Abstract
Complement component 8 γ (C8γ) is a subunit of complement protein 8 (C8), which itself is a subunit of the complement cytolytic membrane attack complex. However, C8γ is also suggested to be a carrier protein for the general clearance of endogenous and exogenous compounds because it belongs to the lipocalin family of small secreted proteins that have the common ability to bind small hydrophobic ligands. Although retinoic acid, a metabolite of vitamin A, has been suggested as a potential ligand of C8γ, it remains unclear which other substances are able to bind to C8γ as ligands. Here, we evaluated the binding affinity of several organotin compounds that are ligands of a receptor of retinoic acid, retinoid X receptor, by using radioligand binding assays. The amount of [14C]triphenyltin (TPT), a tri-substituted organotin, that bound to purified recombinant C8γ was increased with increasing protein concentration, whereas that of [3H]all-trans retinoic acid and [3H]9-cis retinoic acid was unchanged. Scatchard analysis revealed that [14C]TPT bound to C8γ with an equilibrium dissociation constant (Kd) of 56.2 ± 16.2 nM. Non-radiolabeled tributyltin (TBT), another tri-substituted organotin, blocked the binding of [14C]TPT to C8γ in a competitive manner, but non-radiolabeled mono- or di-substituted organotin compounds did not. Together, our present observations indicate that TBT and TPT, but not retinoic acid or mono- or di-substituted organotin compounds, are potent ligands of C8γ, suggesting that C8γ may be involved in the toxicities of these organotin compounds.
INTRODUCTION
The lipocalin protein family is a large group of small extracellular proteins. These proteins have three common structures: An N-terminal 310-like helix; an eight-stranded continuously hydrogen-bonded antiparallel β-barrel; and a C-terminal α-helix called lipocalin. Lipocalins are present in a wide range of species from nematodes to mammals, and have roles in various biological processes including hydrophobic substance transportation, invertebrate cryptic coloration, and prostaglandin synthesis (Kawaguchi et al., 2007; Nagata et al., 1991; Cianci et al., 2002). Although the functions of these proteins are largely unknown, many reports suggest that they bind to hydrophobic biologically active substances such as vitamin A metabolites (retinoids), fatty acids, steroids, thyroid hormone, and pheromones (Kawaguchi et al., 2007; Flower et al., 2000; Schiefner and Skerra, 2015). In particular, studies of retinol binding protein 4, α2u-globulin, and β-lactoglobulin have suggested that retinoids are common ligands for lipocalins (Dufour et al., 1990; Cogan et al., 1976; Cavaggioni et al., 1990; Flower, 1996; Kawaguchi et al., 2007). However, the lipocalin binding spectra are likely to vary greatly because of the many differences in the amino acid sequences of the pockets and loops among the lipocalin proteins. Indeed, xenobiotics such as medicines and environmental chemicals have also been shown to bind to lipocalins. For instance, the lipocalin α1-acid glycoprotein, which consists of a β-barrel surrounded by three lipocalin motifs, can bind to hundreds of chemicals and alter their pharmacokinetics (Israili and Dayton, 2001). Therefore, lipocalins are potential key molecules in the pharmacological effects of medicines and the toxicities of environmental chemicals.
Complement component 8 γ (C8γ) is a 22-kDa subunit of complement component 8 (C8), which itself is one of five components (C5b, C6, C7, C8, C9) that interact to form the cytolytic membrane attack complex (MAC). C8 consists of three nonidentical subunits (α, β, γ), among which C8γ binds to C8α via a disulfide bond between two cysteine residues (Steckel et al., 1980; Haefliger et al., 1991; Lovelace et al., 2011). It has been reported that C8γ is dispensable to the function of MAC (Parker and Sodetz, 2002), but no other physiological function has yet been identified for C8γ. However, C8γ is the only complement component that contains a lipocalin motif, suggesting that it may have a role as a carrier protein for the general clearance of endogenous and exogenous compounds. A few reports have indicated that retinoids are potential endogenous substances that bind to C8γ (Haefliger et al., 1991; Schreck et al., 1998), but it remains unclear which substances specifically bind to C8γ as ligands.
Organotin compounds, such as tributyltin (TBT) and triphenyltin (TPT), are tin-containing environmental contaminants and suspected endocrine-disrupting chemicals (Nakanishi et al., 2002; Nakanishi, 2008; Hiromori et al., 2016b). We previously reported that TBT and TPT at the nanomolar level act as agonists of the nuclear receptor retinoid X receptor (RXR) (Nakanishi et al., 2005; Hiromori et al., 2009). We also demonstrated that these two organotin compounds cause endocrine disruption in human placental cells and female gastropods via RXR-mediated signaling pathways (Nakanishi et al., 2005, 2006; Castro et al., 2007; Hiromori et al., 2015). One of the retinoids, 9-cis retinoic acid (9cRA), is most typical agonist of RXR (Levin et al., 1992), and the ligand activities of both TBT and TPT against RXR are comparable with that of 9cRA (Nakanishi et al., 2005). In addition, it has been reported that TBT is a potential ligand for the fish lipocalin tributyltin-binding protein, which plays a role in reducing the toxicity of TBT (Shimasaki et al., 2002; Satone et al., 2008). The counterparts of tributyltin-binding protein in mammals have not yet been reported, but this previous data suggests that organotin compounds are potential ligands of C8γ.
In the present study, we constructed a recombinant C8γ protein and evaluated the specific binding of several organotin compounds to the recombinant C8γ in comparison with that of two geometric isomers of retinoic acid, 9cRA and all-trans retinoic acid (atRA).
MATERIALS AND METHODS
Chemicals
The organotin compounds used in the present study were purchased from Tokyo Kasei Chemical (Tokyo, Japan) or Sigma-Aldrich (St. Louis, MO, USA) and are listed in Table 1. [3H]9cRA (9-cis-retinoic acid, [11,12-3H]; 1.85 TBq/mmol) was purchased from PerkinElmer (Waltham, MA, USA). [3H]atRA (all-trans retinoic acid [11,12-3H]; 1.665 TBq/mmol) was purchased from American Radiolabeled Chemicals (Saint Louis, MO, USA). [14C]TPT (Tri[U-14C]-phenyltin hydroxide; radiochemical purity > 96.6%, 2.04 GBq/mmol) was custom-made by Amersham Biosciences (Piscataway, NJ, USA) (Nakanishi et al., 2005; Castro et al., 2007; Hiromori et al., 2009, 2016a). All chemicals were dissolved in dimethyl sulfoxide purchased from Nacalai Tesque (Kyoto, Japan).
Table 1. Organotin compounds tested in the present study.
 Plasmid construction
Plasmid construction
Full-length mouse C8γ cDNA (Gene Accession No. NM_027062.2) was amplified by reverse transcription polymerase chain reaction using total RNA from C57BL/6J mouse liver and cloned into pCold-TF expression vector (TaKaRa Bio, Shiga, Japan). The sequences synthesized by polymerase chain reaction were confirmed by DNA sequencing.
Protein expression and purification
6×His-tagged trigger factor (TF)-C8γ hybrid protein (TF-C8γ) was expressed in plasmid-transformed Escherichia coli BL21 (DE3) cells cultured at 30°C in Lysogeny Broth medium containing 500 μg/mL ampicillin to an OD600 of 0.3-0.5. The cells were then induced by the addition of 500 μg/mL ampicillin and isopropyl β-D-1-thiogalactopyranoside to a final concentration of 0.1 mM and cultured at 15°C for a further 24 hr. Cultured cells were harvested by centrifugation and lysed by sonication. The supernatant was applied to a 1-mL HisTrap HP column (GE Healthcare, Chicago, IL, USA) attached to an ÄKTAprime plus chromatography system (GE Healthcare). The column was washed with 5 volumes of wash solution (50 mM sodium phosphate buffer [pH = 7.4] containing 0.5 M NaCl and 5 mM imidazole). The recombinant TF-C8γ protein was eluted with elution solution (50 mM sodium phosphate buffer [pH = 7.4] with 0.5 M NaCl and 0.5 M imidazole). Desalting and imidazole removal of the eluate were accomplished with a PD-10 column (GE Healthcare). TF-tag protein was expressed from empty pCold-TF expression vector and purified by the same method as TF-C8γ protein.
Radioligand binding assay
The purified TF-C8γ protein (750 μg/mL) or TF-tag protein was incubated at 4°C for 1 hr with 200 nM [14C]TPT or 50 nM of [3H]atRA or [3H]9cRA with or without non-radiolabeled ligands or test compounds. After incubation, specific binding was determined by using the hydroxyapatite binding assay (Nakanishi et al., 2005; Castro et al., 2007; Hiromori et al., 2009, 2016a). In brief, hydroxyapatite was added to precipitate the TF-C8γ protein and bind radioactive compounds. The hydroxyapatite was collected by centrifugation. The obtained pellet was washed, and then the radioactivity in the pellet was determined by liquid scintillation counting. Nonspecific binding was defined as binding in the presence of a molar excess of non-radiolabeled TPT (100-fold) or 9cRA or atRA (1000-fold); specific binding was defined as total binding minus nonspecific binding. As a competition binding assay, the purified TF-C8γ protein was incubated with 200 nM [14C]TPT and increasing concentrations of the test compounds.
RESULTS AND DISCUSSION
C8γ specifically binds to TPT, but not retinoic acid
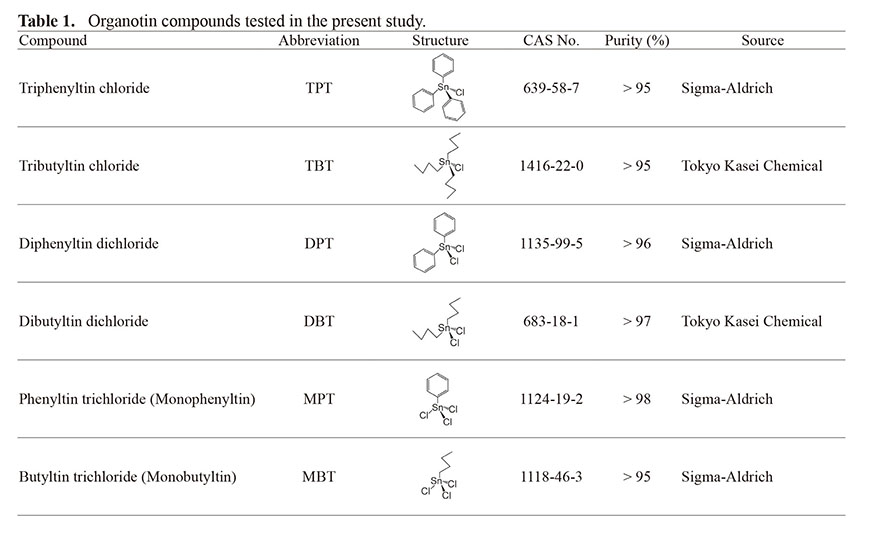
We performed radioligand-binding assays to characterize the binding affinities of TPT and two geometric isomers of retinoic acid, 9cRA and atRA. A constant concentration of radiolabeled substrate was incubated with an increasing concentration of purified TF-C8γ, and the amount of substrate that bound to the protein was measured. The amounts of [3H]9cRA and [3H]atRA that bound to the protein were very small and remained constant irrespective of the concentration of TF-C8γ (Fig. 1), suggesting that neither of these compounds are ligands of C8γ. However, the amount of [14C]TPT that bound to the protein increased with increasing protein concentration, and saturation was achieved at a relative binding rate of about 30% and a TF-C8γ concentration of 400 μg/mL or more (Fig. 1), suggesting that TPT is a ligand of C8γ.
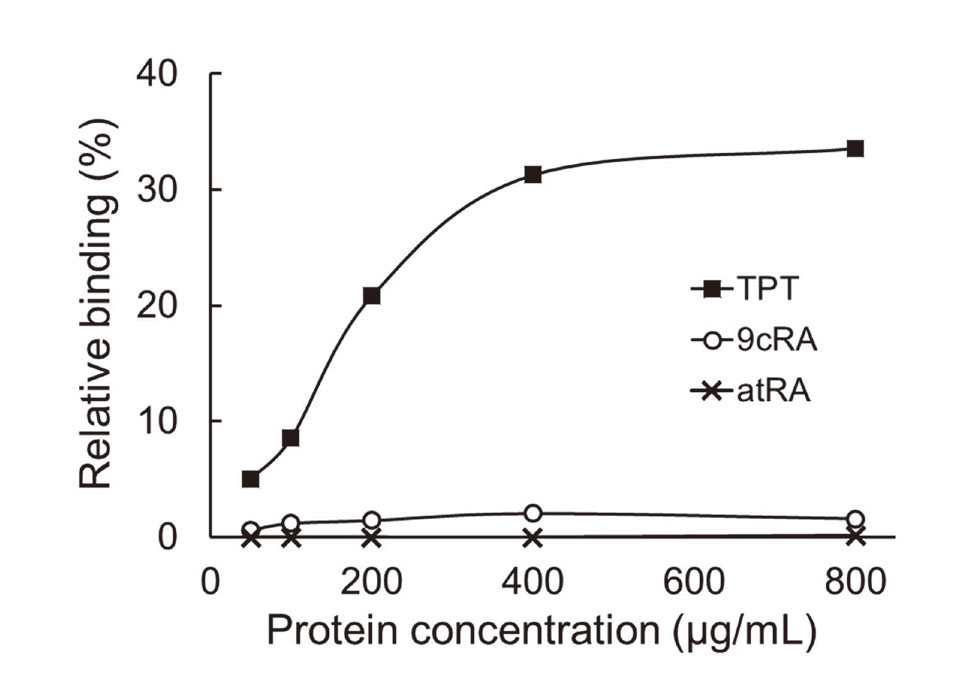
To confirm the binding specificity of [14C]TPT for C8γ, we performed a competitive ligand-binding assay experiment using empty TF-tag protein. Constant concentrations of [14C]TPT and either empty TF-tag protein or TF-C8γ were incubated with or without non-radiolabeled 100-fold molar excess of TPT, and the amount of [14C]TPT that bound to each protein was measured. Non-radiolabeled TPT markedly blocked the binding of [14C]TPT to TF-C8γ, but had no effect on the binding to empty TF-tag protein (Fig. 2), indicating that the observed binding of [14C]TPT to TF-C8γ was specific for C8γ and reversible.
Previously, based on the results of a density gradient assay, Haefliger et al. (1991) reported that retinoic acid and retinol both specifically bind to C8γ, which is inconsistent with our present results. However, it is possible that direct binding of these retinoids to C8γ may have been detected by Haefliger et al. because of their use of crude protein products. In contrast, Schreck et al. examined whether retinol is a ligand of C8γ by using purified recombinant protein and methods commonly used for the characterization of retinol-binding proteins, and they found no evidence that retinol is a ligand of either C8α-γ or C8γ (Schreck et al., 1998). Schreck et al. (1998) considered the binding of retinol to C8γ in the experiments of Haefliger et al. (1991) to be due to nonspecific hydrophobic binding under extremely ionic conditions (2 M NaCl). Indeed, the ionic condition in the experiments of Schreck et al. (1998) (150 mM NaCl) and that of ours (100 mM KCl, 5 mM MgCl2) was not so high as compared with that of Haefliger et al. In addition, Haefliger et al. (1991) used crude proteins from serum as C8γ protein, whereas Schreck et al. (1998) and we used recombinant purified proteins. It suggests that Haefliger et al. might have detected retinol and retinoic acid bound to proteins other than C8γ. Taken together, the previous and present data indicate that retinoids are not ligands of C8γ.
Scatchard analysis of TPT binding to C8γ
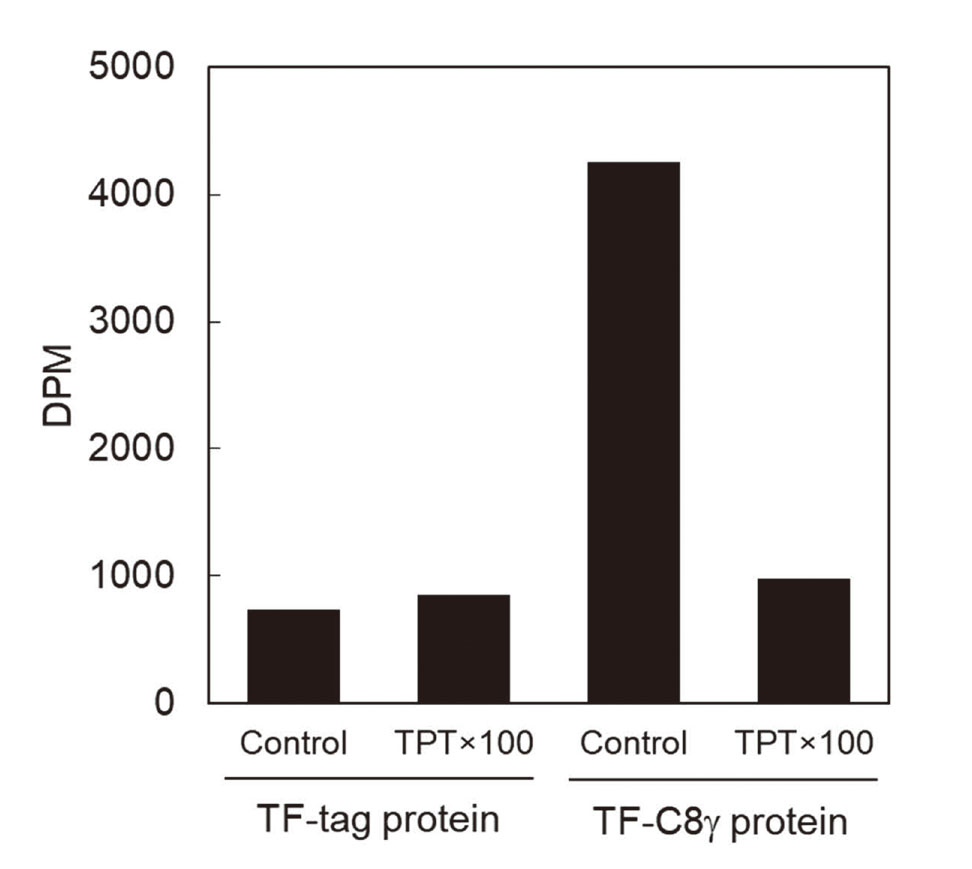
To directly characterize the binding affinity of TPT to C8γ, we further examined the saturation binding of [14C]TPT to TF-C8γ. Representative binding curves and Scatchard plots for [14C]TPT binding to TF-C8γ are shown in Fig. 3. The binding of [14C]TPT to TF-C8γ was specific and saturating. Scatchard analysis yielded an equilibrium dissociation constant (Kd) of 56.2 ± 16.2 nM for the binding of [14C]TPT to TF-C8γ.
We previously reported that TPT is a powerful agonist of RXR and peroxisome proliferator-activated receptor gamma (PPARγ), which are both members of the nuclear receptor superfamily (Kanayama et al., 2005; Nakanishi et al., 2005; Hiromori et al., 2009). We also demonstrated, using X-ray crystallography and mass spectroscopy, that TBT and TPT stably bind to RXR and PPARγ via a coordinate ionic bond between the tin atom of the organotin compounds and a critical cysteine residue in the receptors (Harada et al., 2015). Furthermore, we previously performed Scatchard analyses of [14C]TPT binding to these receptors and reported Kd values for RXRα and PPAR of 55.5 nM (Nakanishi et al., 2005) and 66.6 nM (Hiromori et al., 2009), respectively. The Kd value for the binding of TPT to TF-C8γ obtained in the present experiment was comparable with those for the binding of TPT to RXRα and PPARγ, and suggests that TPT binds very strongly to C8γ.
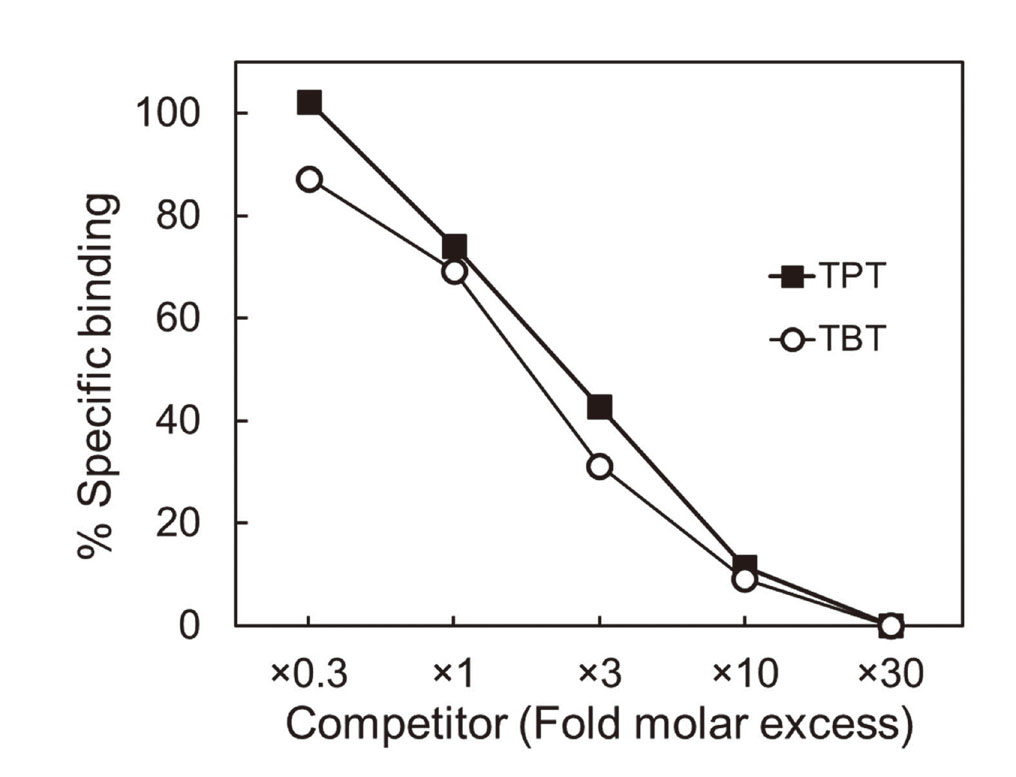
Competition of butyltin and phenyltin compounds with [14C]TPT for binding to C8γ
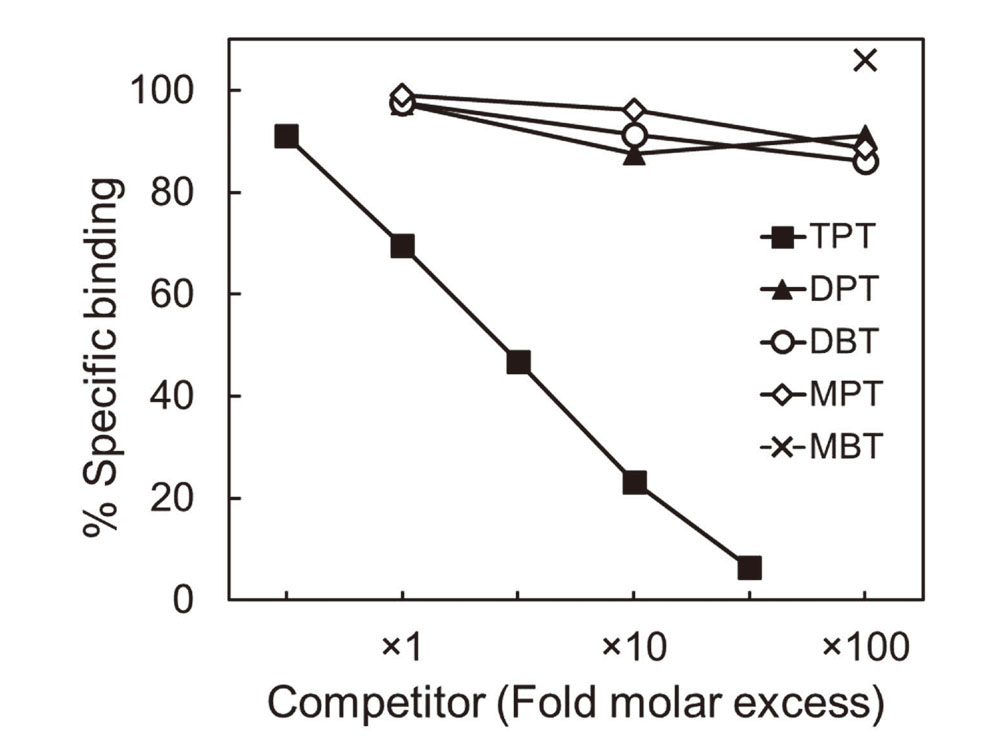
To examine whether other organotin compounds other than TPT bind to C8γ as ligands, we performed a series of competitive ligand-binding assays in which we measured the ability of butyltin and phenyltin compounds to compete with [14C]TPT for binding to TF-C8γ. TBT and non-radiolabeled TPT competed with [14C]TPT for binding to TF-C8γ in a concentration-dependent manner (Fig. 4). The competitive binding kinetics of TBT were comparable with those of TPT, suggesting that the binding affinity of TBT to TF-C8γ was also specific and strong. In contrast, diphenyltin (DPT), dibutyltin (DBT), and monophenyltin (MPT) competed only slightly with [14C]TPT, and monobutyltin (MBT) failed to compete with [14C]TPT (Fig. 5). Although a similar trend was observed in our previous studies in which we examined the binding of organotin compounds to RXR and PPARγ (Nakanishi et al., 2005; Hiromori et al., 2009), our present observations suggest that the ability of organotin compounds to bind to C8γ depends on the number of alkyl or aryl groups in the organotin compound, and also that tri-substituted organotin compounds are the most potent ligands for C8γ.
Lovelace et al. (2011) have suggested that C8γ is unable to bind small hydrophobic molecules because the binding of C8γ to C8α is accomplished via a disulfide bond at cysteine 60, which is located near to the lipocalin binding pocket. However, free C8γ is released from the liver to the blood and exists as a single molecule in tissues and blood (Trojer et al., 1999), so it may be able to bind its ligands before it binds to C8α and becomes part of MAC. Together with our present findings, this suggests that C8γ may act as a carrier protein for TBT and TPT. Furthermore, our previously reported finding that the binding of RXR and PPARγ with TBT and TPT requires the formation of a coordinate ionic bond between a critical cysteine residue of the receptors and the tin atom of the organotin compounds (Harada et al., 2015), suggests that free C8γ may bind to TBT and TPT via cysteine 60 and therefore that it may modulate their toxicities and toxicokinetics. In contrast, because C8γ hardly binds to the metabolites of TBT and TPT (i.e., DBT, DPT, MBT, and MPT), it is unlikely that C8γ modulates the toxicities and toxicokinetics of these compounds.
In the present study, we found that TPT and TBT, but not retinoic acid or mono- or di-substituted organotin compounds, are potent ligands of C8γ. These findings suggest that C8γ may be involved in the toxicity of these organotin compounds. Although C8γ binds specifically and strongly to tri-substituted organotin compounds, these organotins are nothing but xenobiotics. Perhaps C8γ has an endogenous counterpart instead of these organotin compounds. In the future, searches for other ligands of C8γ, and animal experiments to elucidate the significance of C8γ in the toxicities of organotin compounds, are needed.
ACKNOWLEDGMENTS
This research was supported in part by a Grant-in-Aid for Scientific Research (B) (18H03381) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and the Long-range Research Initiative of the Japan Chemical Industry Association.
Conflict of interest
The authors declare that there is no conflict of interest.
REFERENCES
- Castro, L.F., Lima, D., Machado, A., Melo, C., Hiromori, Y., Nishikawa, J., Nakanishi, T., Reis-Henriques, M.A. and Santos, M.M. (2007): Imposex induction is mediated through the Retinoid X Receptor signalling pathway in the neogastropod Nucella lapillus. Aquat. Toxicol., 85, 57-66.
- Cavaggioni, A., Findlay, J.B. and Tirindelli, R. (1990): Ligand binding characteristics of homologous rat and mouse urinary proteins and pyrazine-binding protein of calf. Comp. Biochem. Physiol. B, 96, 513-520.
- Cianci, M., Rizkallah, P.J., Olczak, A., Raftery, J., Chayen, N.E., Zagalsky, P.F. and Helliwell, J.R. (2002): The molecular basis of the coloration mechanism in lobster shell: beta-crustacyanin at 3.2-A resolution. Proc. Natl. Acad. Sci. USA, 99, 9795-9800.
- Cogan, U., Kopelman, M., Mokady, S. and Shinitzky, M. (1976): Binding affinities of retinol and related compounds to retinol binding proteins. Eur. J. Biochem., 65, 71-78.
- Dufour, E., Marden, M.C. and Haertlé, T. (1990): Beta-lactoglobulin binds retinol and protoporphyrin IX at two different binding sites. FEBS Lett., 277, 223-226.
- Flower, D.R. (1996): The lipocalin protein family: structure and function. Biochem. J., 318, 1-14.
- Flower, D.R., North, A.C. and Sansom, C.E. (2000): The lipocalin protein family: structural and sequence overview. Biochim. Biophys. Acta, 1482, 9-24.
- Haefliger, J.A., Peitsch, M.C., Jenne, D.E. and Tschopp, J. (1991): Structural and functional characterization of complement C8 gamma, a member of the lipocalin protein family. Mol. Immunol., 28, 123-131.
- Harada, S., Hiromori, Y., Nakamura, S., Kawahara, K., Fukakusa, S., Maruno, T., Noda, M., Uchiyama, S., Fukui, K., Nishikawa, J., Nagase, H., Kobayashi, Y., Yoshida, T., Ohkubo, T. and Nakanishi, T. (2015): Structural basis for PPARγ transactivation by endocrine-disrupting organotin compounds. Sci. Rep., 5, 8520.
- Hiromori, Y., Aoki, A., Nishikawa, J., Nagase, H. and Nakanishi, T. (2015): Transactivation of the human retinoid X receptor by organotins: use of site-directed mutagenesis to identify critical amino acid residues for organotin-induced transactivation. Metallomics, 7, 1180-1188.
- Hiromori, Y., Ido, A., Aoki, A., Kimura, T., Nagase, H. and Nakanishi, T. (2016a): Ligand Activity of Group 15 Compounds Possessing Triphenyl Substituent for the RXR and PPARγ Nuclear Receptors. Biol. Pharm. Bull., 39, 1596-1603.
- Hiromori, Y., Nishikawa, J., Yoshida, I., Nagase, H. and Nakanishi, T. (2009): Structure-dependent activation of peroxisome proliferator-activated receptor (PPAR) gamma by organotin compounds. Chem. Biol. Interact., 180, 238-244.
- Hiromori, Y., Yui, H., Nishikawa, J., Nagase, H. and Nakanishi, T. (2016b): Organotin compounds cause structure-dependent induction of progesterone in human choriocarcinoma Jar cells. J. Steroid Biochem. Mol. Biol., 155 (Pt B), 190-198.
- Israili, Z.H. and Dayton, P.G. (2001): Human alpha-1-glycoprotein and its interactions with drugs. Drug Metab. Rev., 33, 161-235.
- Kanayama, T., Kobayashi, N., Mamiya, S., Nakanishi, T. and Nishikawa, J. (2005): Organotin compounds promote adipocyte differentiation as agonists of the peroxisome proliferator-activated receptor gamma/retinoid X receptor pathway. Mol. Pharmacol., 67, 766-774.
- Kawaguchi, R., Yu, J., Honda, J., Hu, J., Whitelegge, J., Ping, P., Wiita, P., Bok, D. and Sun, H. (2007): A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science, 315, 820-825.
- Levin, A.A., Sturzenbecker, L.J., Kazmer, S., Bosakowski, T., Huselton, C., Allenby, G., Speck, J., ratzeisen, C., Rosenberger, M., Lovey, A. and Grippo, J.F. (1992): 9-cis retinoic acid stereoisomer binds and activates the nuclear receptor RXR alpha. Nature, 355, 359-361.
- Lovelace, L.L., Cooper, C.L., Sodetz, J.M. and Lebioda, L. (2011): Structure of human C8 protein provides mechanistic insight into membrane pore formation by complement. J. Biol. Chem., 286, 17585-17592.
- Nagata, A., Suzuki, Y., Igarashi, M., Eguchi, N., Toh, H., Urade, Y. and Hayaishi, O. (1991): Human brain prostaglandin D synthase has been evolutionarily differentiated from lipophilic-ligand carrier proteins. Proc. Natl. Acad. Sci. USA, 88, 4020-4024.
- Nakanishi, T. (2008): Endocrine disruption induced by organotin compounds; organotins function as a powerful agonist for nuclear receptors rather than an aromatase inhibitor. J. Toxicol. Sci., 33, 269-276.
- Nakanishi, T., Hiromori, Y., Yokoyama, H., Koyanagi, M., Itoh, N., Nishikawa, J. and Tanaka, K. (2006): Organotin compounds enhance 17beta-hydroxysteroid dehydrogenase type I activity in human choriocarcinoma JAr cells: potential promotion of 17beta-estradiol biosynthesis in human placenta. Biochem. Pharmacol., 71, 1349-1357.
- Nakanishi, T., Kohroki, J., Suzuki, S., Ishizaki, J., Hiromori, Y., Takasuga, S., Itoh, N., Watanabe, Y., Utoguchi, N. and Tanaka, K. (2002): Trialkyltin compounds enhance human CG secretion and aromatase activity in human placental choriocarcinoma cells. J. Clin. Endocrinol. Metab., 87, 2830-2837.
- Nakanishi, T., Nishikawa, J., Hiromori, Y., Yokoyama, H., Koyanagi, M., Takasuga, S., Ishizaki, J., Watanabe, M., Isa, S., Utoguchi, N., Itoh, N., Kohno, Y., Nishihara, T. and Tanaka, K. (2005): Trialkyltin compounds bind retinoid X receptor to alter human placental endocrine functions. Mol. Endocrinol., 19, 2502-2516.
- Parker, C.L. and Sodetz, J.M. (2002): Role of the human C8 subunits in complement-mediated bacterial killing: evidence that C8 gamma is not essential. Mol. Immunol., 39, 453-458.
- Satone, H., Oshima, Y., Shimasaki, Y., Tawaratsumida, T., Oba, Y., Takahashi, E., Kitano, T., Kawabata, S., Kakuta, Y. and Honjo, T. (2008): Tributyltin-binding protein type 1 has a distinctive lipocalin-like structure and is involved in the excretion of tributyltin in Japanese flounder, Paralichthys olivaceus. Aquat. Toxicol., 90, 292-299.
- Schiefner, A. and Skerra, A. (2015): The menagerie of human lipocalins: a natural protein scaffold for molecular recognition of physiological compounds. Acc. Chem. Res., 48, 976-985.
- Schreck, S.F., Plumb, M.E., Platteborze, P.L., Kaufman, K.M., Michelotti, G.A., Letson, C.S. and Sodetz, J.M. (1998): Expression and characterization of recombinant subunits of human complement component C8: further analysis of the function of C8 alpha and C8 gamma. J. Immunol., 161, 311-318.
- Shimasaki, Y., Oshima, Y., Yokota, Y., Kitano, T., Nakao, M., Kawabata, S., Imada, N. and Honjo, T. (2002): Purification and identification of a tributyltin-binding protein from serum of Japanese flounder, Paralichthys olivaceus. Environ. Toxicol. Chem., 21, 1229-1235.
- Steckel, E.W., York, R.G., Monahan, J.B. and Sodetz, J.M. (1980): The eighth component of human complement. Purification and physicochemical characterization of its unusual subunit structure. J. Biol. Chem., 255, 11997-12005.
- Trojer, P., Wojnar, P., Merschak, P. and Redl, B. (1999): Complement component C8gamma is expressed in human fetal and adult kidney independent of C8alpha. FEBS Lett., 446, 243-246.






