2022 Volume 47 Issue 11 Pages 493-501
2022 Volume 47 Issue 11 Pages 493-501
Lead (Pb) is an environmental pollutant that adversely affects various organs in the human body and is a well-known risk factor for cardiovascular diseases, caused by the dysfunction of vascular endothelial cells that cover the luminal surface of the blood vessels. The Zrt- and Irt-like related protein (ZIP) transporter ZIP8 is one of the primary importers of zinc, iron, manganese, and cadmium, and its expression appears to be important for the metabolism of these metals. In the present study, we investigated the influence of ZIP8 on Pb-induced cytotoxicity in vascular endothelial cells, induction of ZIP8 expression by Pb, and its mechanism of action in vascular endothelial cells. The study revealed the following: (1) Pb cytotoxicity in vascular endothelial cells was potentiated by the knockdown of ZIP8, but the intracellular accumulation of Pb in the cells remain unaffected; (2) Pb induced the expression of ZIP8; (3) the induction of ZIP8 expression by Pb was mediated by nuclear factor (NF)-κB signaling pathway; and (4) Pb activated p38, mitogen-activated protein kinase (MAPK), and c-jun N-terminal kinase (JNK), but the activation of these MAPKs was not involved in the induction of ZIP8 by Pb. Therefore, the study shows that Pb induces the expression of endothelial ZIP8 and this induction appears to be involved in the protection against Pb cytotoxicity by intracellular Pb accumulation independent mechanisms.
Vascular endothelial cells are a cell type that covers the luminal surface of blood vessels and are in direct contact with blood. These cells regulate the blood coagulation-fibrinolytic system via synthesizing tissue factors (Maynard et al., 1977) and plasminogen activator inhibitor-1 (van Mourik et al., 1984) as the procoagulant and antifibrinolytic factors, and prostacyclin (Weksler et al., 1977), thrombomodulin (Esmon and Owen, 1981), heparan and dermatan sulfate proteoglycans (Yamamoto et al., 2005), and plasminogen activators (Levin and Loskutoff, 1982) as anticoagulant and fibrinolytic factors. Vascular endothelial cells also regulate inflammation, including the response to histamine (Andriopoulou et al., 1999), and blood vessel permeability via vascular endothelial growth factor (Nomi et al., 2006), vascular endothelium-cadherin (Dejana, 2004), and prostacyclin (Langeler et al., 1991). The initiation and progression of cardiovascular diseases are attributed to imbalance between the procoagulant/antifibrinolytic factors and the anticoagulant/fibrinolytic factors by abnormal functions of vascular endothelial cells (Ross, 1999).
Lead (Pb) is a heavy metal that pollutes the environment and food and is a risk factor for cardiovascular disease (Revis et al., 1981), associated with cardiovascular disease-related mortality in the United States (Schwartz, 1991; Brody et al., 1994; Lanphear et al., 2018). Approximately 75% people in Kabwe, Zambia, have over 5 μg/dL Pb in their blood and are contaminated with high concentrations of Pb at 10 µg/dL, which is a serious concern (Yamada et al., 2020). Our previous studies have shown that Pb suppresses fibrinolytic activity by decreasing the expression of tissue-type plasminogen activator (Kaji et al., 1992), inhibiting the proliferation of vascular endothelial cells (Fujiwara and Kaji, 1999a), and inhibiting the synthesis of perlecan, a large heparan sulfate proteoglycan (Fujiwara and Kaji, 1999b). Pb also induces endoplasmic reticulum stress and glucose-regulated proteins 78 and 94 via c-Jun N-terminal kinase (JNK)-activator protein-1 signaling in vascular endothelial cells (Shinkai et al., 2010).
