Abstract
The objective of this study was to investigate an appropriate observation period for an evaluation of tumorigenicity in NOD/Shi-scid IL-2 Rγnull (NOG) mice. At SNBL, 19 male and 19 female NOG mice were observed the general condition from 7 weeks old up to 68 weeks old and at FBRI, 7 male and 16 female NOG mice were observed the general condition throughout the lifespan from 7 weeks old. The survival rate started to decline rapidly around 54 to 56 weeks of age in both facilities without a facility difference. Based on these survival data, it seems reasonable to terminate a tumorigenicity study at 52 weeks of age.
INTRODUCTION
Immunodeficient rodents are commonly used in preclinical safety studies of human cell-based therapy products (CTP) to avoid an unwanted immune response elicited by xenogeneic transplant of human-derived cells. Amongst the rodents, the NOD/Shi-scid IL-2 Rγnull (NOG) mouse is one of the most commonly used models in safety studies of CTP in Japan, having been created as a severely immunodeficient mouse model in 2000 by Ito at the Central Institute for Experimental Animals. The model was established by back-cross mating of a CL57BL/6J-IL-2Rγnull mouse and NOD/Shi-scid mouse (Ito et al., 2002). However, other immunodeficient rodent models such as NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG) mice, nude mice, and NOD/scid mice are more commonly used in other countries.
NOG mice carry multiple immunodeficiencies that are derived from 3 strains, NOD/ShiJcl inbred, Prkdcscid (SCID), and IL-2Rγnull (Brayton et al., 2012), and also have a higher heterologous engraftment success rate compared with other immunodeficient mouse models (Ito et al., 2002). Characteristics of NOG mice include a lack of T, B, and natural killer (NK) cell activity, dysfunction of dendritic cells and macrophages, reduced complement system activity, and the absence of T and B cell leakiness associated with aging (Ito et al., 2012). When the development of a CTP proceeds to the clinical phase as first-in-human, an appropriate lifelong preclinical tumorigenicity study needs to be conducted to assess the risk of adverse effects caused by the CTP, although preclinical data cannot be directly translated to clinical, particularly with high risk CTP, such as induced pluripotent stem cells. To avoid high development costs and/or delays in delivery of CTP to patients in desperate need, a reasonable lifelong period for preclinical tumorigenicity studies needs to be set based on scientific data.
Background data on NOG mice up to 52 weeks old have been reported, including survival rate, blood chemistry, and pathological data, suggesting that NOG mice are capable of living healthily up to 52 weeks old in a well-maintained facility (Kasahara et al., 2017). However, such data are not available for animals older than 52 weeks old, at which the onset of organ dysfunction or age-related lesions may appear and obstruct the correct interpretation of tumorigenicity caused by CTP. Thus, in an effort to find the longest possible lifelong period suitable as the duration of a tumorigenicity study, we observed the general condition of NOG mice for more than 52 weeks old in 2 different facilities (SNBL and FBRI), and histopathological examination was conducted at termination.
MATERIALS AND METHODS
Facilities and Animals
This study was conducted at 2 facilities: Shin Nippon Biomedical Laboratories, Ltd. Drug Safety Research Laboratories (SNBL, Kagoshima, Japan) and the Research & Development Center for Cell Therapy at the Foundation for Biomedical Research and Innovation (FBRI, Hyogo, Japan). At SNBL, 19 male and 19 female NOG mice were selected in order to secure at least 10 animals per sex as specified in the guidelines on tumorigenicity tests and genetic stability evaluation (PMDA, 2019) when accounting for the possibility of earlier or accidental death. Data acquired from FBRI were used to evaluate differences between facilities.
