2021 Volume 46 Issue 12 Pages 561-568
2021 Volume 46 Issue 12 Pages 561-568
An anesthetic mixture of medetomidine, midazolam and butorphanol (MMB) has been recently used in laboratory animals. We observed corneal opacity in nephrectomized rats that had undergone two operations under MMB anesthesia at 4 and 5 weeks of age. To evaluate the features of this corneal opacity, ophthalmic examinations were conducted in 83 nephrectomized rats, and 8 representative animals with corneal opacity were evaluated histopathologically 4 weeks after operation. The ophthalmic examinations revealed that 66/83 animals had corneal opacity, which was characterized histopathologically by mineralization with or without inflammation in the corneal stroma. In addition, to examine the possible causes of this corneal opacity, we investigated whether similar corneal changes were induced by the MMB anesthetic treatment in normal rats. The MMB anesthetic was administered twice to 4- and 5-week-old normal SD rats (5 animals/age) in the same manner as for the nephrectomized rats. Ophthalmic examinations were conducted in all the animals once a week, and the animals were necropsied 4 weeks after the first administration. In normal rats, similar corneal opacity was observed after the first administration, and increases in the severity and size of the corneal opacity were noted after the second administration. In conclusion, this study revealed the features of corneal opacity in rats undergoing nephrectomy under MMB anesthesia and the occurrence of similar corneal opacity in normal rats treated with MMB anesthetic. To the best of our knowledge, this is the first report of corneal opacity related to MMB anesthetic treatment in rats.
Anesthesia is important for alleviating pain and distress during animal experiments, and ketamine or pentobarbital sodium are commonly used for anesthesia. The combination of medetomidine, midazolam and butorphanol (MMB) has been recently introduced as an injectable anesthetic agent in laboratory animals (Kawai et al., 2011; Kirihara et al., 2016; Ochi et al., 2014; Verstegen and Petcho, 1993). In Japan, MMB anesthetic is widely used in experimental animal studies as a substitute for ketamine or pentobarbital sodium: ketamine is categorized as a narcotic drug and the regulation of its purchasing, storage and associated record-keeping procedures have become quite strict, while pentobarbital sodium is inappropriate as a general anesthetic because of its minimal analgesic action and narrow safety margin. In addition, the anesthetic duration of MMB is longer than those of ketamine or pentobarbital sodium. Finally, the largest advantage is that the injection of atipamezole, which is a synthetic α2-adrenoreceptor antagonist that can antagonize medetomidine, can cause rapid recovery from MMB anesthesia.
The effects of MMB anesthetic on physiological conditions such as body temperature, respiration and circulation have been reported, but few studies have investigated its effects on the eye. On the other hand, effects on the eye have been reported for other injectable anesthetic agents. Corneal opacity induced by the combination of ketamine and xylazine (KET/XYL) has been reported in rats and mice (Fabian et al., 1967; Guillet et al., 1988; Koehn et al., 2015; Tita et al., 2001; Turner and Albassam, 2005). Although the effects of MMB anesthetic on the cornea have not been reported, we observed corneal opacity in rats that had undergone a nephrectomy under MMB anesthesia. The cause of the corneal opacity in the nephrectomized rats was suspected to have been the MMB anesthetic treatment, since these nephrectomized rats had not received any treatment other than the surgical operation under MMB anesthesia and no reports have suggested that a nephrectomy can cause corneal opacity in rats.
The purposes of this study were to evaluate the features of the corneal opacity in nephrectomized rats that had undergone operations under MMB anesthesia and to confirm that the cause of the corneal opacity was the effects of the MMB anesthetic treatment. In the present study, two experiments were conducted. In Experiment 1, corneal opacity was evaluated in nephrectomized rats by performing ophthalmic and histopathological examinations. In Experiment 2, the MMB anesthetic was administered to normal rats to determine whether similar corneal changes are induced by the MMB anesthetic treatment in normal rats.
Nephrectomized rats were used for Experiment 1. Six-week-old male nephrectomized SD rats (n = 86) were purchased from a Japanese supplier. These rats had undergone two nephrectomies under MMB anesthesia at 4 weeks (removal of two-thirds of left kidney) and 5 weeks (removal of right kidney) of age by the supplier. The supplier used MMB anesthetic administered by intraperitoneal injection at a dosing volume of 10 mL/kg (medetomidine hydrochloride [MED]: 0.15 mg/kg, midazolam [MID]: 2 mg/kg, butorphanol [BUT]: 2.5 mg/kg) during the operations. Atipamezole was not used to induce rapid recovery from the MMB anesthesia.
Normal rats were used for Experiment 2. Four- and 5-week-old male normal SD rats (5 animals/age) were purchased from the same supplier that had provided the nephrectomized rats used in Experiment 1. In Experiment 2, the MMB anesthetic was prepared using the following reagents: MED (Dorbene®; Kyoritsu Seiyaku Corp., Tokyo, Japan), MID (Dormicum®; Astellas Pharma Inc., Tokyo, Japan), BUT (Vetorphale®; Meiji Seika Pharma Co., Ltd., Tokyo, Japan), and saline (Otsuka Normal Saline®; Otsuka Pharmaceutical Factory, Inc., Tokushima, Japan). MED, MID and BUT were mixed and diluted with saline to concentrations of 0.015, 0.2 and 0.25 mg/mL, respectively.
All the rats were maintained in an air-conditioned animal room (temperature, 23°C ± 3°C; relative humidity, 50% ± 20%) with a 12-hr light/dark cycle. The animals were fed a pellet basal diet (MF; Oriental Yeast Co., Ltd., Tokyo, Japan) and tap water ad libitum. All animal experiments were conducted in accordance with the Guidelines for Animal Experimentation specified by the Research Center of Taisho Pharmaceutical Co., Ltd. (Saitama, Japan).
Experimental designs Experiment 1: Evaluation of the features of corneal opacity in nephrectomized rats (Fig. 1)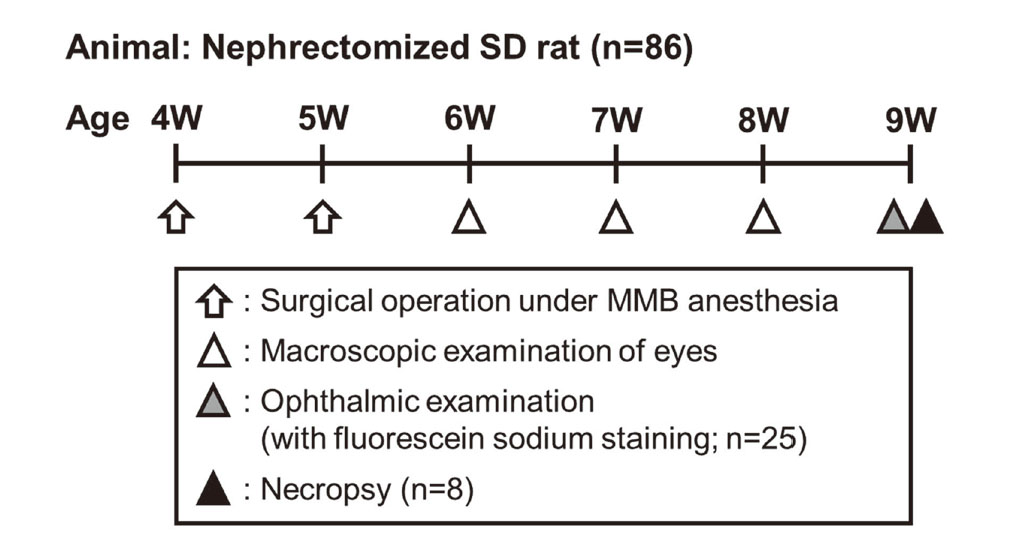
Experimental design of Experiment 1. Male SD rats (n = 86) underwent two surgical operations for nephrectomies under MMB anesthesia at 4 weeks of age (removal of two-thirds of left kidney) and 5 weeks of age (removal of right kidney). In all the nephrectomized rats, the appearance of the eyes was examined macroscopically once a week at 1 to 3 weeks after the last operation (6 to 8 weeks of age), and an ophthalmic examination was conducted prior to necropsy (8 or 9 weeks of age). In addition, corneal fluorescein sodium staining was performed to detect damage to the corneal epithelium in 25 nephrectomized rats at the time of the ophthalmic examination. Eight animals with various sizes of corneal opacity were selected, and these animals were euthanized for necroscopy at 4 weeks after the last operation (9 weeks of age).