Zrt-and Irt-like protein (ZIP)-8 is a transporter that transports heavy metals, including zinc (Zn), iron (Fe), manganese (Mn), and cadmium (Cd), from the extracellular space into the cytosol (He et al., 2006; Himeno et al., 2009; Wang et al., 2012). Strains of mice with higher expression of ZIP8 in vascular endothelial cells of the testis exhibit higher susceptibility to Cd toxicity (Dalton et al., 2005). In our previous study, either overexpression of ZIP8 (Fujie et al., 2022) or transforming growth factor-β1(TGF-β1) that induced endothelial ZIP8 expression (Ito et al., 2021) potentiated Cd toxicity in vascular endothelial cells, suggesting that the level of ZIP8 expression is important for determining the susceptibility of endothelial cells to Cd toxicity. Moreover, we also showed that Cd induced ZIP8 expression in endothelial cells by activating, accumulating, and stabilizing the nuclear factor (NF)-κB; in addition, Pb increased the expression of ZIP8 mRNA (Fujie et al., 2022), suggesting that Pb may induce ZIP8 expression, and this could be associated with Pb toxicity in vascular endothelial cells.
Thus, in this study, we investigated the influence of ZIP8 on Pb-induced cytotoxicity in vascular endothelial cells, induction of ZIP8 expression, and mechanisms underlying this induction.
Bovine aortic endothelial cells were purchased from Cell Applications (San Diego, CA, USA). Dulbecco’s modified Eagle’s medium (DMEM) and calcium- and magnesium-free phosphate-buffered saline (CMF-PBS) were obtained from Nissui Pharmaceutical (Tokyo, Japan). Fetal bovine serum (FBS), OPTI-MEM reduced serum medium, Lipofectamine RNAiMAX transfection reagent, Lipofectamine LTX and Plus reagent, high-capacity cDNA reverse transcription kit, NE-PER Nuclear and Cytoplasmic Extraction Reagents, and Protein Assay Bicinchoninate Kit were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Mouse monoclonal anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody conjugated with horseradish peroxidase was purchased from FUJIFILM Wako Pure Chemical Industries (Osaka, Japan). May-Grünwald and Giemsa staining solutions were obtained from Merck KGaA (Darmstadt, Germany). Rabbit polyclonal anti-ZIP8 antibody was purchased from ABclonal (Tokyo, Japan). Rabbit polyclonal anti-phospho-extracellular signal-regulated kinase 1/2 (ERK1/2) (Thr202/Tyr204) antibody (#9101), rabbit polyclonal anti-ERK1/2 antibody (#9102), rabbit polyclonal anti-phospho-p38 mitogen-activated protein kinase (MAPK) (Thr180/Tyr182) antibody (#9211), rabbit polyclonal anti-p38 MAPK antibody (#9212), mouse monoclonal anti-phospho-JNK (Thr183/Tyr185) antibody (#9255), rabbit polyclonal anti-JNK antibody (#9252), mouse monoclonal anti-phospho-IκBα antibody (#9246), mouse monoclonal anti-IκBα antibody (#4814), mouse monoclonal anti-p65 antibody (#6956), horseradish peroxidase-conjugated anti-rabbit IgG antibody (#7074), and horseradish peroxidase-conjugated anti-mouse IgG antibody (#7076) were purchased from Cell Signaling Technology (Danvers, MA, USA). Mouse monoclonal anti-Lamin A/C antibody (sc-7292) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). ISOGEN II and GeneAce SYBR qPCR Mixα were obtained from Nippon Gene (Tokyo, Japan). ERK inhibitor U0126, p38 MAPK inhibitor SB203580, and JNK inhibitor SP600125 were purchased from Cayman Chemical (Ann Arbor, MI, USA). Lead nitrate and other reagents were purchased from Nacalai Tesque Inc. (Kyoto, Japan).
Cell culture and treatmentVascular endothelial cells were cultured at 37°C in 5% carbon dioxide in DMEM supplemented with 10% FBS until they reached confluency. The medium was removed and the cells were washed twice with serum-free DMEM. The cells were then incubated in the presence or absence of Pb (5, 10, 20, or 50 μM) for 3, 6, 12, 24, and 48 hr in serum-free DMEM in 100-mm dishes or 6- or 24-well culture plates.