At SNBL, 19 male and 19 female 6-week-old NOG mice were purchased from In-Vivo Science Inc. (Kanagawa, Japan) and were monitored from 7 weeks old up to 68 weeks old (the end of the observation period). The NOG mice were individually housed in TPX plastic cages (W 120 mm x D 250 mm x H 130 mm) equipped with a filter cap and kept in a specific-pathogen-free (SPF) facility. Conditions in the animal room were maintained as follows: room temperature between 19°C and 25°C, relative humidity between 30% and 70%, air circulation 15 times per hr, and illumination 12 hr per day. Solid food, CRF-1 (Oriental Yeast Co., Ltd., Tokyo, Japan), and ultrafiltered tap water in water bottles were available ad libitum to each animal. This study was reviewed and approved by the Institutional Animal Care and Use Committee and was performed in accordance with the animal welfare regulations at SNBL, which is accredited by AAALAC International. The following examinations were conducted at SNBL: clinical signs observations, body weight and food consumption measurements, ophthalmology, hematology, peripheral blood immunophenotyping, blood chemistry, necropsy, and histopathology (these examinations were conducted only in SNBL except for clinical signs observations and body weight measurement, which were conducted at both facilities). In addition, ICR mice were individually housed in TPX plastic cages or suspended stainless steel cages (W 120 mm x D 250 mm x H 130 mm). Thirty-two male and sixteen female ICR mice were examined in the range of 34 to 59 weeks old and 150 male and 150 female mice were examined at 108 weeks old.
At FBRI, 7 male and 16 female 6-week-old NOG mice were purchased from CLEA Japan Inc. (Shizuoka, Japan) or In-Vivo Science Inc., and the animals were monitored for long-term survival from 7 weeks old. A maximum of 5 animals were group housed in a plastic cage (W 210 mm x D 270 mm x H 130 mm) with a filter cap and kept in a SPF room. The conditions of the animal room were maintained as follows: room temperature between 20°C and 26°C, relative humidity between 40% and 70%, air circulation 20 to 30 times per hr, and 12 hr per day of illumination. Solid food, CRF-1, and ultrafiltered tap water in water bottles were available ad libitum to each animal. FBRI conducted clinical signs observations until the animals died from natural causes. A body weight decrease of 20% or an observation of clinical signs such as colpoptosis was established as humane endpoints. This study was reviewed and approved by the committee for animal experiments at FBRI.
Clinical signs observations, body weight, and food consumption measurements
At SNBL, clinical signs observations were conducted at least once daily until the animals were found dead or were euthanized, and body weight and food consumption measurements were conducted once weekly during the observation period. When animals exhibited significant weight loss, the animals were euthanized at the relevant time point based on welfare reasons. Surviving animals were euthanized at 68 weeks old. Unless otherwise specified, animals euthanized due to deteriorated health and animals found dead are described as having ‘died’ hereafter.
Ophthalmology
Ophthalmologic examinations were conducted at 6 and 68 weeks old. Gross observations and pupillary light reflex examinations were conducted using a portable slit lamp (SL-15, Kowa Co., Ltd., Aichi, Japan). After instillation of a mydriatic (Mydrin-P ophthalmic solution, Santen Pharmaceutical, Co., Ltd., Osaka, Japan), the anterior ocular segment and optic media were examined using a portable slit lamp, and the ocular fundi were examined using an indirect ophthalmoscope (IO-α Small Pupil, Neitz Instruments Co., Ltd., Tokyo, Japan).
Hematology
Hematological examination was conducted at 68 weeks old. Blood was drawn from the caudal vena cava with a syringe containing heparin sodium from animals anesthetized for necropsy. Approximately 0.2 mL of blood was treated with EDTA-2K as an anticoagulant and examined for red blood cell count (RBC), hemoglobin concentration (HGB), hematocrit value (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), reticulocyte ratio, differential leukocytes, white blood cell count (WBC), and neutrophil count (NEUT) using a Sysmex XT-2000iV automated hematology analyzer (Sysmex Corp., Hyogo, Japan). Wright-stained blood smears were prepared, and blood cell morphology was examined under a microscope.
Peripheral blood immunophenotyping
Blood cells collected at 68 weeks old were used. Cells were labeled with antibodies against specific cell surface antigens, and CD3e+, CD3e+CD4+, CD3e+CD8a+, CD3e-CD49b/Pan NK cell+, CD3e-CD45R/B220+, and CD3e+CD4+CD25+ were measured using a flow cytometer (FACSCanto II, BD Biosciences, Franklin Lakes, NJ, USA), and analyzed with FACSDiva Software (ver. 8.0. 1, BD Biosciences). Absolute counts were calculated for each parameter using the number of the lymphocytes obtained from hematological data.