In all the nephrectomized rats, the appearance of the eyes was examined macroscopically once a week at 1 to 3 weeks after the last operation (6 to 8 weeks of age), and an ophthalmic examination using a slit lamp (SL-5 or 15; Kowa Co., Ltd., Aichi, Japan) was conducted prior to necropsy (8 or 9 weeks of age). In addition, staining with corneal fluorescein sodium (AYUMI Pharmaceutical Corp., Tokyo, Japan) was performed to detect any damage to the corneal epithelium in 25 nephrectomized rats at the time of the ophthalmic examination. Eight animals with various sizes of corneal opacity were selected, and these animals were euthanized by exsanguination from the abdominal aorta under deep isoflurane anesthesia (ISOFLURANE Inhalation Solution [Pfizer]®; Pfizer Japan Inc., Tokyo, Japan) and necropsied at 4 weeks after the last operation (9 weeks of age). The eyeballs were removed bilaterally at the time of necropsy and fixed in 10% neutral buffered formalin (FUJIFILM Wako Pure Chemical Corp., Osaka, Japan) or formaldehyde-glutaraldehyde solution (F-G solution) for approximately 24 hr (the eyeballs were cut approximately 4 hr after the initiation of F-G solution-fixation) before being immersed in 10% neutral buffered formalin. After fixation, the eyeballs were cut at the site of the corneal opacity, routinely embedded in paraffin, sectioned, and stained with hematoxylin and eosin for histopathological examination.
Experiment 2: Examination of the effect of MMB anesthetic on the cornea in normal rats (Fig. 2)
Experimental design of Experiment 2. MMB anesthetic was administered on Days 1 and 16 to 4- and 5-week-old male normal SD rats (5 animals/age group) in the same dose and manner as those used for the nephrectomized rats. Before the administration, the integrity of each animal’s corneas was checked by macroscopic examination (Day-5) and ophthalmic examination (Day-3). At the second administration, all the rats were treated with hydroxyethyl cellulose eye solution in their eyes to prevent drying during anesthesia. In all the animals, the appearance of the eyes was examined macroscopically every day excluding holidays, and an ophthalmic examination with a slit lamp was conducted once a week. All the animals were euthanized for necropsy on Day 30.
MMB anesthetic was administered to 4- and 5-week-old male normal SD rats (5 animals/age) on Days 1 and 16 at the same dose and in the same manner as those used for the nephrectomized rats. Before the administration, the integrity of each animal’s corneas was checked by macroscopic examination upon arrival (Day-5) and ophthalmic examination using a slit lamp (SL-17; Kowa Co., Ltd.) on Day-3. Atipamezole was not used in this experiment, based on the surgical protocol used for the nephrectomized rats in Experiment 1. During the second administration, the eyes of all the rats were treated with a hydroxyethyl cellulose eye solution (Scopisol 15®; Senju Pharmaceutical Co., Ltd., Osaka, Japan) to prevent drying during the anesthesia. In all the animals, the appearance of the eyes was examined macroscopically every day excluding weekends and holidays, and an ophthalmic examination using a slit lamp was conducted once a week. All the animals were euthanized by exsanguination from the abdominal aorta under deep isoflurane anesthesia and necropsied on Day 30. A histopathological examination was not conducted in Experiment 2.
Three animals died during the experiment. The causes of deaths were considered to be a worsening of their conditions caused by the nephrectomies.
In the macroscopic examination, corneal opacity was observed unilaterally or bilaterally in 29.1% to 46.4% of the animals at 1 to 3 weeks after the last operation (Fig. 3 and Table 1). The incidence of corneal opacity gradually increased every week.

Macroscopic (A) and ophthalmic (B) examinations in a nephrectomized rat. A white focus is clearly visible in the eyeball (A). An ophthalmic examination showed a large, irregular and white focus (arrow) with neovascularization (arrowhead) in the center of the cornea (B).
| Week after last surgical operation (Age) |
1 week (6 weeks old) |
2 weeks (7 weeks old) |
3 weeks (8 weeks old) |
4 weeks (9 weeks old) |
||
|---|---|---|---|---|---|---|
| Examination | Macroscopic | Ophthalmic | ||||
| Number of animals with corneal opacity | Unilateral | 20/86 | 28/85 | 29/84 | 24/83 | |
| Bilateral | 5/86 | 9/85 | 10/84 | 42/83 | ||
| Total | 25/86 | 37/85 | 39/84 | 66/83 | ||
| Incidence of corneal opacity | 29.1% | 43.5% | 46.4% | 79.5% | ||
Three animals died during the experiment period.
The severity of all the corneal opacities observed in the ophthalmic examinations was severe.
The ophthalmic examination revealed that 79.5% of the animals had severe, unilateral or bilateral corneal opacity at 4 weeks after the last operation (Table 1). The corneal opacity was mainly located in the center of the cornea and was large, white, and irregularly shaped (Fig. 3). The sizes of the corneal opacities ranged from less than one-eighth to between one-eighth and one-quarter of the cornea in most animals (Table 2). In some animals, the opacity was extensive and covered over a quarter of the cornea with neovascularization (Fig. 3). When the eyes were examined using corneal fluorescein staining, no staining reaction was seen in 21/25 animals. Positive staining was observed in 4 animals in areas of corneal opacity; this staining area was focal and very small, compared with the size of the corneal opacity.
| Size of corneal opacity | |||||
|---|---|---|---|---|---|
| Absent | < 1/8 | 1/8-1/4 | 1/4-1/2 | > 1/2 | |
| Number of eyeballs | 58 | 62 | 29 | 7 | 10 |
| (n = 83, Total eyeballs = 166) | |||||
| Percentage of total eyeballs | 34.9% | 37.3% | 17.5% | 4.2% | 6.0% |
Necropsies and histopathological examinations were conducted in 8 rats with various sizes of corneal opacity upon ophthalmic examination. In the necropsies, corneal opacity in the eyeball was also macroscopically observed in all the animals. No macroscopic abnormalities were observed in any other organ, except for changes related to the surgical nephrectomy such as adhesions around the surgical site. Histopathologically, focal mineralization with or without inflammatory cell infiltration in the corneal stroma, ulcer, focal degeneration and thickening in the corneal epithelium, and focal inflammatory cell infiltration and hypertrophy in the corneal endothelium were observed in these animals (Fig. 4 and Table 3). Corneal stromal lesions were observed in almost all animals.

Histopathological findings of the cornea in normal and nephrectomized rats. (A) Normal rat in historical control. (B) Nephrectomized rat with corneal opacity extending from between one-eighth and one-quarter of the cornea’s area. (C) Nephrectomized rat with corneal opacity extending to more than half of the cornea. Focal mineralization (arrow) in the corneal stroma was observed (B). Focal mineralization (arrow) with inflammatory cell infiltration and neovascularization was observed in the corneal stroma (C). Focal thickening of the corneal epithelium and focal inflammatory cell infiltration in the corneal endothelium were also observed (C). HE staining. Bar = 100 μm.
| Size of corneal opacity* | Absent | < 1/8 | 1/8-1/4 | 1/4-1/2 | > 1/2 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of eyeballs (n = 8, Total eyeballs = 16) | 2 | 5 | 4 | 1 | 4 | ||||||||||||||||
| Grade | - | ± | + | - | ± | + | - | ± | + | - | ± | + | - | ± | + | ||||||
| Corneal stroma | |||||||||||||||||||||
| Mineralization, Focal | 2 | 0 | 0 | 3 | 0 | 2 | 0 | 0 | 4 | 0 | 0 | 1 | 0 | 0 | 4 | ||||||
| Inflammatory cell infiltration, Focal** | 2 | 0 | 0 | 4 | 1 | 0 | 4 | 0 | 0 | 0 | 0 | 1 | 0 | 3 | 1 | ||||||
| Corneal epithelium | |||||||||||||||||||||
| Degeneration, Focal | 2 | 0 | 0 | 4 | 1 | 0 | 2 | 2 | 0 | 1 | 0 | 0 | 4 | 0 | 0 | ||||||
| Ulcer | 2 | 0 | 0 | 5 | 0 | 0 | 4 | 0 | 0 | 1 | 0 | 0 | 3 | 0 | 1 | ||||||
| Thickening, Focal | 2 | 0 | 0 | 5 | 0 | 0 | 4 | 0 | 0 | 1 | 0 | 0 | 3 | 1 | 0 | ||||||
| Corneal endothelium | |||||||||||||||||||||
| Inflammatory cell infiltration, Focal | 2 | 0 | 0 | 4 | 1 | 0 | 4 | 0 | 0 | 1 | 0 | 0 | 1 | 2 | 1 | ||||||
| Hypertrophy | 2 | 0 | 0 | 4 | 1 | 0 | 4 | 0 | 0 | 1 | 0 | 0 | 4 | 0 | 0 | ||||||
-: None/Negligible, ±: Minimal/Slight, +: Mild
*: Evaluated in ophthalmic examination
**: With or without neovascularization
After the first administration, corneal opacity was first observed on Day 3 in the macroscopic examination, and the number of animals with macroscopically observed corneal opacity increased over time (Table 4). Corneal opacity was macroscopically observed in 3/5 animals in both the 4- and 5-week-old groups on Day 14 (Table 4). The ophthalmic examinations revealed that all the animals in both age groups had corneal opacities similar to those observed in the nephrectomized rats on Day 14 (Table 4). The corneal opacity was mainly located in the center of the cornea and was white and irregularly shaped. The severity of the corneal opacity was moderate to severe, and the size of the corneal opacity was less than one-eighth of the cornea in most animals on Day 14 (Tables 5 and 6).
| Examination day | Pre | Period after first administration | Period after second administration | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 3 | Day 7 or 8 | Day 14 | Day 22 | Day 29 | Day 30 | ||||||||
| Examination | MA | OP | MA | MA | OP | MA | OP | MA | OP | MA | OP | NE | |
| 4 weeks old* (n = 5) | 0 | 0 | 0 | 3 | 5 | 3 | 5 | 4 | 5 | 4 | 5 | 5 | |
| 5 weeks old* (n = 5) | 0 | 0 | 1 | 2 | 3 | 3 | 5 | 3 | 5 | 3 | 5 | 3 | |
MA: Macroscopic examination, OP: Ophthalmic examination, NE: Necropsy
*: Age at first administration
| Examination day | Pre | Period after first administration | Period after second administration | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 7 | Day 14 | Day 22 | Day 29 | ||||||||
| Grade | + | 2+ | + | 2+ | + | 2+ | + | 2+ | + | 2+ | |
| 4 weeks old* (n = 5) | 0 | 0 | 4 | 1 | 4 | 1 | 2 | 3 | 2 | 3 | |
| 5 weeks old* (n = 5) | 0 | 0 | 1 | 2 | 3 | 2 | 3 | 2 | 3 | 2 | |
+: Moderate, 2+: Severe
*: Age at first administration
| Examination day | Period after first administration | Period after second administration | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 14 | Day 29 | ||||||||||
| Size of corneal opacity | Absent | < 1/8 | 1/8-1/4 | 1/4-1/2 | > 1/2 | - | < 1/8 | 1/8-1/4 | 1/4-1/2 | > 1/2 | |
| 4 weeks old* (n = 5, Total eyeballs = 10) |
3 | 6 | 1 | 0 | 0 | 1 | 4 | 4 | 1 | 0 | |
| 5 weeks old* (n = 5, Total eyeballs = 10) |
2 | 7 | 0 | 1 | 0 | 2 | 5 | 2 | 0 | 1 | |
*: Age at first administration
After the second administration, the macroscopic examinations showed that the number of animals with corneal opacity had increased in the 4-week-old group on Day 22 (Table 4). In the ophthalmic examinations, some animals in both age groups showed an increased severity and/or size of the corneal opacity, compared with the observations that were made after the first administration, even though hydroxyethyl cellulose eye solution had been dropped into both eyes of all the animals to prevent drying during anesthesia (Tables 5 and 6).
At the time of necropsy, corneal opacity was observed in 5/5 and 3/5 animals in the 4- and 5-week-old groups, respectively (Table 4). No other abnormalities in any of the other organs were seen in any of the animals.
Differences in the incidence, severity or size of the corneal opacities were not observed between the 4- and 5-week-old groups.
In our present study, we examined the ophthalmic and histopathological features of corneal opacity in nephrectomized rats that had undergone surgical operations under MMB anesthesia. These corneal opacities were large, white, and irregularly shaped and were centrally located in the cornea. Histopathologically, focal mineralization with or without inflammatory cell infiltration in the corneal stroma was observed in almost all the animals, and corneal epithelial and endothelial lesions were sporadically noted. These ophthalmic and histopathological features were similar to those of the corneal opacities induced by KET/XYL anesthetic in rodents (Fabian et al., 1967; Guillet et al., 1988; Koehn et al., 2015; Tita et al., 2001; Turner and Albassam, 2005). On the other hand, the features of the corneal changes in a dry eye model were punctate lesions spreading diffusely to the cornea and positively stained with fluorescein solution, as well as a thinning of the corneal epithelium upon histopathological examination (Chen et al., 2008; Nakamura et al., 2007; Li et al., 2013). Thus, the size, distribution and histopathological findings of the corneal opacities in the nephrectomized rats differed from those of the corneal lesions in the dry eye model. The positive fluorescein staining in the corneal opacities observed in Experiment 1 was considered to be a reaction to corneal epithelial lesions.
We also showed that the corneal opacities observed in normal young adult rats (4 and 5 weeks of age) treated with MMB anesthetic were ophthalmically similar to those in the nephrectomized rats. The occurrence of corneal opacity related to MMB anesthetic treatment was also confirmed in normal SD rats purchased from another supplier (data not shown). No differences in the incidence, severity, or size of the corneal opacities were seen between the different age groups (4 or 5 weeks old at the initiation of MMB anesthetic administration).
Corneal lesions related to MMB anesthetic treatment have not been previously reported in experimental animals, and the pathogenesis of the corneal opacity occurred by MMB anesthetic treatment is unknown. The desiccation of the corneal surface is known to contribute to the occurrence of corneal opacity. The contribution of desiccation to the corneal opacity related to MMB anesthetic treatment was speculated to be unlikely because of the differences in the ophthalmic and histopathological features that were observed between the corneal opacities in nephrectomized rats and the corneal lesions in the dry eye model as well as the results of Experiment 2, which showed an increase in the severity and size of the corneal opacities despite the use of hydroxyethyl cellulose eye solution to protect the eyes from drying. However, the contribution of desiccation could not be completely ruled out since hydroxyethyl cellulose eye solution was not treated during the first administration in Experiment 2. In order to evaluate the effects of desiccation, further studies are needed, such as treatment to prevent drying immediately after MMB anesthetic treatment and evaluation of tear fluid volume and composition. On the other hand, the ophthalmic and histopathological features of the corneal opacities related to MMB anesthetic treatment were similar to those produced by KET/XYL anesthetic. The detailed pathogenesis of corneal opacity induced by KET/XYL anesthetic remains uncertain, but the effects of XYL, which is an α2-adrenoreceptor agonist, have been proposed as causes of the corneal opacity (Fabian et al., 1967; Guillet et al., 1988; Koehn et al., 2015; Tita et al., 2001; Turner and Albassam, 2005). Many studies investigating the effects of XYL have been reported, and the mechanisms responsible for the induction of corneal opacity were suggested to be related to changes in oxygen partial pressure or osmotic pressure in the aqueous humor, intraocular pressure, or interactions with the local α-adrenoreceptor for corneal homeostasis, such as the transport of chloride ions (Arnbjerg and Eriksen, 1990; Chalabi et al., 1987; Ding et al., 2011; Koehn et al., 2015; Melamed et al., 1980; Qiu et al., 2014; Tita et al., 2001; Turner and Albassam, 2005). Since MMB anesthetic also contains an α2-adrenoreceptor agonist (MED), the mechanism responsible for the induction of corneal opacity by MMB anesthetic might also involve α2-adrenoreceptor agonistic effects, similar to those induced by KET/XYL anesthetic. In addition, yohimbine, a synthetic α2-adrenoreceptor antagonist that can antagonize XYL, reportedly suppresses the occurrence of KET/XYL anesthetic-induced corneal opacity (Guillet et al., 1988; Koehn et al., 2015; Tita et al., 2001; Turner and Albassam, 2005). When MMB anesthetic is used, the injection of atipamezole, which is a synthetic α2-adrenoreceptor antagonist that can antagonize MED, has been recommended to allow animals to recover from the anesthesia quickly (Kirihara et al., 2016; Verstegen and Petcho, 1993). Therefore, atipamezole might be capable of suppressing corneal opacity related to MMB anesthetic treatment, similar to the effects of yohimbine on KET/XYL anesthetic-induced corneal opacity. However, further studies are needed to elucidate the mechanism of corneal opacity related to MMB anesthetic treatment, since very few studies have focused on the effects of MMB anesthetic on the eye such as oxygen partial pressure or osmotic pressure in the aqueous humor, intraocular pressure, or interactions with the local α-adrenoreceptor for corneal homeostasis as investigated in XYL.
As mentioned in the histopathological findings, focal mineralization was observed in the corneal stroma in almost all the nephrectomized rats. This finding may suggest a possibility of the contribution of metastatic calcification related to secondary hyperparathyroidism due to nephrectomy. The mean serum calcium and inorganic phosphorus values in nephrectomized rats were within the historical control data (data not shown), and there were no obvious increases in the serum calcium and inorganic phosphorus causing metastatic calcification in any of the treated rats (Block et al., 1998; Slatopolsky et al., 2001). In addition, there were no low levels of serum inorganic phosphorus which was reported to occur in nephrectomized rats with secondary hyperparathyroidism (Naveh-Many et al., 1995), although pathological examination of the parathyroid was not performed. Therefore, it was unlikely that focal mineralization in the corneal stroma was induced by metastatic calcification related to secondary hyperparathyroidism in the nephrectomized rats.
In conclusion, we examined the features of corneal opacity in nephrectomized rats that had undergone operations under MMB anesthesia and noted the occurrence of similar corneal opacities in normal rats administered MMB anesthetic. To the best of our knowledge, this is the first report to describe corneal opacity related to MMB anesthetic treatment in rats. We believe that this report will provide useful information and that the likelihood of corneal opacity should be taken into consideration when using MMB anesthetic, especially in rat studies involving the eye and/or vision-dependent behavioral assays.
We thank Kenta Matsue and Kazuhiro Taguchi for their laboratory assistance.
Conflict of interestThe authors declare that there is no conflict of interest.