TransfectionTransfection of small interfering RNAs (siRNAs) (Bioneer, Daejeon, Korea) was performed using Lipofectamine RNAiMAX according to the manufacturer’s protocol. Briefly, the annealed siRNA duplex and Lipofectamine RNAiMAX were dissolved in Opti-MEM in separate tubes and incubated for 5 min at room temperature, followed by mixing and incubation for 20 min at room temperature. The vascular endothelial cells were grown to 70–80% confluency in DMEM supplemented with 10% FBS and then incubated for 4 hr in fresh DMEM supplemented with 10% FBS and the siRNA/Lipofectamine RNAiMAX mixture. The final concentration of siRNA and Lipofectamine RNAiMAX were 40 nM and 0.2%, respectively. After 24 hr, the cells were incubated at 37°C for 24 hr and then treated with or without Pb. The sequences of the sense and antisense strands of the siRNAs were as follows: bovine ZIP8 siRNA, 5′-GGUUUUGACUCCAUUGAUATT-3′ (sense) and 5′-UAUCAAUGGAGUCAAAACCTT-3′ (antisense); bovine p65 siRNA, 5′-AUUGAAAGGGCUCUUUUUCAU-3′ (sense) and 5′-GAAAAAGAGCCCUUUCAAUGGTT-3′ (antisense); inositol-requiring enzyme 1α (IRE1α) siRNA, 5′-ACAAAGAGUAGACUCUUUG-3′ (sense) and 5′-CAAAGAGUCUACUCUUUGU-3′ (antisense); PKR-like ER kinase (PERK) siRNA, 5′-UAUGUAGUGAGAGACUUUC-3′ (sense) and 5′-GAAAGUCUCUCACUACAUA-3′ (antisense); and activating transcription factor 6 (ATF6) siRNA, 5′-AAUAUCUGUACAGAAUUGG-3′ (sense) and 5′-CCAAUUCUGUACAGAUAUU-3′ (antisense). A nonspecific sequence was used as a negative control siRNA (Nippon Gene).
Morphological appearance and lactate dehydrogenase (LDH) activityVascular endothelial cells cultured in 24-well culture plates were transfected with control or ZIP8 siRNA for 24 hr and then incubated in the presence or absence of Pb (5, 10, 20, or 50 μM) for 24 hr. After incubation, the conditioned medium was transferred to new 96-well plates and the supernatant was used to determine LDH activity. The cell layers were washed with CMF-PBS, fixed with methanol, and stained with Giemsa for morphological observations.
Intracellular accumulation of metalsVascular endothelial cells cultured in 6-well culture plates were transfected with the control or ZIP8 siRNA for 24 hr and then incubated in the presence or absence of Pb (5, 10, 20, or 50 μM) for 24 hr. After incubation, the conditioned medium was discarded and the cells were washed twice with ice-cold CMF-PBS and harvested in 1 mL of CMF-PBS. The cell suspensions were sonicated to prepare the homogenates; 0.6 mL of the homogenates were added to 4.4 mL of 0.1 M nitric acid and used for the detection of intracellular Pb (m/z = 208) and Zn (m/z = 66) by inductively coupled plasma mass spectrometry (NexION 300S; PerkinElmer, Waltham, MA, USA). Another portion of the lysate was analyzed for DNA content using the fluorometric method (Kissane and Robins, 1958); Pb content was expressed as pmol/μg DNA.
Extraction of the membrane fractionMembrane fraction extraction was performed as previously described (Fujie et al., 2022). Vascular endothelial cells cultured in 100-mm culture dishes were exposed to Pb (5, 10, or 20 μM) for 24 hr. Then, the cell layer was washed twice with CMF-PBS and harvested in 1.3 mL of ice-cold CMF-PBS. The cell suspensions were centrifuged at 12,000 × g for 3 min, the supernatant was discarded, and the pellets suspended in 20 mM 4-(2-hydroxyethyl)-1-piperazineëthanesulfonic acid (HEPES) solution containing 250 mM sucrose and 1 mM ethylenediaminetetraacetic acid (EDTA). Sonicated for three cycles (5 sec pulse and 5 sec rest, on ice) using an Ultrasonic Homogenizer UR-20P (TOMY SEIKO, Tokyo, Japan). The homogenates were centrifuged at 5,000 × g for 5 min, and the supernatants were transferred into new tubes and centrifuged at 15,000 × g for 30 min. The supernatants were discarded and the pellets were lysed in 50 mM HEPES solution (pH 7.5) containing 150 mM sodium chloride (NaCl), 0.5% CHAPS, 0.1 mM ethylene glycol tetraacetic acid (EGTA), and 0.1 mM EDTA, and analyzed by western blotting analysis as described below.
Western blotting analysisVascular endothelial cells in 6-well culture plates were exposed to Pb (5, 10, or 20 μM) for 3, 6, 12, and 24 hr. The conditioned medium was discarded, and the cells were lysed in sodium dodecyl sulfate sample buffer (50 mM Tris-HCl buffer solution containing 2% sodium dodecyl sulfate and 10% glycerol, pH 6.8) and incubated at 95°C for 5 min. Thereafter, 2-Mercaptoethanol and bromophenol blue (1.67% each) were added to the samples (10 µg protein) and incubated at 95°C for 3 min. The cellular proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 12% polyacrylamide gels and electrotransferred to polyvinylidene difluoride membranes at 2 mA/cm2 for 1 hr. The membranes were blocked with 0.5% skim milk in 20 mM Tris-HCl buffer solution containing 15 mM NaCl and 0.1% Tween 20 (pH 7.5) and then incubated with primary antibodies (1:1000) at 4°C overnight. After washing with 20 mM Tris-HCl buffer solution containing 15 mM NaCl and 0.1% Tween 20 (pH 7.5), the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (1:5000) for 1 hr at room temperature. Immunoreactive bands were visualized by enhanced chemiluminescence using Chemi-Lumi One Super and scanned with an Amersham Imager 600 (GE Healthcare, Little Chalfont, UK). Nuclear protein fractions were extracted using NE-PER Nuclear and Cytoplasmic Extraction Reagents according to the manufacturer’s protocol and analyzed by western blotting as described here.
Real-time reverse transcription-polymerase chain reaction (RT-PCR)Vascular endothelial cells in 6-well culture plates were treated with or without U0126, SB203580, or SP600125 (5 or 10 μM each) for 3 hr, followed by incubation in the presence or absence of Pb (5, 10, 20, or 50 μM) for 24 hr. After incubation, the conditioned medium was discarded, and the cell layer was lysed with 300 μL ISOGEN II. Deionized distilled water (120 μL) was mixed with the lysate and the mixture was centrifuged at 15,000 × g for 15 min. The supernatant was harvested, mixed with the same volume of 2-propanol, centrifuged at 15,000 × g for 10 min, and discarded. The precipitate was resuspended in 70% ethanol, centrifuged at 12,000 × g for 5 min, and the precipitate containing the total RNA was dried. Complementary DNA (cDNA) was synthesized using a high-capacity cDNA reverse transcription kit. Real-time PCR was performed using GeneAce SYBR qPCR Mix α with 10 ng of cDNA and primers on a StepOnePlus Real-Time PCR System (Thermo Fisher Scientific). The thermal cycling parameters were as follows: 95°C for 10 min, followed by 45 cycles of 95°C for 30 sec, and annealing/ enzymatic extension at 60°C for 1 min. The mRNA levels of ZIP1, ZIP2, ZIP3, ZIP4, ZIP6, ZIP7, ZIP8, ZIP9, ZIP10, ZIP11, ZIP12, ZIP13, ZIP14, p65, IRE1α, PERK, ATF6, and GAPDH were quantified using the standard curve method. The fold-change in the intensity value of the target gene was normalized to that of GAPDH. Sequences of the forward and reverse primers are listed in Table 1.
| Gene | Forward primer (5′→3′) | Reverse primer (3′→5′) |
|---|---|---|
| ZIP1 | TTCTCTACATCACCTTCCTGG | AACCTTCCTTGCCTGTCTTG |
| ZIP2 | GCTCTCGCTCTCCTTTCAC | ACCAGCCGCAGTCCTACA |
| ZIP3 | GGACACACTCACCTCAACGC | CTCAAGGCTCCAAGCAGAAC |
| ZIP4 | GACAGCCACAGTGACGACAG | CAGACATTCCGTACACAGCC |
| ZIP6 | CCTGAAAATGATGATGATGTGG | CAAGATTGCTGGCTGCTGAG |
| ZIP7 | TATTCTATGTAGCAACGGTGTC | CGAGGTGGCAATCAAC |
| ZIP8 | GAATGAGCACTCGACAAGCC | TAGAGGAACATGCCTCCAGC |
| ZIP9 | CAGCCTCTTGTCTCGCCTTG | ATGTCTGTATCCTTCGCAGTGTG |
| ZIP10 | TTCTATCACTGTCATTAGCCTGC | GCGTCTCCACTCATTGTTCC |
| ZIP11 | CCATCACCATCCACAACATC | TACCAGAAGGCTCTCCAGG |
| ZIP13 | CTGGACAGTAAGGAGAGCGAG | GAGCAGGTGGAAGAAACACG |
| ZIP14 | TCTCGGTAGTGCCTCTGTCC | GAATGTCTCAGTGCTGGTTGG |
| p65 | GATGGCTTCTAATGAGGCTGAG | TTGTTGTTGGTCTGGATGC |
| IRE1α | TTTAGCCAGCGTGCTTTGAC | GGAAGTTTCGTCAGGCCTTC |
| PERK | TGATTGCAGCCGAAGTGACG | ATCACTAATGATCTGCCGCAC |
| ATF6 | GTGGGTTCTGATATTGCTGTGT | GCTGCCTTTAACCTTGCCTC |
| GAPDH | CAATGACCCCTTCATTGACCTTC | GGATCTCGCTCCTGGAAGATG |
The data were analyzed using Student’s t-test or analysis of variance with Dunnett’s or Tukey-Kramer’s test for multiple comparisons with Statcel3 (OMS, Tokyo, Japan). Statistical significance was set at p < 0.05.
The influence of ZIP8 expression on the susceptibility of vascular endothelial cells to Pb was investigated by morphological observations and LDH leakage. In vascular endothelial cells transfected with ZIP8 siRNA, the expression levels of ZIP8 mRNA were observed to have decreased (Fig. 1A). Morphologically, the cytotoxicity of Pb in vascular endothelial cells transfected with ZIP8 siRNA was significantly higher than that in cells transfected with the control siRNA (Fig. 1B). LDH activity in ZIP8-knockdown cells was also significantly higher than that in cells transfected with the control siRNA (Fig. 1C). Knockdown of ZIP8 in vascular endothelial cells, however, did not affect the intracellular accumulation of Pb and Zn (Fig. 1D), suggesting that the knockdown of ZIP8 potentiated Pb cytotoxicity, which was independent of the intracellular accumulation of Pb and Zn in vascular endothelial cells.
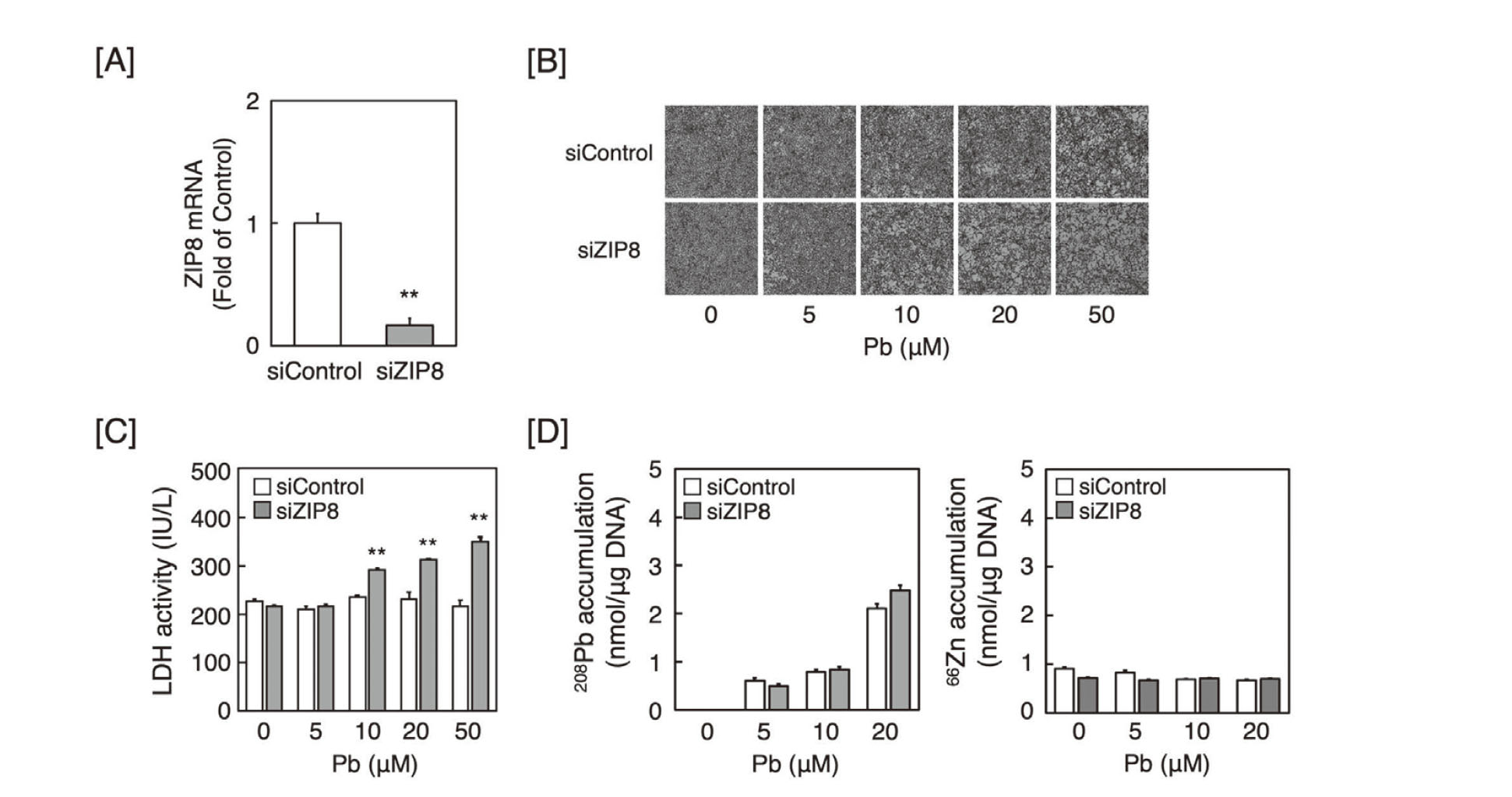
Effects of ZIP8 on susceptibility of vascular endothelial cells to lead (Pb) cytotoxicity. [A, B, C, and D] Vascular endothelial cells were transfected with the control or ZIP8 small interfering RNA (siRNA) for 24 hr. They were then incubated in the presence or absence of Pb (5, 10, 20, or 50 μM) for a further 24 hr. [A] Expression levels of ZIP8 mRNA were determined by real-time RT-PCR. Each value represents the mean ± standard error (S.E.) of three technical replicates; **p < 0.01 compared with the control. [B] The cell layer was stained with Giemsa. [C] The activity of lactate dehydrogenase (LDH) in the medium was measured. Each value represents the mean ± S.E. of four independent samples; **p < 0.01 compared with the control. [D] The intracellular accumulation of Pb and Zn was measured by inductively coupled plasma-mass spectrometry. Each value represents the mean ± S.E. of four independent samples; **p < 0.01 compared with the control.