Blood chemistry
Blood chemistry examination was conducted at 68 weeks old using the blood remaining after hematology. Plasma was obtained by centrifugation (4°C, 1700 × g, 10 minutes) and then examined for aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, creatine kinase, total bilirubin, total protein, total cholesterol, triglycerides, glucose, urea nitrogen, creatinine, inorganic phosphorus, calcium, sodium, potassium, and chloride by using a JCA-BM6070 automated biochemical analyzer (JEOL Ltd., Tokyo, Japan) and for protein fractions by using a AES320 automated electrophoresis apparatus (Beckman Coulter, Inc., Brea, CA, USA). Albumin and globulin concentrations were determined using the calculations of [(total protein) x (albumin ratio / 100)] and (total protein – albumin), respectively.
Necropsy
At the time of termination at SNBL, mice were euthanized by exsanguination after blood sampling for examinations while anesthetized by inhalation (2.0 to 4.0%) of isoflurane (Isoflu, Zoetis Japan Inc., Tokyo, Japan). In all animals (including animals found dead or euthanized due to deteriorated health), external appearance, and internal organs and tissues were examined macroscopically. The lungs, submandibular glands/uniforate sublingual glands, liver, heart, kidneys, testes, epididymides, prostate, seminal vesicles, ovaries, uterus, brain, spleen, thymus, pituitary, thyroids/parathyroids, and adrenals were weighed using an electronic balance (HR-200, A&D Co., Ltd., Tokyo, Japan). Relative organ weight was calculated from the body weight on the day of necropsy. At FBRI, necropsy was not conducted on the animals found dead.
Histopathology
The eyeballs and optic nerves were fixed in a mixture of 3% glutaraldehyde and 2.5% formalin, and the testes were fixed in formalin-sucrose-acetic acid solution. Other organs were fixed in 10% neutral buffered formalin. The fixed organs and tissues were trimmed, embedded in paraffin, sectioned, and stained with hematoxylin-eosin. The trachea, sternum, sternal bone marrow, femurs, femoral bone marrow, and spinal cord (including dorsal root ganglion) were decalcified with formic acid-formalin.
RESULTS
Survival rate
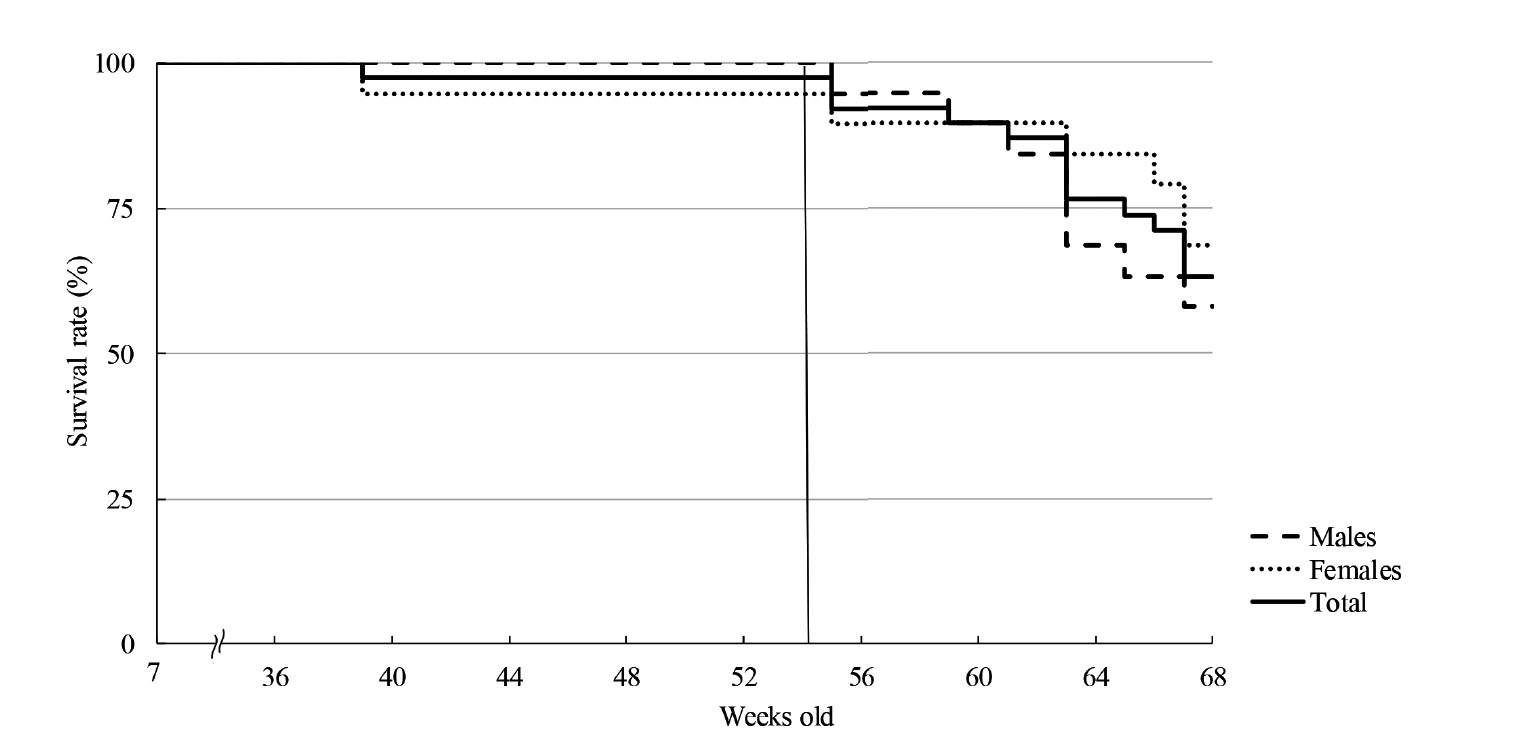
At SNBL, 1 female died at 39 weeks old, which was 5.3% (1/19) of females and 2.6% (1/38) of total mice. The survival curve started to decline rapidly at 56 weeks old for both sexes (Fig. 1-1). By 68 weeks old, 8 out of 19 male mice (42.1%) and 6 out of 19 female mice (31.6%, including 1 female that died at 39 weeks old), a total of 14 out of 38 mice (36.8%), had died.
