2022 年 47 巻 11 号 p. 457-466
2022 年 47 巻 11 号 p. 457-466
Although both o-toluidine and o-anisidine are known as aromatic amines with bladder carcinogenicity, the specific metabolites involved in carcinogenesis are still unclear. Here, we examined the toxicological effects of head-to-tail dimers of o-toluidine and o-anisidine, 2-methyl-N4-(2-methylphenyl) benzene-1,4-diamine (MMBD) and 2-methoxy-N4-(2-methoxyphenyl) benzene-1,4-diamine (MxMxBD), respectively, in rats. Six-week-old male F344 rats were orally administered MMBD, MxMxBD, o-toluidine, and o-anisidine at a dose of 100 mg/kg/day for 28 days. Rats administered 400 mg/kg o-toluidine and 600 mg/kg/day o-anisidine were set as high-dose groups for comparison. Histopathology and immunohistochemistry for γ-H2AX, a DNA damage biomarker, and bladder stem cell markers, including aldehyde dehydrogenase 1A1 (ALDH1A1), were performed. MMBD and MxMxBD caused different toxicities than their monomers, inducing hepatotoxicity such as vacuolar degeneration but not splenic lesions due to methemoglobinemia. Bladder lesions, including urothelial hyperplasia, were observed in the high-dose o-toluidine and o-anisidine groups, whereas no obvious changes were induced in the low-dose groups or their dimers. Although γ-H2AX formation was significantly increased by o-toluidine and o-anisidine treatment, γ-H2AX formation did not differ among the MMBD, MxMxBD, and control groups. Notably, immunohistochemistry revealed marked increases in ALDH1A1 expression in the bladder urothelium of the MMBD and MxMxBD groups and in the o-toluidine and o-anisidine groups, suggesting that the two dimers may contribute to the bladder carcinogenic effects of o-toluidine and o-anisidine to some extent. The degrees of bladder lesions and γ-H2AX formation did not correlate with the amount of unchanged o-toluidine and o-anisidine in urine, indicating the presence of other metabolites responsible for these findings.
Urinary bladder cancer is the sixth most common cancer in men worldwide, and smoking and occupational exposure are considered major risk factors for the development of transitional cell carcinoma (Bray et al., 2018). Several epidemiological and experimental studies have suggested that some of the aromatic amines are closely associated with the risk of bladder carcinogenesis (Cumberbatch et al., 2015; Nakano et al., 2018; Cohen, 1998; Cohen and Arnold, 2016). Among aromatic amines, o-toluidine, an intermediate in the production of herbicides, dyes, and pigments, is known to cause bladder cancer in both experimental animals and human workers and is therefore classified as a Group 1 carcinogen (“carcinogenic to humans”) by the International Agency for Research on Cancer (International Agency for Research on Cancer, 2010). By contrast, o-anisidine, a chemical intermediate in the synthesis of dyes, pigments, and pharmaceuticals, induces urinary bladder tumors in rodents, whereas there is limited epidemiological evidence of carcinogenicity in humans (International Agency for Research on Cancer, 2021).
Despite the research accumulated to date, the specific metabolites responsible for the bladder carcinogenicity of o-toluidine and o-anisidine are still unknown. We have recently identified two head-to-tail dimers, 2-methyl-N4-(2-methylphenyl) benzene-1,4-diamine (MMBD) and 2-methoxy-N4-(2-methoxyphenyl) benzene-1,4-diamine (MxMxBD), as novel metabolites in urine of rats treated with o-toluidine and o-anisidine, respectively (Tajima et al., 2020; Kobayashi et al., 2021). In these studies, we have proposed a mechanism for dimer formation: the unstable 4-aminophenol structure generated by oxidation of o-toluidine and o-anisidine by P450 monooxygenase is converted to the p-quinone imine structure by autoxidation, and the carbonyl carbon in the structure undergoes nucleophilic attack from the nitrogen atom of the unchanged form, resulting in the p-semidine dimer formation (Tajima et al., 2020; Kobayashi et al., 2021). Both MMBD and MxMxBD showed more potent genotoxicity and cytotoxicity in in vitro tests than their parent monomers, suggesting the possibility that the two dimers may contribute to bladder carcinogenesis. In addition, there is no information on the in vivo toxicity of these metabolites. Therefore, in the current study, we investigated the toxicological effects of MMBD and MxMxBD in rat tissues, including the urinary bladder, after oral administration for 28 days.
o-Toluidine (CAS no. 95-53-4; lot no. NXSDJ, purity 99.6%) and o-anisidine (CAS no. 90-04-0; lot no. ZCUXL, 99.5%) were purchased from Tokyo Chemical Industry (Tokyo, Japan). MMBD (CAS no. 109688-61-1; batch no. E60414-19046-004; 98.1%) and MxMxBD (CAS no. 93045-45-5; batch no. E02500-18391-050; 99.5%) are head-to-tail dimers of o-toluidine and o-anisidine, respectively, and were synthesized by Sundia MediTech (Shanghai, China; Fig. 1). Thirty-five male specific pathogen-free rats (F344/DuCrlCrlj, 5 weeks old) were obtained from Charles River Japan (Yokohama, Japan) and used after 1 week of acclimation. The animals were housed in plastic cages with soft chip bedding in a room with a barrier system controlled for the light/dark cycle (12 hr), ventilation (air exchange rate 20 times/hr), temperature (23 ± 1°C), and relative humidity (50% ± 5%). The cages and chip bedding were exchanged twice a week. All animals had free access to a basal diet (CRF-1; Oriental Yeast, Tokyo, Japan) and water throughout the experiment. The diet and water were changed once and twice per week, respectively.

Chemical structures of o-toluidine, 2-methyl-N4-(2-methylphenyl) benzene-1,4-diamine (MMBD), o-anisidine, and 2-methoxy-N4-(2-methoxyphenyl) benzene-1,4-diamine (MxMxBD).
Animals were randomly divided into seven groups (5 rats per group) based on their body weights measured just before starting chemical treatment. The animals were administered 100 mg/kg body weight (bw)/day MMBD, 100 or 400 mg/kg bw/day o-toluidine, 100 mg/kg bw/day MxMxBD, or 100 or 600 mg/kg bw/day o-anisidine by gavage using corn oil (FUJIFILM Wako Pure Chemical, Osaka, Japan) as vehicle for 28 days. Dosing solutions were prepared daily, immediately before administration. The dimers used in this study were not commercially available because they were novel substances that we have recently found (Kobayashi et al., 2021; Tajima et al., 2020), and thus had to be synthesized artificially. We set the administration dose of dimers based on the maximum amount that could be synthesized and also set the same dose (low-dose) of o-toluidine and o-anisidine. For comparison, we also evaluated the effects of high doses of o-toluidine and o-anisidine, based on our previous report (Toyoda et al., 2019). General conditions and mortality were checked daily, and body weights and the amounts of supplied and residual diet were measured once a week. All rats were fasted overnight at the completion of the treatment, and blood samples for serum biochemistry were collected from the abdominal aorta under deep anesthesia with inhalation of isoflurane. After blood collection, the animals were immediately exsanguinated under deep anesthesia and subjected to laparotomy with excision of the urinary bladder, liver, and spleen for histopathology and immunohistochemistry. The experimental design was approved by the Animal Care and Utilization Committee of National Institute of Health Sciences, Japan, and the animals were cared for in accordance with institutional guidelines.
Urinalysis and analysis of metabolites in urineUrine samples were collected for about 4 hr using metabolic cages during the last week of administration. The urine samples were tested for protein, glucose, ketone, bilirubin, urobilinogen, occult blood, and pH using an Aution Eleven AE-4021 semi-automatic urinalysis analyzer (Arkray, Kyoto, Japan).
An aliquot (20 µL) of urine was added to an equal volume of methanol containing 0.2% formic acid, vortexed, and centrifuged at 21,500 × g for 5 min at 4°C. The supernatant was subjected to liquid chromatography-mass spectrometry (LC-MS), and LC-MS/MS was performed on an Agilent 1290 infinity LC system using a TSK gel ODS-120H (1.9 µm, 50 × 2.0 mm; TOSOH, Tokyo, Japan) at 40°C and an Agilent G6410B triple quadrupole tandem mass spectrometer with an electrospray ionization device running in the positive ionization mode. A linear gradient system was used to separate aromatic amines. A gradient was set using solvent A (0.1% formic acid in water) and solvent B (0.1% formic acid in methanol) as follows: 1% B at 0–3 min, 50% B at 10 min, and 99% B at 10.01–12 min. The flow rate of the mobile phase was 0.4 mL/min. The multiple reaction monitoring transition for the detection and standard curves, calibration range, recoveries, and relative standard deviations for the quantification of aromatic amines are summarized in Supplementary Table 1.
Serum biochemistrySerum biochemical analysis was performed by Oriental Yeast for the following parameters: total protein (TP), albumin (Alb), albumin/globulin ratio (A/G), glucose, total cholesterol (T-Chol), triglyceride (TG), aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyl transpeptidase (γ-GTP), blood urea nitrogen (BUN), creatinine (Cre), total bilirubin (Bil), alkaline phosphatase (ALP), inorganic phosphorus (IP), calcium (Ca), sodium (Na), potassium (K), and chlorine (Cl).
Histopathology and immunohistochemistryFor histopathological and immunohistochemical examination, the urinary bladders were inflated with 10% neutral-buffered formalin and carefully removed for immersion fixation. After fixation for 24 hr, the urinary bladders were sliced into six strips of equal width along the longitudinal axis and embedded in paraffin. The liver, kidney, and spleen were weighed, and histopathological analysis was performed on the liver and spleen along with the urinary bladder. Serial sections (4 μm thick) were prepared and stained with hematoxylin and eosin for histological observation. The grading criteria for histopathological examination were as follows: slight (±), mild (+), moderate (++), and marked (+++).
For immunohistochemistry, the bladder sections were deparaffinized, hydrated, and autoclaved in 10 mM citrate buffer (pH 6.0) for 15 min at 121°C for antigen retrieval. For inactivation of endogenous peroxidase activity, all sections were immersed in 3% H2O2/methanol solution for 10 min at room temperature. After blocking nonspecific reactions with 10% normal goat serum, the sections were incubated with primary antibodies targeting γ-H2AX (diluted 1:1000; anti-phospho-histone H2A.X [Ser139] mouse monoclonal antibody; Cell Signaling Technology, Danvers, MA, USA), aldehyde dehydrogenase 1A1 (ALDH1A1; diluted 1:2000; anti-ALDH1A1 goat polyclonal antibody; Abcam, Cambridge, UK), cytokeratin 14 (KRT14; diluted 1:8000; anti-cytokeratin 14 [C-14] goat polyclonal antibody; Santa Cruz Biotechnology, Dallas, TX, USA), and CD44 (diluted 1:10,000; anti-CD44 rabbit polyclonal antibody; Abcam) overnight at 4°C. Visualization of antibody binding was performed using a Histofine Simple Stain Rat MAX PO Kit (Nichirei, Tokyo, Japan) for γ-H2AX and CD44 or a VectaStain Elite ABC Kit (Vector Laboratories, Burlingame, CA, USA) for ALDH1A1 and KRT14, combined with 3,3’-diaminobenzidine. All sections were counterstained with hematoxylin.
γ-H2AX-positive cells in the urinary bladder were counted under a light microscope. Whole epithelial cells in each strip were sequentially counted, and the ratios of γ-H2AX-positive cells among 1000 cells were calculated based on a minimum total count of 1800 cells from each animal. The degrees of expression of ALDH1A1, KRT14, and CD44 in the bladder urothelium were scored using the following criteria: for ALDH1A1, ± (≤ 5%), + (6–25%), ++ (26–50%), +++ (51-75%), and ++++ (≥ 75%); for KRT14, ± (≤ 25%), + (26–50%), ++ (51–75%), and +++ (≥ 76%); and for CD44, ± (≤ 20%), + (21–40%), ++ (41–60%), and +++ (≥ 61%) (Yamada et al., 2021).
Statistical analysisQuantitative values were expressed as means ± standard deviations (SDs), and differences between the control and each treatment group were statistically analyzed using Student’s t-tests for MMBD and MxMxBD groups or Dunnett’s multiple comparison tests for o-toluidine and o-anisidine groups. For incidences of histopathological findings, Fisher’s exact probability test was applied. Differences with P values of less than 0.05 were considered statistically significant.
No obvious clinical signs were noted throughout the experiment, and all animals survived until the scheduled necropsy. Body weight gain was significantly reduced in rats receiving 400 mg/kg o-toluidine and 600 mg/kg o-anisidine as compared with that in rats in the control group (Table 1). There were no significant differences in daily food consumption among all groups. Data for organ weights are included in Table 1. Absolute and relative liver weights were significantly increased in the MMBD, MxMxBD, 400 mg/kg o-toluidine, and 600 mg/kg o-anisidine groups (Table 1). Significant increases in relative liver weights were also observed in the 100 mg/kg o-toluidine and o-anisidine groups. Relative kidney weights were increased in the 100 and 400 mg/kg o-toluidine, MxMxBD, and 600 mg/kg o-anisidine groups. Absolute and relative spleen weights were significantly increased in both low- and high-dose o-toluidine and o-anisidine groups in a dose-dependent manner, whereas a decrease in absolute spleen weight was detected in the MMBD group.
 Urinalysis and urine metabolites
Urinalysis and urine metabolites
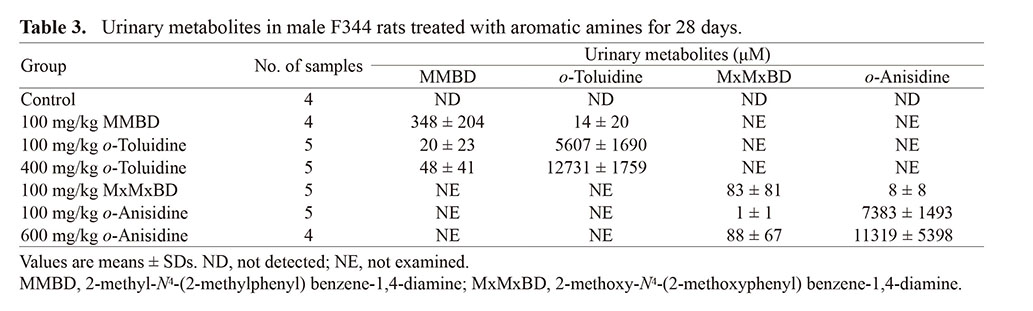
Urinalysis data are summarized in Table 2. The MxMxBD group showed decreased protein and increased bilirubin as in the 600 mg/kg o-anisidine group, whereas the MMBD group only showed increased bilirubin, which differed from the o-toluidine groups. LC-MS analysis data for metabolites in urine are shown in Table 3. Levels of unchanged forms in the MMBD and MxMxBD groups were higher or comparable to those in the 400 mg/kg o-toluidine and 600 mg/kg o-anisidine groups, respectively. By contrast, the conversion from dimers to their monomer was found to occur less frequently, at least as urinary metabolites.

 Serum biochemistry
Serum biochemistry
Serum biochemistry data are shown in Table 4. In the MMBD group, significant increases in T-Chol, ALT, BUN, and IP and decreases in TG and AST were observed. In the MxMxBD group, significant increases in glucose, T-Chol, ALT, BUN, and Ca and decreases in TG, AST, Cre, and ALP were observed. In the o-toluidine groups, significant increases in A/G and Bil in both the low- and high-dose groups and increases in TG, AST, ALT, BUN, and Na in the high-dose group were detected. In the o-anisidine groups, a significant increase in Bil in both the low- and high-dose groups and increases in Alb, A/G, ALT, and Ca and a decrease in ALP in the high-dose group were detected. Although we observed a significant increase in glucose and a decrease in TP in the 100 mg/kg o-tolidine group and increases in glucose and IP and decreases in TG and Na in the 100 mg/kg o-anisidine group, the lack of dose relationships suggested that these differences were not associated with exposure to the chemicals.
 Histopathology in the rat urinary bladder, liver, and spleen
Histopathology in the rat urinary bladder, liver, and spleen
Histopathological findings in the urinary bladder, liver, and spleen are summarized in Table 5. In the urinary bladder, edematous changes, intramucosal hemorrhage, formation of granulation tissue, submucosal inflammation, and urothelial hyperplasia were observed in the 400 mg/kg o-toluidine group, and urothelial hyperplasia was also induced in the 600 mg/kg o-anisidine group (Fig. 2, upper column). By contrast, there were no obvious bladder lesions in the MMBD and MxMxBD groups. In the liver, centrilobular hepatocellular hypertrophy was detected in the MMBD, MxMxBD, and high-dose o-toluidine and o-anisidine groups (Fig. 2, center column). In addition, MMBD caused vacuolar degeneration of hepatocytes, and the high-dose o-toluidine and o-anisidine groups showed yellow-brown pigment deposition in hepatocytes and Kupffer cells. In the spleen, MMBD and MxMxBD, unlike o-toluidine and o-anisidine, did not induce methemoglobinemia characterized by splenomegaly and severe congestion with pigment deposition in the red pulp, extramedullary hematopoiesis, and occasional capsulitis (Fig. 2, lower column).

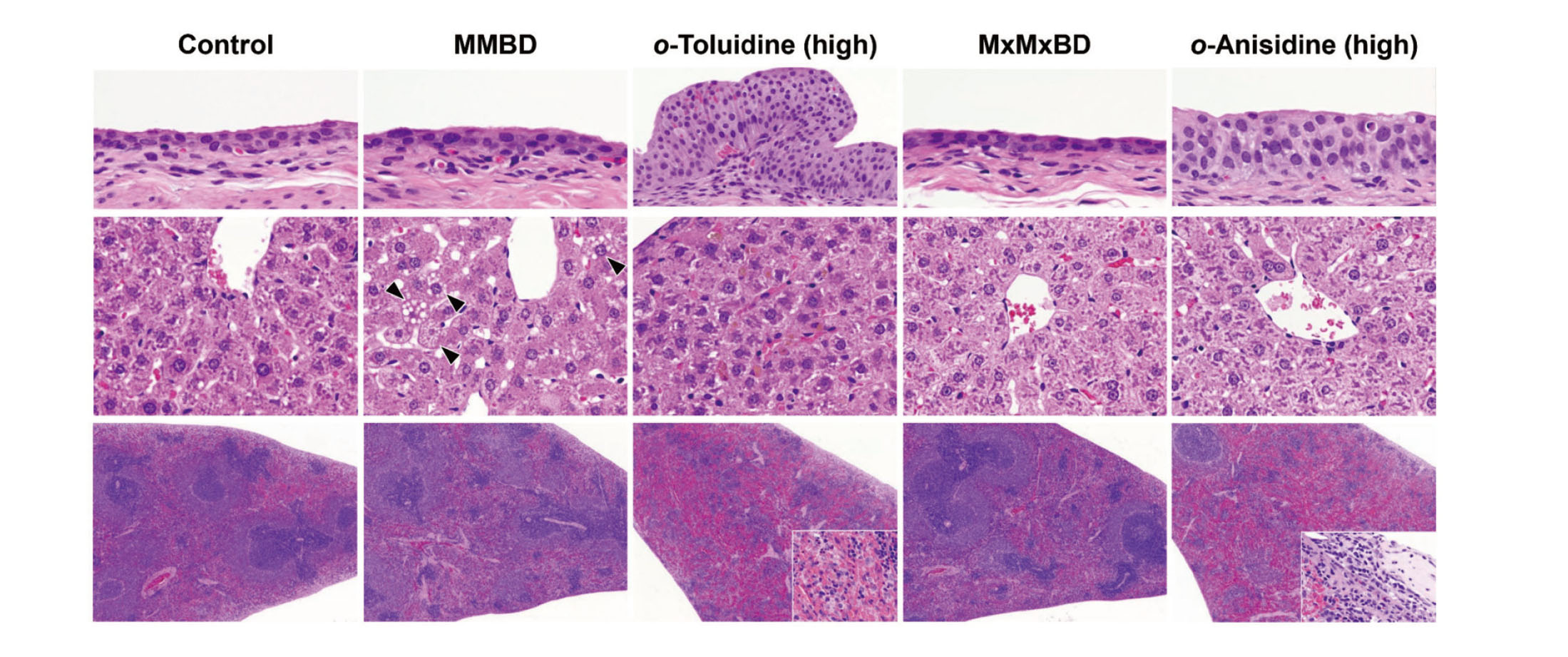
Representative histopathological findings in the urinary bladder, liver, and spleen of male F344 rats. In the urinary bladder (upper column), high doses of o-toluidine and o-anisidine induced urothelial hyperplasia, whereas 2-methyl-N4-(2-methylphenyl) benzene-1,4-diamine (MMBD) and 2-methoxy-N4-(2-methoxyphenyl) benzene-1,4-diamine (MxMxBD) did not cause any obvious bladder lesions. In the liver (center column), MMBD induced vacuolar degeneration of hepatocytes (arrowheads), o-toluidine caused deposition of brown pigments, and MxMxBD and o-anisidine induced centrilobular hypertrophy of hepatocytes. In the spleen (lower column), high doses of o-toluidine and o-anisidine induced splenomegaly owing to severe congestion of red pulp with hemosiderosis (insert of o-toluidine) and focal capsulitis (insert of o-anisidine), whereas MMBD and MxMxBD did not. Original magnification: 400× (urinary bladder and liver) or 40× (spleen).
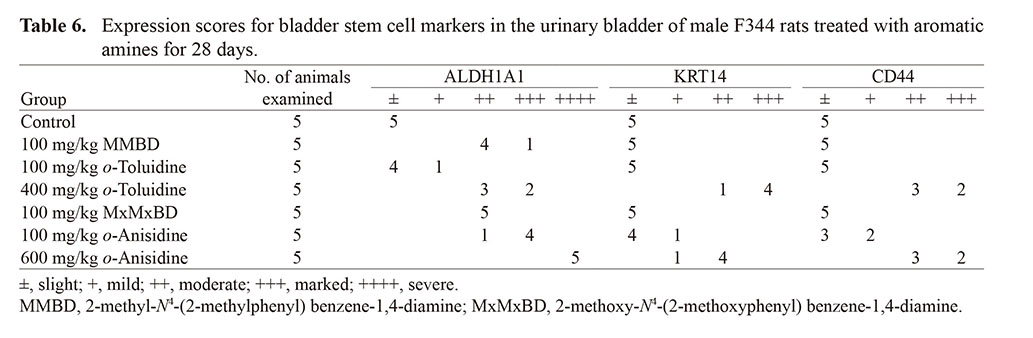
γ-H2AX formation and expression of stem cell markers in bladder epithelial cells were investigated by immunohistochemistry (Fig. 3). γ-H2AX-positive cells with characteristic intranuclear dot-like foci were detected throughout the bladder epithelium in rats treated with 400 mg/kg o-toluidine and 600 mg/kg o-anisidine, whereas γ-H2AX formation was rarely observed in other groups, as confirmed by quantitative analysis (Fig. 4). Although ALDH1A1-, KRT14-, and CD44-positive cells were only occasionally scattered in the basal layer of the urothelium in the control group, they were clearly increased in the 400 mg/kg o-toluidine and 600 mg/kg o-anisidine groups. In the MMBD and MxMxBD groups, ALDH1A1 expression scores were higher than those in the control (Table 6).
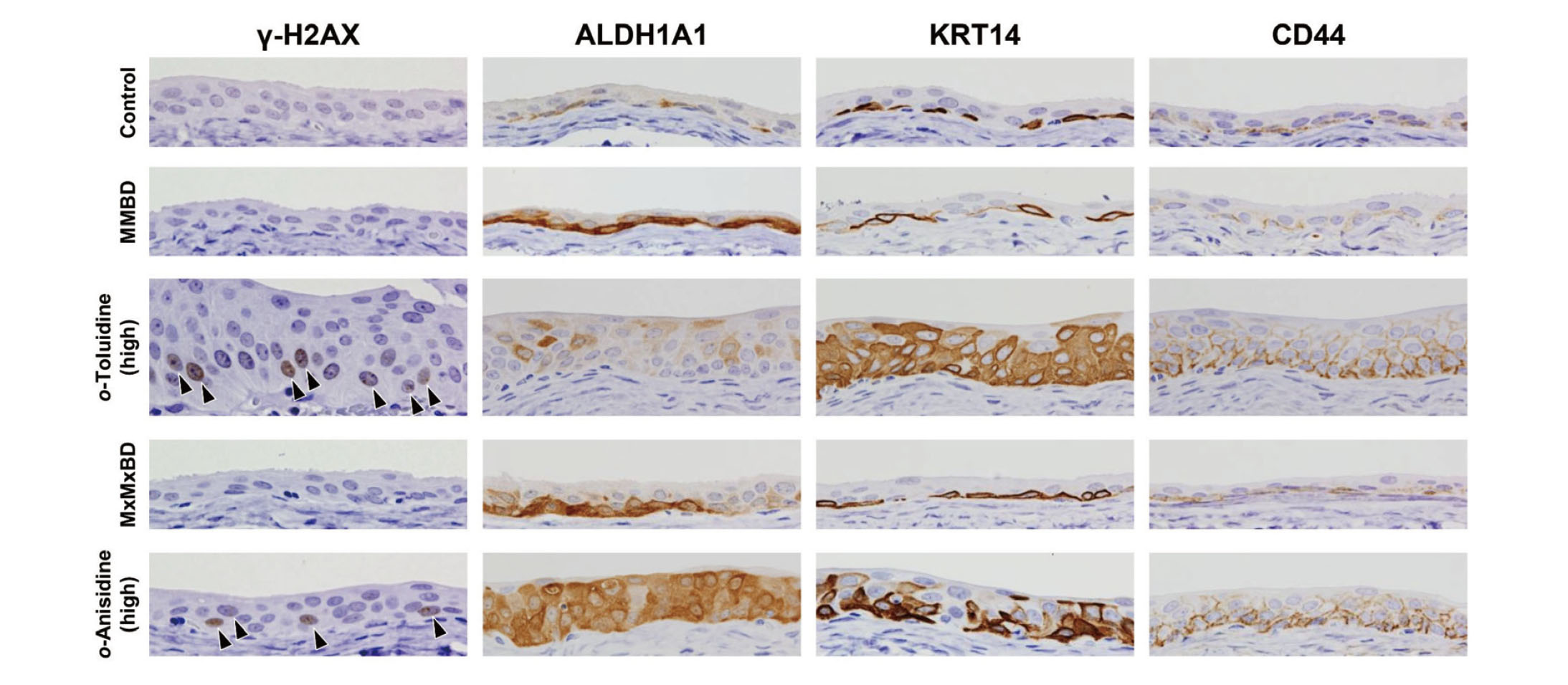
Representative immunohistochemical findings for γ-H2AX, aldehyde dehydrogenase 1A1 (ALDH1A1), cytokeratin 14 (KRT14), and CD44 in the urinary bladder of male F344 rats. γ-H2AX-positive cells with characteristic dot-like foci (arrowheads) were increased in the high-dose o-toluidine and o-anisidine groups. ALDH1A1-, KRT14-, and CD44-positive cells were scattered in the basal layer of the urothelium in the control group. Increased expression of ALDH1A1 was observed in the 2-methyl-N4-(2-methylphenyl) benzene-1,4-diamine (MMBD) and 2-methoxy-N4-(2-methoxyphenyl) benzene-1,4-diamine (MxMxBD) groups and in the o-toluidine and o-anisidine groups. Original magnification: 400×.
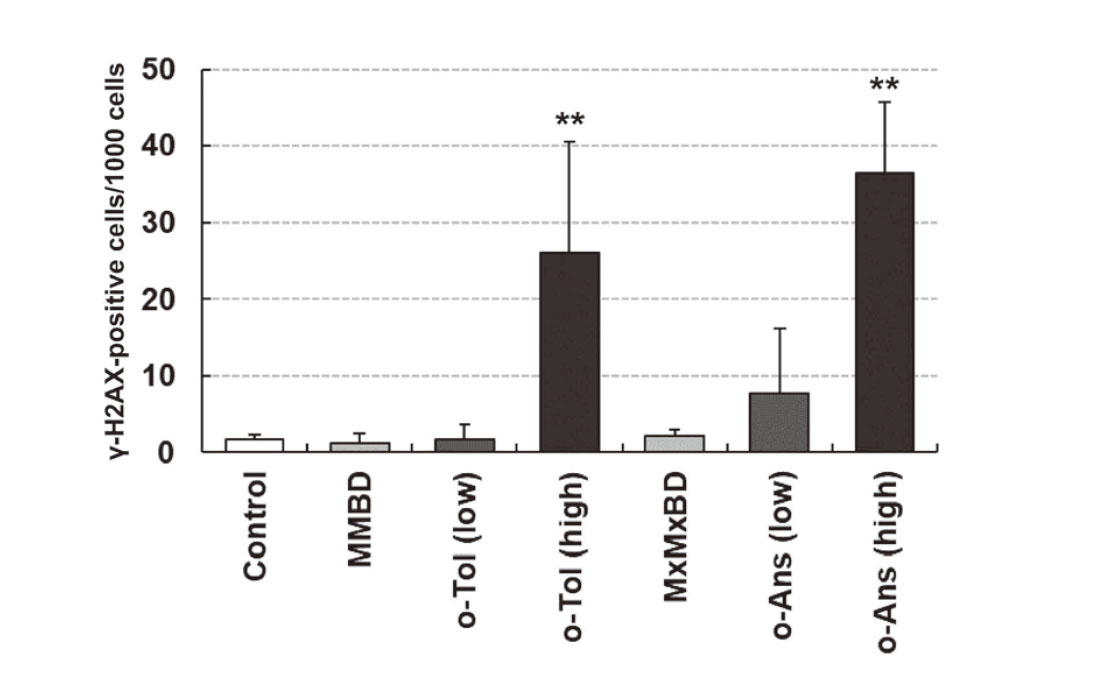
Quantitative analysis of γ-H2AX formation in the urothelium of male F344 rats treated with 2-methyl-N4-(2-methylphenyl) benzene-1,4-diamine (MMBD), o-toluidine (o-Tol), 2-methoxy-N4-(2-methoxyphenyl) benzene-1,4-diamine (MxMxBD), and o-anisidine (o-Ans). γ-H2AX staining was evaluated by determining the average number of γ-H2AX-positive epithelial cells per 1000 cells. Each group contained five rats. Values are the means ± SDs. **: significantly different from the control at P < 0.01.
Several carcinogenicity studies of o-toluidine and o-anisidine have been published (National Toxicology Program, 1978, 1979; Weisburger et al., 1978; Hecht et al., 1982), and the relationships of both of these substances with urinary bladder carcinogenesis in rodents are well established (International Agency for Research on Cancer, 2010, 2021). In the carcinogenic mechanism of both chemicals, although the active metabolites are assumed to be covalently bound to a DNA base, direct detection of DNA adducts from human specimen or identification of the responsible metabolites has not yet been reported, whereas there have been several reports on the in vitro generation of o-toluidine and calf thymus DNA adducts (Guo et al., 2018), the indirect detection of o-toluidine from hydrolyzed DNA samples of human bladder cancer tissue (Böhm et al., 2011), and the detection of o-anisidine adducts in the rat urinary bladder (Naiman et al., 2012). In the current study, we examined the toxic effects of MMBD and MxMxBD, recently reported as novel urine metabolites derived from o-toluidine and o-anisidine, respectively, in rats (Tajima et al., 2020; Kobayashi et al., 2021).
Although significant bladder lesions, such as urothelial hyperplasia with increased γ-H2AX formation, were observed in the high-dose o-toluidine and o-anisidine groups, similar to our previous report (Toyoda et al., 2019), none were found in the MMBD, MxMxBD, and low-dose o-toluidine and o-anisidine groups, except slight to mild inflammation in the submucosa. γ-H2AX is a well-established biomarker of DNA damage, particularly DNA double-strand breaks (Rogakou et al., 1998), and immunostaining for γ-H2AX is expected to be a useful tool to evaluate the genotoxicity and/or carcinogenicity of chemicals (Redon et al., 2012; Motoyama et al., 2018). We have demonstrated that bladder carcinogens significantly increase the number of γ-H2AX-positive urothelial cells after administration for 28 days, whereas non-bladder carcinogens do not induce this change (Toyoda et al., 2015; Yamada et al., 2020, 2022; Toyoda and Ogawa, 2022). Immunohistochemistry for γ-H2AX can detect bladder carcinogens with high sensitivity even as a single marker, and some bladder carcinogens with false-negative results can be detected using bladder stem cell markers, including ALDH1A1, as complementary markers (Yamada et al., 2021). In fact, 4-amino-2-nitrophenol, a bladder carcinogenic aromatic amine, increases ALDH1A1 expression but does not induce γ-H2AX formation (Yamada et al., 2021), similar to MMBD and MxMxBD in the current study. Our results showed that ALDH1A1 expression was also increased in the low-dose o-anisidine group without significant increases in γ-H2AX-positive cells or bladder lesions. Although further studies are needed, these results suggested that these two dimers may be involved to some extent in bladder carcinogenesis induced by o-toluidine and o-anisidine.
In addition to the urinary bladder described above, histopathological and serum biochemical analyses of the spleen and liver highlighted differences in the toxic effects of the dimers and their parent compounds. In the spleen, MMBD and MxMxBD, unlike o-toluidine and o-anisidine, did not induce methemoglobinemia characterized by splenomegaly and severe congestion in the red pulp with hemosiderosis. In the liver, toxic effects, such as hepatocellular hypertrophy and increased serum ALT, were observed in the MMBD and MxMxBD groups and high-dose o-toluidine and o-anisidine groups. By contrast, the MMBD and MxMxBD groups did not show hemosiderin deposition, which may be related to methemoglobinemia, and vacuolar degeneration of hepatocytes was observed only in the MMBD group; no specific liver lesions were found in the MxMxBD group, but significant changes in T-Chol and TG were commonly induced in both dimer groups, supporting the toxic effects of these compounds on hepatic lipid metabolism.
In our analysis of urinary metabolites, the urinary exposure of MMBD and MxMxBD in each dimer group was similar to or higher than that in the high-dose o-toluidine and o-anisidine groups, confirming that the doses used in this study were appropriate. Moreover, the concentrations of the unchanged form detected in the MMBD and MxMxBD groups were much lower than those in the o-toluidine and o-anisidine groups, suggesting extensive conversion to other metabolites. In the low-dose o-toluidine and o-anisidine groups, approximately half of the unchanged form was detected compared with that in the high-dose groups, whereas bladder lesions and γ-H2AX formation were rarely observed, indicating that these findings were related to the presence of other metabolites rather than the unchanged forms of the compounds. We have recently identified several novel metabolites of o-toluidine and o-anisidine (Kobayashi et al., 2022), and it may need to be investigated on the potential of bladder toxicity of these metabolites.
In conclusion, MMBD and MxMxBD showed different toxic effects on the spleen and liver compared with their parent monomers o-toluidine and o-anisidine, respectively, in a 28-day repeated-dose study in male F344 rats. Both MMBD and MxMxBD did not induce bladder lesions or increase γ-H2AX formation, whereas both increased ALDH1A1 expression in the bladder urothelium, suggesting that these dimers may contribute to the bladder carcinogenic effects of o-toluidine and o-anisidine to some extent. However, because the amount of the unchanged forms in the urine of rats in the o-toluidine and o-anisidine groups did not correlate with the degree of bladder damage or γ-H2AX formation, further identification of the metabolites responsible for these findings may be needed.
This work was supported by a Health and Labour Sciences Research Grant from the Ministry of Health, Labour and Welfare, Japan (grant no. H30-Roudou-Ippan-010). We thank Ayako Saikawa and Yoshimi Komatsu for expert technical assistance in processing histological materials.
Conflict of interestThe authors declare that there is no conflict of interest.