Thereafter, we studied the effect of Pb on the expression of ZIP8 in vascular endothelial cells, and it was observed that expression levels of ZIP8 protein in the membrane fraction of vascular endothelial cells exposed to Pb increased at 5 µM and higher (Fig. 2A). The expression level of ZIP8 mRNA also increased in a concentration- and time-dependent manner following Pb exposure (Fig. 2B), suggesting that ZIP8 expression was indeed induced by Pb in vascular endothelial cells. In case of other ZIP transporters, even though Pb increased the expression levels of ZIP1, ZIP3, ZIP9, and ZIP13 mRNAs, the increase was not marked (Fig. 2C); the increased expression of ZIP8 mRNA by Pb was much higher than that of the other ZIP transporters. The mRNA expression level of ZIP10 was decreased by Pb. Our previous study showed that mRNA expression levels of ZIP5 and ZIP12 were not detected in the cells (Fujie et al., 2022).
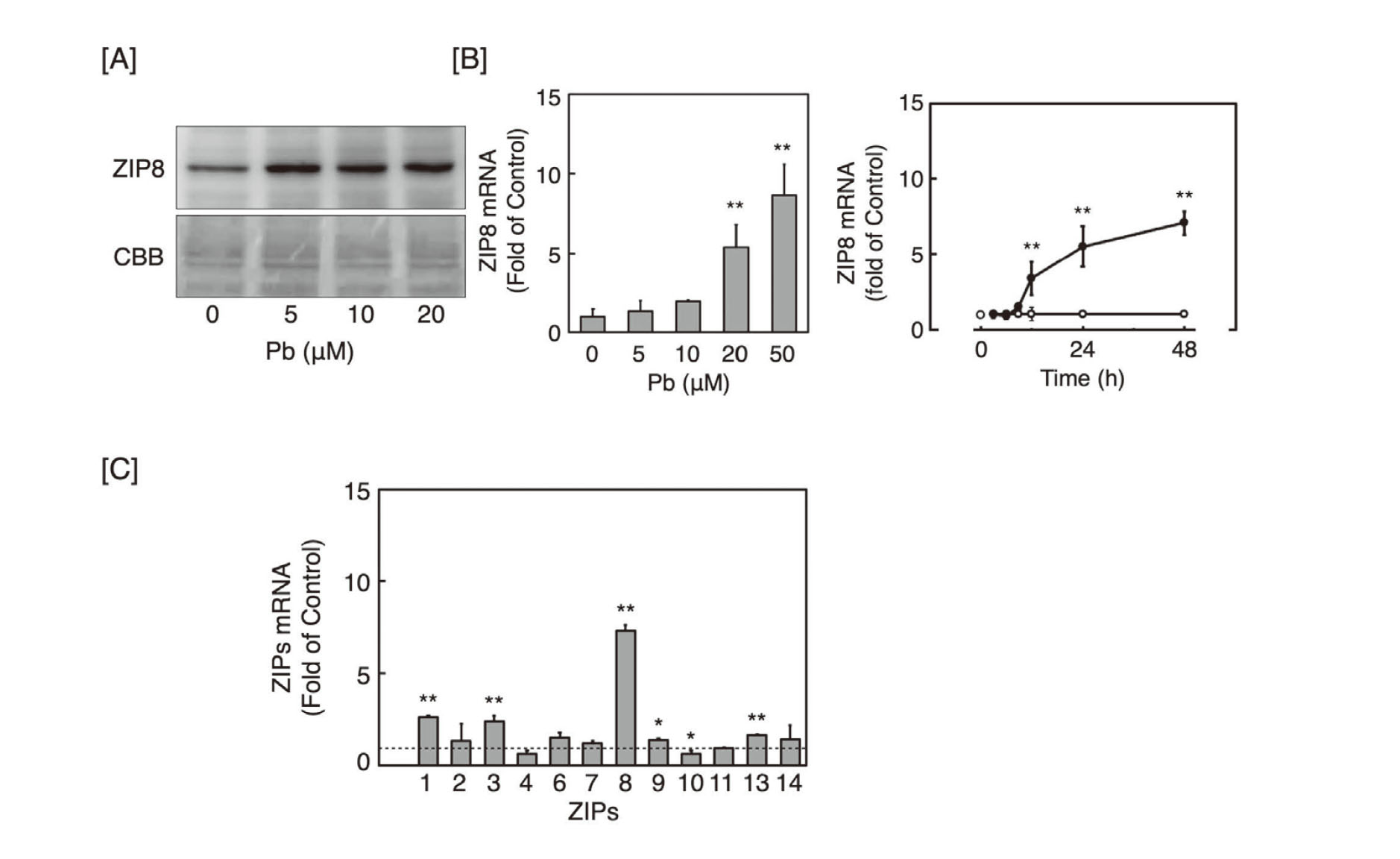
Induction of ZIP8 in vascular endothelial cells after exposure to Pb. [A] Vascular endothelial cells were incubated in the presence or absence of Pb (5, 10, or 20 μM) for 24 hr, and the expression levels of ZIP8 protein of the membrane fraction were determined by western blotting analysis. [B] Vascular endothelial cells were incubated in the presence or absence of Pb (5, 10, 20, or 50 μM) for 24 hr (left panel) or Pb (20 μM each) for 3, 6, 9, 12, 24, and 48 hr (right panel), and the expression levels of ZIP8 mRNA were determined by real-time RT-PCR. Each value represents the mean ± S.E. of three technical replicates; **p < 0.01 compared with the corresponding control. [C] Vascular endothelial cells were incubated in the presence or absence of Pb (20 μM) for 24 hr, and the expression levels of ZIP1, ZIP2, ZIP3, ZIP4, ZIP6, ZIP7, ZIP8, ZIP9, ZIP10, ZIP11, ZIP13, and ZIP14 mRNAs were determined by real-time RT-PCR. Each value represents the mean ± S.E. of three technical replicates; *p < 0.05; **p < 0.01 compared with the control.
Further, we investigated the role of NF-κB signaling in the Pb-mediated induction of ZIP8 in vascular endothelial cells because the promoter region of ZIP8 has five κB binding sites and the expression of ZIP8 is regulated by NF-κB signaling (Liu et al., 2013). We observed that Pb exposure increased the expression levels of phosphorylated IκBα protein, while decreasing the expression levels of total IκBα protein in a concentration- and time-dependent manner (Figs. 3A and B). The accumulation of p65 protein in the nuclear fraction also increased 3, 6, and 12 hr after exposure to Pb (Fig. 3C), suggesting that p65 was translocated into the nuclei of vascular endothelial cells in the presence of Pb. Furthermore, when p65 was knocked down (Fig. 3D, left panel), Pb-induced ZIP8 mRNA expression was suppressed (Fig. 3D, right panel), suggesting that NF-κB signaling is involved in Pb-induced ZIP8 expression in vascular endothelial cells.

Involvement of the NF-κB signaling in the induction of ZIP8 in vascular endothelial cells after exposure to Pb. [A, B, and C] Vascular endothelial cells were incubated in the presence or absence of Pb (5, 10, 20, or 50 μM) for 6 hr (A) or Pb (20 μM) for 3, 6, 12, and 24 hr (B and C), and the expression levels of phosphorylated IκBα (P-IκBα), total IκBα, and GAPDH proteins (A and B) or p65 and Lamin A/C proteins of nuclear fractions (C) were determined by western blotting analysis. [D] