2021 Volume 46 Issue 7 Pages 329-339
2021 Volume 46 Issue 7 Pages 329-339
Lidocaine has been shown to inhibit the invasion and metastasis of breast cancer, but the mechanism still remains unclear. This study explored the relationship between lidocaine and circulating seeding of breast cancer cells from the perspective of nerve fiber formation. The cell lines MDA-MB-231 and 4T1 were subcutaneously inoculated in mice to simulate the tumor self-seeding by circulating cancer cells. Lidocaine was used to treat these mice and tumor growth was observed. Silver staining was performed to observe the distribution of nerve fibers in tumor-bearing tissues, and immunohistochemical analysis was performed to observe the expression levels of nerve-related proteins. The results showed that lidocaine treatment effectively inhibited tumor growth and nerve fiber formation, and down-regulated the expression levels of protein gene product 9.5, neurofilament, nerve growth factor (NGF), and neuronatin (Nnat). Overexpression NGF and Nnat both could reverse the therapeutic effects of lidocaine. These results suggest that the effect of lidocaine on inhibiting breast cancer invasion and metastasis may be achieved by targeting Nnat, regulating the production of NGFs in cancer cells, and subsequently inhibiting the formation of nerve fibers.
Breast cancer is one of the main causes of cancer-related death in women. Early diagnosis and resection of primary tumor is the current conventional treatment, but more than 20% of patients have postoperative metastasis and cancer recurrence following surgery. This seriously affects the quality of life of patients (Bray et al., 2018). During surgery, tumor cells can accidentally be introduced into the circulation, and thus move to distant organs and colonize at suitable growth sites to cause metastases (Azevedo et al., 2015). Studies have shown that anesthetics used during surgery can directly or indirectly affect the circulating cancer cells, thus interfering with the metastasis and seeding of cancer cells (Heaney and Buggy, 2012; Grandhi and Perona, 2020).
Lidocaine is a widely used amide derivative used as a local anesthetic in clinical practice. It can also be used to treat ventricular arrhythmias (Grandhi and Perona, 2020). In recent years, many studies have shown that lidocaine can directly inhibit the growth and metastasis of various cancers, including breast cancer. Tran et al. observed the effect of lidocaine on a variety of breast cancer cell lines. Their results showed that lidocaine can effectively inhibit the growth and proliferation of breast cancer cells, and improve the survival duration of MDA-MB-231 tumor-bearing mice (Chamaraux-Tran et al., 2018). Wall et al. (2019) have reported that systemic lidocaine reduces postoperative pulmonary metastases in a murine breast cancer surgery model. Agostino et al. have confirmed that lidocaine can significantly inhibit CXCR4 signaling, thereby inhibiting the migration of human breast cancer cells (D’Agostino et al., 2018). A recent study suggested that TRPM7 may be the target for lidocaine reducing the viability and migration of human breast cancer cell lines (Liu et al., 2021). Some studies have also reported that the mechanism underlying the inhibition of breast cancer cell metastasis by lidocaine may be associated with its anti-inflammatory and anti-angiogenesis effects (Johnson et al., 2018). However, the mechanism of lidocaine in inhibiting the invasion and metastasis of breast cancer is not yet fully understood, and needs further research and exploration.
Studies have shown that there are abundant nerve fibers and electrical activity in breast cancer tissues (McCallum et al., 2020), which are associated with the progression and metastasis of breast cancer (Huang et al., 2014). This may be associated with the ability of breast cancer cells to produce neurotrophic growth factors (Hondermarck, 2012). Breast cancer cells can secrete nerve growth factors (NGFs), which can stimulate the growth of sympathetic and sensory nerves. This may attract nerve fibers to extend into breast tumors (Adriaenssens et al., 2008; Pundavela et al., 2015). Thus, inhibiting the production of NGF and controlling the formation of nerve fibers may be a strategy to prevent and treat breast cancer metastasis and recurrence.
In preliminary experiments, we have observed that lidocaine effectively inhibited the formation of nerve fibers in a tumor-bearing MDA-MB-231 tissue and significantly down-regulated the expression level of the neuronatin (Nnat) protein. Nnat is a neurodevelopment-related gene, which is involved in the pathophysiological process of neurodevelopment and metabolism (Pitale et al., 2017). A retrospective study including clinical samples showed that the high expression of Nnat in breast cancer may be an independent prognostic marker for breast cancer (Nass et al., 2017). We speculate that the mechanism of lidocaine with regard to inhibition of breast cancer invasion and metastasis may be associated with its ability to inhibit nerve fiber formation, and may involve Nnat. This study explored the mechanism of lidocaine in inhibiting the tumor self-seeding by circulating breast cancer cells from the perspective of neurogenesis.
Lidocaine was obtained from Macklin (Shanghai, China) and dissolved in normal saline (15 mg/mL). Mouse nerve growth factor for injection was purchased from Staidson (Beijing, China). Nnat antibodies (26905-1-AP), protein gene product 9.5 (PGP9.5, 14730-1-AP), neurofilament medium polypeptide (NF, 25805-1-AP), and NGF (55014-1-AP) were purchased from Proteintech (Rosemont, IL, USA).
Cell cultureThe human breast cancer cell line MDA-MB-231 and mouse breast cancer cell line 4T1 were obtained from Procell (Wuhan, HB, China). MDA-MB-231 cells were grown in Leibovitz’s L-15 with 10% calf bovine serum and 1% penicillin-streptomycin at 37°C with 5% CO2 (v/v). 4T1 cells were grown in Roswell Park Memorial Institute (RPMI) 1640 with 10% calf bovine serum and 1% penicillin-streptomycin at 37°C with 5% CO2 (v/v). The medium was replaced every two to three days and the cells were passaged when the cell adherence area at 80% confluence.
AnimalsAdult male Balb/c nude mice and adult male Balb/c mice aged 4 to 6 weeks and weighing 18 to 22 g were obtained from the Nanchang University Laboratory Animal Center. The animals were allowed to access food and water ad libitum and maintained under a 12 hr dark/light cycle at 22°C to 25°C. Experiments were performed according to the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH Publication No. 85-23, revised in 1996), and approved by the Ethics Committee of Nanchang University (No. 2020-0019).
Construction of recombinant adenovirusNnat (human gene ID: 4826; mouse gene ID: 18111) was cloned into GV287 vector (Genechem, Shanghai, China) via site-specific recombination cloning, and then transfected into 293T cells using envelope and packaging plasmids. The detailed information regarding the construction of recombinant adenovirus is shown in Table 1. The virus was harvested from the supernatant after density gradient centrifugation and stored at -80°C. Virus titer was calculated using a 50% tissue culture infective dose. Protein expression was confirmed via western blotting as shown in Supplemental Fig. 1.
| Gene name | Neuronatin | |
|---|---|---|
| Species | Human | Mouse |
| GenBank ID | NM_005386.4 | NM_010923.3 |
| Length (nt) | 1259 | 1254 |
| Upstream cloning restriction sites | BamHI | BamHI |
| Downstream cloning restriction site | AgeI | AgeI |
| Vector | Ubi-MCS-3FLAG-SV40-Cherry | Ubi-MCS-3FLAG-SV40-EGFP |
| Coding region element | Ubi-hNnat-3FLAG-SV40-Cherry | Ubi- mNnat -3FLAG-SV40-EGFP |
| Forward sequencing primer sequence | GCCCATTGCGAGAAGTGTTC | CGCAAATGGGCGGTAGGCGTG |
| Reverse sequencing primer sequence | CTCAACTGTGCCCTCCAGTC | CGTCGCCGTCCAGCTCGACCAG |
Cells in the logarithmic growth phase were collected and quantified at 106/mL. Each balb/c nude mouse was subcutaneously injected with 50 μL of MDA-MB-231 cell suspension in the right anterior scapula and each balb/c mouse was subcutaneously injected with 50 μL of 4T1 cell suspension. After 72 hr, cell line-derived xenograft (CDX) mice were randomly divided into the following six groups: the control group (Control, n = 6), in which the mice were injected with the vehicle intraperitoneally every two days; lidocaine treatment group (Lidocaine, n = 6), in which the mice were injected with lidocaine (100 mg/kg) intraperitoneally every two days (Chamaraux-Tran et al., 2018); lidocaine combined with NGF group (Lidocaine + NGF, n = 6), in which the mice were injected with lidocaine (100 mg/kg) intraperitoneally and NGF (10 μg/kg) intramuscularly every two days; NGF treatment group (NGF, n = 6), in which the mice were injected with NGF (10 μg/kg) intramuscularly every two days; lidocaine combined with Nnat over-expression group (Lidocaine + Nnat, n = 6), in which the cells were first infected with adenovirus containing Nnat gene and implanted subcutaneously into the right anterior scapula of mice after 72 hr, and 72 hr after implanting, the mice were injected with lidocaine (100 mg/kg) intraperitoneally every two days; Nnat over-expression group (Nnat, n = 6), in which the cells were first infected with an adenovirus containing Nnat gene and implanted subcutaneously into the right anterior scapula of mice after 72 hr, and 72 hr after implanting, the mice were injected with vehicle intraperitoneally every two days.
The tumor volumes and body weights were recorded every two days. On the 14th day, the mice were euthanized with CO2. Tumors were weighed and soaked in 4% paraformaldehyde for 12 hr, and then embedded in paraffin and sectioned for subsequent experiments.
Silver stainingAfter dewaxing and hydration, the paraffin sections were soaked in acid formaldehyde, washed with ultrapure water, stained with silver glycine, developed using a reducing solution, dehydrated and sealed. For each paraffin section, five different areas randomly were selected under the microscope and took images. The Image Pro Plus 6.0 software (Media Cybernetics, Rockville, MA, USA) was used to mark the nerve fibers according to the difference between the color of the nerve fiber and the color of background, and the software automatically counted and analyzed them.
Immunohistochemical assayAfter dewaxing and hydration, the paraffin sections were immunostained using antibodies for PGP9.5 (1:200), NF (1:100), NGF (1:100), and Nnat (1:100). All sections were observed and photographed using an optical microscope. For each paraffin section, five different areas randomly were selected under the microscope and took images. The Image Pro Plus 6.0 software (Media Cybernetics) was used to measure integrated optic density (IOD) and area. According to the formula IOD/area, the mean density value was obtained, which represented the expression level of proteins.
Statistical analysisData are presented as means ± standard error. SPSS version 20.0 (IBM Corp., Armonk, NY, USA) was used to perform the variance homogeneity test and one-way analysis of variance. The least significant difference method was used to compare between groups. P < 0.05 was considered to indicate statistically significant differences.
As shown in Fig. 1, the administration of lidocaine significantly inhibited the tumor growth in mice bearing MBA-MB-231 or 4T1 tumors compared with the control mice (P < 0.05). However, NGF could significantly reverse the effects of lidocaine on tumor volumes of CDXs (P < 0.05 vs. lidocaine group). NGF treatment alone could obviously promote tumor growth in mice bearing MBA-MB-231 or 4T1 tumors (P < 0.05 vs. control group).

Effects of lidocaine on CDXs tumor volumes. (A) Representative image of bearing tumors of MDA-MB-231 in each experimental group. (B) Growth curves of bearing tumors of MDA-MB-231 in each experimental group. (C) Representative image of bearing tumors of 4T1 in each experimental group. (D) Growth curves of bearing tumors of 4T1 in each experimental group. Values were expressed as the means ± SD (n = 6 for each group). *P < 0.05 vs. Control; #P < 0.05 vs. Lidocaine.
Silver staining was used to evaluate the formation of nerve fibers in mice bearing tumors. As shown in Fig. 2, there were abundant nerve fibers in the tumor tissues in the control group, whereas the numbers of nerve fibers were significantly decreased in the lidocanine group (P < 0.05 vs. control group). NGF treatment significantly reversed the effect of lidocaine on the formation of nerve fibers in tumor tissues (P < 0.05 vs. lidocaine group). Furthermore, treatment with NGF alone could obviously promote the formation of nerve fibers in mice bearing MBA-MB-231 or 4T1 tumors (P < 0.05 vs. control group).
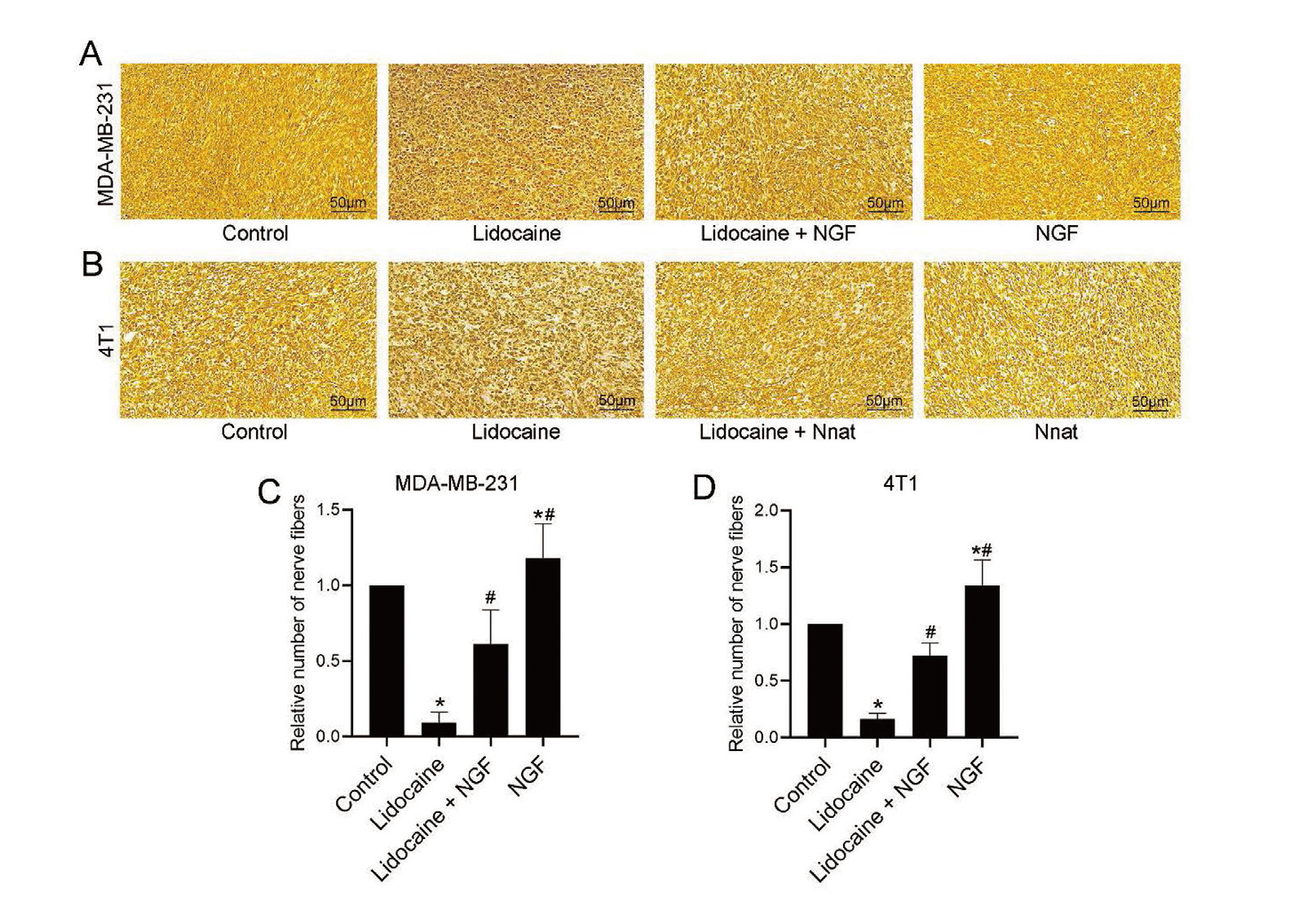
Effects of lidocaine on the formation of nerve fiber in tumors. The nerve fibers in the paraffin section stained dark brown or black after silver staining. (A) Representative silver staining images of bearing tumors of MDA-MB-231 in each experimental group. (B) Representative silver staining images of bearing tumors of 4T1 in each experimental group. (C) The relative numbers of nerve fibers in bearing tumors of MDA-MB-231 in each experimental group. (D) The relative numbers of nerve fibers in bearing tumors of 4T1 in each experimental group. Values were expressed as the means ± SD (n = 6 for each group). *P < 0.05 vs. Control; #P < 0.05 vs. Lidocaine.
Immunohistochemical analysis was performed to determine the protein expression levels of PGP9.5, NF, NGF, and Nnat in tumors. As shown in Fig. 3, lidocaine administration significantly down-regulated the levels of PGP9.5, NF, NGF, and Nnat in mice bearing MBA-MB-231 or 4T1 tumors compared with the control mice (P < 0.05), whereas the expression levels of the abovementioned proteins were significantly up-regulated in mice treated with lidocanine + NGF and NGF alone (P < 0.05 vs. lidocaine group).

Effects of lidocaine on the expression levels of nerve-related proteins in tumors. The proteins in the paraffin section stained brown in different shades after immunohistochemical staining. (A) Representative immunohistochemical images of PGP9.5 in bearing tumors of MDA-MB-231 and 4T1 in each experimental group. (B) Representative immunohistochemical images of NF in bearing tumors of MDA-MB-231 and 4T1 in each experimental group. (C) Representative immunohistochemical images of NGF in bearing tumors of MDA-MB-231 and 4T1 in each experimental group. (D) Representative immunohistochemical images of Nnat in bearing tumors of MDA-MB-231 and 4T1 in each experimental group. (E) PGP9.5 mean intensity of MDA-MB-231 in each experimental group. (F) NF mean intensity of MDA-MB-231 in each experimental group. (G) NGF mean intensity of MDA-MB-231 in each experimental group. (H) Nnat mean intensity of MDA-MB-231 in each experimental group. (I) PGP9.5 mean intensity of 4T1 in each experimental group. (J) NF mean intensity of 4T1 in each experimental group. (K) NGF mean intensity of 4T1 in each experimental group. (L) Nnat mean intensity of 4T1 in each experimental group. Values were expressed as the means ± SD (n = 6 for each group). *P < 0.05 vs. Control; #P < 0.05 vs. Lidocaine.
The tumor growth curves of CDXs over-expressing Nnat were monitored. As shown in Fig. 4, lidocaine treatment significantly inhibited the tumor growth in mice bearing MBA-MB-231 or 4T1 tumors compared with the control mice (P < 0.05), whereas the over-expression of Nnat could significantly reverse the effect of lidocaine on CDX tumor volumes (P < 0.05 vs. Lidocaine group). Over-expression of Nnat alone also obviously promoted tumor growth in mice bearing MBA-MB-231 or 4T1 tumors.

Effects of Nnat on CDXs tumor volumes. (A) Representative image of bearing tumors of MDA-MB-231 in each experimental group. (B) Growth curves of bearing tumors of MDA-MB-231 in each experimental group. (C) Representative image of bearing tumors of 4T1 in each experimental group. (D) Growth curves of bearing tumors of 4T1 in each experimental group. Values were expressed as the means ± SD (n = 6 for each group). *P < 0.05 vs. Control; #P < 0.05 vs. Lidocaine.
As shown in Fig. 5, lidocaine treatment significantly inhibited the formation of nerve fibers in mice bearing MBA-MB-231 or 4T1 tumors compared with the control mice (P < 0.05), whereas the over-expression of Nnat could significantly reverse the effect of lidocaine on nerve fiber formation (P < 0.05 vs. lidocaine group). Moreover, over-expression of Nnat alone could obviously promote the formation of nerve fibers in mice bearing MBA-MB-231 or 4T1 tumors.

Effects of Nnat on the formation of nerve fiber in tumors. The nerve fibers in the paraffin section stained dark brown or black after silver staining. (A) Representative silver staining images of bearing tumors of MDA-MB-231 in each experimental group. (B) Representative silver staining images of bearing tumors of 4T1 in each experimental group. (C) The relative numbers of nerve fibers in bearing tumors of MDA-MB-231 in each experimental group. (D) The relative numbers of nerve fibers in bearing tumors of 4T1 in each experimental group. Values were expressed as the means ± SD (n = 6 for each group). *P < 0.05 vs. Control; #P < 0.05 vs. Lidocaine.
Immunohistochemical analysis was used to analyze the expression levels of PGP9.5, NF, NGF and Nnat in tumors. As shown in Fig. 6, lidocaine administration significantly down-regulated the expression levels of PGP9.5, NF, NGF and Nnat in mice bearing MBA-MB-231 or 4T1 tumors compared with the control mice (P < 0.05), whereas the expression levels of the abovementioned were significantly up-regulated in mice treated with Lidocanine + Nnat or Nnat alone (P < 0.05 vs. lidocaine group).

Effects of Nnat on the expression levels of nerve-related proteins in tumors. The proteins in the paraffin section stained brown in different shades after immunohistochemical staining. (A) Representative immunohistochemical images of PGP9.5 in bearing tumors of MDA-MB-231 and 4T1 in each experimental group. (B) Representative immunohistochemical images of NF in bearing tumors of MDA-MB-231 and 4T1 in each experimental group. (C) Representative immunohistochemical images of NGF in bearing tumors of MDA-MB-231 and 4T1 in each experimental group. (D) Representative immunohistochemical images of Nnat in bearing tumors of MDA-MB-231 and 4T1 in each experimental group. (E) PGP9.5 mean intensity of MDA-MB-231 in each experimental group. (F) NF mean intensity of MDA-MB-231 in each experimental group. (G) NGF mean intensity of MDA-MB-231 in each experimental group. (H) Nnat mean intensity of MDA-MB-231 in each experimental group. (I) PGP9.5 mean intensity of 4T1 in each experimental group. (J) NF mean intensity of 4T1 in each experimental group. (K) NGF mean intensity of 4T1 in each experimental group. (L) Nnat mean intensity of 4T1 in each experimental group. Values were expressed as the means ± SD (n = 6 for each group). *P < 0.05 vs. Control; #P < 0.05 vs. Lidocaine.
Studies have shown that the nervous system is affected by cancer tissues and is a conduit for metastasis, invasion, and pain associated with cancers (Saloman et al., 2016). In the stage of metastasis, cancer cells enter the circulatory system and seed after encountering suitable soil. NGF secreted from cancer cells attracts peripheral nerve fibers and neural progenitor cells, promoting tumor growth and guiding the further spread and metastasis of cancer cells together with immune cells, fibroblasts, and endothelial cells (Talmadge and Fidler, 2010; Cervantes-Villagrana et al., 2020). Inhibition of tumor-induced neurogenesis is a recognized anti-cancer strategy. Studies have shown that the nervous systems, particularly the sympathetic nervous system, interact with tumor cells to promote the progression of breast cancer (Austin et al., 2017). A retrospective analysis of human breast cancer samples has shown that an increase in sympathetic nerve density and the decrease in parasympathetic nerve density in breast cancer is associated with poor clinical outcomes (Kamiya et al., 2019). Sloan et al. (2010) observed that the sympathetic nerves of mice inoculated with breast cancer cells were activated, thereby inducing the expression of cancer metastasis-related genes and promoting distant metastasis of cancer cells. Continuous sympathetic nerve stimulation can also promote the colonization of breast cancer cells in the bones of mice (Campbell et al., 2012). In this study, we simulated the process of tumor self-seeding by circulating breast cancer cells by subcutaneously inoculating breast cancer cells in mice. Silver staining results showed that there were abundant nerve fibers in the tumor-bearing tissues of 4T1 and MDA-MB-231, and results of immunohistochemical analysis showed that the expressions of nerve-related proteins, including PGP9.5, NF, NGF, and Nnat, all were significantly increased.
Lidocaine mainly reduces the excitability of neurons by reversibly inhibiting voltage-gated sodium channels to prevent or reduce the perception of pain (Scholz, 2002). Lidocaine has been found to have a high level of neurotoxicity and its effects may be achieved by promoting mitochondrial dysfunction, inducing neuronal apoptosis, and activating p38 mitogen activated protein kinase (Lirk et al., 2007; Werdehausen et al., 2007). The neurotoxicity of lidocaine is harmful to normal tissues, but it may also play an active role in tumor tissues. NGF has a neuroprotective effect on lidocaine-induced neurotoxicity (Zhao et al., 2017). In breast cancer, lidocaine may directly inhibit neural activity (McCallum et al., 2020). In this study, we observed that continuous intervention with lidocaine can significantly reduce the density of nerve fibers in mice bearing MBA-MB-231 or 4T1 tumors and down-regulate the expression levels of nerve-related proteins such as PGP9.5, NF, NGF, and Nnat. The growth rates of tumor-bearing tissues were also significantly lower than those in control groups. However, the abovementioned effects of lidocaine can significantly be reversed by NGF. These results suggest that the mechanism of lidocaine for inhibiting the invasion and metastasis of breast cancer may be associated with its ability to inhibit the accumulation of nerve fibers in breast cancer tissue.
Nnat is a paternally imprinted gene, which is expressed during the development of the nervous system (Sel et al., 2017). Nnat is mainly expressed in the adult cerebral cortex, endocrine tissue, placenta, and adipose tissue (Usui et al., 1997; Joseph et al., 1994). The abnormal expression of Nnat is associated with diabetes, obesity, and Lafora disease (Joseph, 2014). Nnat is also translated in situ within dendrites and is observed in parvalbumin-positive GABAergic neurons, raising the possibility of a role in learning and synaptic plasticity, and may be the downstream regulatory mechanism that leads to abnormal neuronal growth (Joseph, 2014). High expression of Nnat is associated with poor prognosis in various solid tumors, including glioblastoma multiforme, medulloblastoma, lung cancer, and breast cancer (Nass et al., 2017; Xu et al., 2012; Renner et al., 2013; Uchihara et al., 2007; Okubo et al., 2006; Siu et al., 2008). In breast cancer, high levels of Nnat reflect cell migration and metastasis rather than cell proliferation. Down-regulating the expression of Nnat protein can reduce the Ca2+ ions in breast cancer cells and inhibit the invasion and metastasis of cancer cells (Ryu et al., 2013). To further clarify the molecular mechanism underlying the role of lidocaine in breast cancer, we constructed breast cancer cells with a high expression of Nnat through adenovirus infection. The results showed that Nnat over-expression can significantly reverse the effects of lidocaine with regard to inhibiting the growth of tumor volumes and the abundance of nerve fibers in tumor tissues. The expression levels of PGP9.5, NF, and NGF were also significantly reversed in tumor tissues with Nnat over-expression, indicating that lidocaine targets Nnat to regulate the production of NGF in cancer cells, inhibit nerve fiber formation, and thereby inhibit breast cancer cell invasion and metastasis.
In conclusion, by subcutaneously inoculating breast cancer cells in mice to simulate tumor self-seeding by circulating breast cancer cells, we found that continuous intervention with lidocaine can significantly inhibit the growth of tumors and the nerve fibers in tumor-bearing tissues; the expression levels of nerve-related proteins in tumor tissues were also significantly inhibited. However, over-expression of Nnat could significantly attenuate the abovementioned effects of lidocaine. We speculate that the inhibitory effect of lidocaine on breast cancer invasion and metastasis may be achieved by targeting Nnat, regulating the production of NGFs in cancer cells, and consequently inhibiting the formation of nerve fibers. However, the kind of nerve fibers targeted by lidocaine are yet to be determined. This requires in-depth exploration in the future.
Conflict of interestThe authors declare that there is no conflict of interest.