At FBRI, 2 out of 7 male mice (28.6%) and 2 out of 16 female mice (12.5%) died by 54 weeks old. The survival curve started to decline rapidly at 54 weeks old (Fig. 1-2). The longest lifespan of males and females were 83 (1/7) and 104 weeks old (1/16), respectively.
Clinical signs observations
At both SNBL and FBRI, almost all the animals that died before termination started to show abnormal signs such as emaciation, pallor, and decreased locomotive activity before death. These findings were also observed in some surviving animals.
At SNBL, a female that died at 40 weeks old exhibited emaciation and a mass in the lateral region before death. Sixteen other animals that died before 68 weeks old showed decreased locomotive activity, emaciation, pallor, decrease of stool, lacrimation, colpoptosis, and/or mass growth.
At FBRI, 14 animals showed similar clinical signs to those described above for SNBL. Five animals that died at 44, 47, 58, 63, or 68 weeks old showed no obvious clinical signs before death. Four animals that died between 57 and 75 weeks old showed anal hemorrhage, uterine tumor, edema in groin, and/or hematoma in the abdomen or uterus in addition to decreased locomotive activity, emaciation, pallor, or colpoptosis before death.
The results of the observations and examinations on NOG mice conducted at SNBL are described below.
Body weight
At the first week of the study at 7 weeks old, the average body weight of males and females was 21.51 and 17.69 g, respectively. Thereafter, the body weight increased rapidly up to 17 weeks old in both sexes, and then gradually increased up to 37 weeks old, at which the average weight of males and females stabilized at approximately 28 and 25 g, respectively. At 68 weeks old, the average body weight of males and females was 27.74 and 25.30 g, respectively (Fig. 2).
Food consumption
The average food consumption of males and females was 2.6 and 2.4 g, respectively, at 7 weeks old. Thereafter, food consumption gradually increased in healthy animals. At 68 weeks old, the average food consumption of males and females was 3.0 and 3.1 g, respectively (Fig. 3). In animals that exhibited deterioration of health, food consumption tended to be low just before death.
Ophthalmology
In gross ophthalmological and funduscopic examinations, no abnormalities were observed at 6 or 68 weeks old in any animal. In the slit-lamp examination, lens opacity was observed in both eyes of all surviving animals at 68 weeks old, although only 2 males showed lens opacity at 6 weeks old.
