not shown).Collectively, these findings suggest that for the intestinal Cd uptake, the induction of the transporters of these homologous elements in the periodic table is promoted, and Cd is taken up into the intestinal tissues through the function of these transporters. Considering together with the results of our study and the other study suggesting that DMT1 is not the only transporter of intestinal Cd absorption (Suzuki et al., 2008), it was thought that multiple metal transporters are involved in the intestinal Cd uptake.
It has been reported in the previously conducted in situ experiments that small amounts of Cd-MT are selectively transported and accumulated in the kidney; however, the mechanism of efflux and the transfer from small intestinal tissue to the bloodstream remain unknown (Ohta and Cherian, 1991).
As no increase in ZnT1 gene expression was observed in response to the increase in Cd concentration, it was thought that the Cd taken up into the intestinal tissue was bound by the MT synthesized in the duodenum and was maintained a dynamic equilibrium, and mainly accumulated. After that, it was speculated that Cd may have been slowly released from the MT or the Cd unbound to MT may have been transferred to the bloodstream via transporters, such as ZnT1, thereby transferring to various internal organs for accumulation.
The detailed mechanism of Cd transport from the intestinal tissue to the bloodstream, including other metal transporters and factors related to metal transport such as megalin, should be examined in a future study (Zalups and Ahmad, 2003; Onodera et al., 2012).
The authors wish to thank Soshiki Kagaku lab Inc. (Hodogaya, Yokohama, Japan) for the technical support provided for immunohistochemical staining. We also thank Mr. Kai Ri and Miss Sae Shimamura at the Department of Occupational, Environmental Health and Toxicology, School of Allied Health Sciences, Kitasato University, for their technical assistance.
Conflict of interestThe authors declare that there is no conflict of interest.