Hematology
Table 1 shows that hematological parameters widely vary among NOG mice with high standard deviations. When compared with the same data set from ICR mice, RBC, HGB, HCT, MCV, and MCH were lower and WBC and NEUT were higher in NOG mice (data not shown). There was no clear sex difference in any of these parameters (Table 1). In blood cell morphology, neutrophilic myelocytes and neutrophilic metamyelocytes were observed in 6 out of 34 animals. Occurrence of these abnormal blood cell morphologies was comparable between animals that died by 68 weeks old and animals euthanized on schedule at 68 weeks old.
Table 1-1. Hematology of NOG mice.

Table 1-2. Hematology of ICR mice.
 Peripheral blood immunophenotyping
Peripheral blood immunophenotyping
Except for CD45R+ cells, the absolute count with indicated surface antigens was lower than those in ICR mice. The large number of CD45R+ cells in NOG mice may reflect the number of the granulocytes and monocytes, as well as B cells. There was no clear sex difference in any of these parameters (Table 2).
Table 2. Peripheral blood immunophenotyping of NOG mice.
 Blood chemistry
Blood chemistry
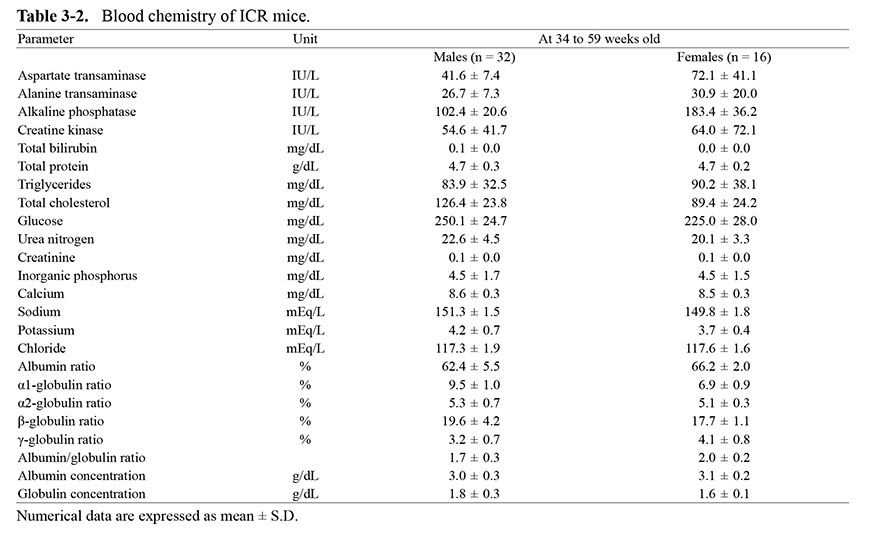
When compared with the data in ICR mice, glucose and adipose parameters, including total cholesterol and triglycerides, were higher in NOG mice. In other parameters, there were no noteworthy trends (Table 3). No clear sex differences were noted in any of these parameters.
Table 3-1. Blood chemistry of NOG mice.
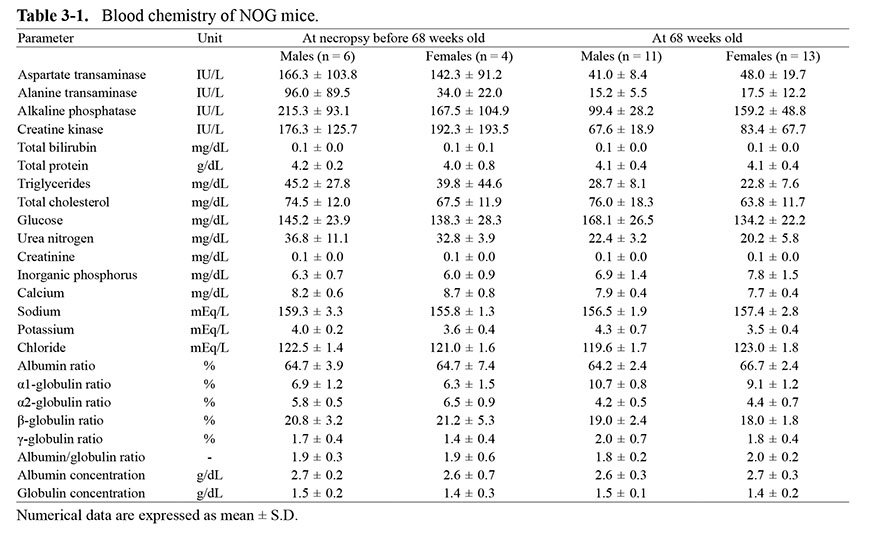
Table 3-2. Blood chemistry of ICR mice.
 Necropsy
Necropsy
One female that died at 40 weeks old developed a mass in the right abdomen and exhibited enlargement of the spleen. This finding was previously observed in 1 out of 150 18-week-old female ICR mice at SNBL (data not shown). Among 13 mice that died between 56 and 68 weeks old, 7 animals were anemic. Macroscopic findings observed in these animals include the following: pleural fluid in the thoracic cavity; white foci and/or mass in the lungs; enlargement of the spleen and/or white foci in the spleen; white foci and nodules in the liver; mass in the pancreas; softening and dark red discoloration of the testes; colpoptosis; enlargement, nodules, or red foci in the uterus; enlargement of the submandibular lymph nodes; and mass in the subcutaneous tissue. In the surviving animals at 68 weeks old, the same findings as those above were observed in the respiratory, digestive, reproductive, and immune systems. In addition to the above findings, a mass in the thyroid, white nodules in the sphenoid bone, bent tail, and nodules in the hypodermis were also observed at 68 weeks old (Table 4).
Table 4. Macroscopic findings in 68-week-old NOG mice.
 Histopathology
Histopathology
In the female mouse described above with a mass in the right abdomen that died at 40 weeks old, histopathology showed sarcoma that was classified as “not otherwise specified” (NOS) in the subcutaneous tissue at the corresponding lesion. Immunohistochemistry for osterix, a biomarker for osteogenesis, was negative, with the exception of several regions in which osseous tissue was present. In other markers examined, αSMA and vimentin were positive, while p63 was negative. Based on these results, the animal was diagnosed with sarcoma NOS (Fig. 4). Corresponding to enlargement of the spleen in this animal, a moderate increase in extramedullary hematopoiesis was observed. Histopathological findings observed at 68 weeks old in surviving animals are listed in Table 5. Some of the findings are found in both the present ICR and NOG mice, while others were novel findings in the present NOG mouse study. These are indicated in Tables 5-1 and 5-2. Malignant lymphoma was noted in 4 males and 2 females that died between 52 and 67 weeks old. Malignant lymphoma was also observed in 1 female at 68 weeks old (Table 6).

Table 5-1. Novel histopathological findings in NOG mice necropsied at 68 weeks old.

Table 5-2. Novel histopathological findings (except lymphoma) in NOG mice euthanized before 68 weeks old.

Table 5-3. Histopathological findings in NOG mice terminated at 68 weeks old that have been previously observed in NOG mice.

Table 5-4. Histopathological findings in NOG mice terminated before 68 weeks old that have been previously observed in NOG mice.

Table 5-5. Histopathological findings in NOG mice terminated at 68 weeks old that have been previously observed in ICR mice.

Table 5-6. Histopathological findings in NOG mice terminated before 68 weeks old that have been previously observed in ICR mice.

Table 6. Distribution of malignant lymphoma in NOG mice.

DISCUSSION
The survival rate started to decline rapidly at 56 weeks old at SNBL and 54 weeks old at FBRI. At 52 weeks old, the survival rate at SNBL was 100% (19/19) of males, 95% (18/19) of females, and 97% (37/38) of all mice, and the survival rate at FBRI was 71% (5/7) of males, 94% (15/16) of females, and 87% (20/23) of all mice. A previous study also reported that the survival rate of NOG mice at 52 weeks old was 95% (Kasahara et al., 2017). Although the survival rate of males at FBRI was slightly lower, it was considered to be due to the small number of males at FBRI, and the death of one animal accounted for a 14% change in survival rate. The NOG mice were individually housed at SNBL, and were group housed at FBRI; however, no adverse effects of group housing were noted such as trauma due to fighting at FBRI. Therefore, it was unclear how much the difference in experimental conditions affected the survival rate. In addition, the housing environment may affect the survival rate, and both facilities were SPF, and the effect was also unclear. However, it was considered that the period up to 52 weeks old is an appropriate period that can be fully evaluated. For accurate safety assessment, examination of at least 10 animals in each group at the end of the study for all required parameters is recommended (PMDA). When starting a tumorigenicity study with 12 animals per group, the survival rate drops to 83.3% when 2 out of 12 animals die before the end of in-life study, which is equivalent to the survival rate at FBRI at 53 weeks old or at SNBL at 62 weeks old. Based on these survival data, it seems reasonable to terminate a tumorigenicity study at 52 weeks old, not only from time and cost standpoints, but also from the viewpoints of animal welfare and science. However, when the age of the animal is a criterion for the duration of the study, a specific time window for dosing must be set to maximize the duration of CTP presence in the specific microenvironment for unbiased assessment.
In hematological data, when compared with ICR mice, on which abundant background data are available, the hematological parameters (e.g. erythrocyte count, hemoglobin concentration, and hematocrit value) in NOG mice were comparatively low and indicate anemia. A similar result has been previously reported by Kasahara et al. (2017); however, lymphoma was not observed in any of the animals in which hematopoietic parameters were low. Therefore, there was considered to be no clear relationship between the hematopoietic parameters and lymphoma formation.
One female mouse developed a mass in the right abdomen at 35 weeks old and was euthanized at 40 weeks old. Histopathological examination of the animal showed edema in the abdomen and ventral neck. Enlargement of the spleen was considered to be due to extramedullary hematopoiesis. In the female, the hematopoietic parameters was low and the extramedullary hematopoiesis in the spleen was considered to be caused by anemia. However, no findings suggestive of hemorrhage in clinical signs observations, necropsy, or histopathology were observed in the female, and it was not considered to be due to hemorrhage. These pathological findings related to mass have also been noted in ICR mice at SNBL. Therefore, these findings are considered relatively common not only in NOG mice but also in non-immunodeficient mice when they are reared until old age.
In 13 animals that died between 52 and 68 weeks old, the following signs were observed before death: emaciation, pallor, decreased locomotive activity, and decreased body weight. Six of these animals developed malignant lymphoma. Lymphoma cells were observed in the parenchyma of organs or tissues such as the liver, kidneys, heart, lungs, cerebrum, cerebellum, small intestine, colon, epididymides, ovaries, submandibular lymph nodes, mesenteric lymph nodes, and/or optic nerves. Although the primary lesions of these tumors were unclear in our histopathology data, it has been previously reported that, based on immunohistochemical and histopathological data, many lymphomas in NOG mice are CD3-positive and CD45R-negative T cell thymic lymphoma (Yasuda et al., 2017). In this study, immunostaining of lymphocyte surface antigens on T or B cells was not performed, and the origin was therefore unknown. Malignant lymphoma was considered to be the cause of death in animals in which enlarged spleen, white foci in the spleen, anemia, and/or increased leukocyte count were observed. It has been reported that the incidences of lymphoma in male and female B6C3F1 mice at 104 weeks old are 9.6% and 23.7%, respectively, and that in male and female ICR mice at 78 weeks old the incidences are 6.6% and 15.4%, respectively (Iwata et al., 2017). The incidences of lymphoma in male and female NOG mice in the present study by 68 weeks old at SNBL were 21% and 16%, respectively, which is slightly higher in males than that in other strains. While it has been previously reported that the incidence of lymphoma in NOG mice is extremely low (Yasuda et al., 2017), the cause of the high incidence in the present study was not identified. A female animal exhibited hemorrhage due to colpoptosis, which caused anemia and, ultimately death. In other animals that died, adenocarcinoma in the bronchiolo-alveoli or mammary gland, high leukocyte count, and/or high platelet count were observed, but the causes of death were not identified.
Among pathological findings that were observed at 68 weeks old, the following were unique to NOG mice: myopathy in the skeletal muscle and degeneration of the neurons in the medulla oblongata. The following findings were also considered to be unique to NOG mice since they occurred at a much higher rate in NOG mice than ICR mice: hematopoietic hyperplasia in the liver, kidney, spleen, uterus, vagina, and lacrimal gland accompanied by emperipolesis of megakaryocytes in the femoral bone marrow, sternal bone marrow, and spleen; chondromucinous degeneration in the growth plate in the femur, sternum, and tail; osteoma in the spine and presphenoid bone; osseous metaplasia in the spleen; swelling of the lens fiber in the posterior and anterior poles in the eyeball; and atrophy of the seminiferous tubules and hyperplasia of the interstitial cells in the testis. Other findings are commonly seen in ICR and/or NOG mice (Kasahara et al., 2017), and no clear differences were seen in their frequency or degree when compared with those in the Kasahara study; therefore, it is out of the scope of discussion.
When evaluating CTP, including induced pluripotent stem cell (iPSC)-derived products, with a higher risk of tumorigenicity for first-in-human clinical applications, it may be necessary to conduct life-long tumorigenicity studies using immunodeficient animals before the clinical phase. In order to deliver valuable and safe products to patients in need in a timely manner, tumorigenicity studies should be executed in the most efficient and scientifically reasonable way. The “Technical guidance on product quality and conducting non-clinical and clinical trials of cell therapy products”, published by the Japanese Ministry of Health, Labour and Welfare, states that an appropriate maximum duration for tumorigenicity studies is one at which the assessment is not affected by spontaneous lesions or mortality (PMDA, 2016). To comply with this guidance, the monitoring period for tumorigenicity tests in mice should be sufficiently long to permit assessment, but not so long that the occurrence of age-related background findings are observed. However, no specific study duration has ever been mentioned due to the lack of NOG mice background data. Based on the data obtained from the 2 facilities, we here propose that termination age in tumorigenicity studies using NOG mice should not exceed 52 weeks old. Beyond this age, the rapid decrease in survival rate of NOG mice may cause a lack of sufficient number of samples, and pathological findings caused by the nature of NOG mice could be mistaken as the effects of the CTP. In addition, from an animal welfare viewpoint, an unreasonably long study is not recommended.
Conflict of interest
The authors declare that there is no conflict of interest.
REFERENCES
- Brayton, C.F., Treuting, P.M. and Ward, J.M. (2012): Pathobiology of aging mice and GEM: background strains and experimental design. Vet. Pathol., 49, 85-105.
- Ito, M., Hiramatsu, H., Kobayashi, K., Suzue, K., Kawahata, M., Hioki, K., Ueyama, Y., Koyanagi, Y., Sugamura, K., Tsuji, K., Heike, T. and Nakahata, T. (2002): NOD/SCID/γcnull mouse: an excellent recipient mouse model for engraftment of human cells. Blood, 100, 3175-3182.
- Ito, R., Takahashi, T., Katano, I. and Ito, M. (2012): Current advances in humanized mouse models. Cell. Mol. Immunol., 9, 208-214.
- Iwata, H., Maekawa, A., Tsuchiya, M. and Harada, T. (2017): Incidence of Spontaneous Tumors in Control Mice (Supplement). In: TOXICOLOGIC HISTOPATHOLOGY. Japanese Society of Toxicologic Pathology (JSTP), pp.725-735, Nishimura Co. Ltd., Tokyo, Japan.
- Kasahara, K., Fukunaga, Y., Igura, S., Andoh, R., Saito, T., Suzuki, I., Kanemitsu, H., Suzuki, D., Goto, K., Nakamura, D., Mochizuki, M., Yasuda, M., Inoue, R., Tamura, K. and Nagatani, M. (2017): Background data on NOD/Shi-scid IL-2Rγnull mice (NOG mice). J. Toxicol. Sci., 42, 689-705.
- Kato, C., Fujii, E., Chen, Y.J., Endaya, B.B., Matsubara, K., Suzuki, M., Ohnishi, Y. and Tamaoki, N. (2009): Spontaneous thymic lymphomas in the non-obese diabetic/Shi-scid, IL-2R γ (null) mouse. Lab. Anim., 43, 402-404.
- Yasuda, M., Ogura, T., Goto, T., Yagoto, M., Kamai, Y., Shimomura, C., Hayashimoto, N., Kiyokawa, Y., Shinohara, H., Takahashi, R. and Kawai, K. (2017): Incidence of spontaneous lymphomas in non-experimental NOD/Shi-scid, IL-2Rγnull (NOG) mice. Exp. Anim., 66, 425-435.
- Pharmaceuticals and Medical Devices Agency’s (PMDA’s) Notification 0614043. (2016): Website in Japanese. https://www.pmda.go.jp/files/000212850.pdf
- Pharmaceuticals and Medical Devices Agency’s (PMDA’s) Notification 0627-1. (2019): Website in Japanese. https://www.mhlw.go.jp/hourei/doc/tsuchi/T190705I0010.pdf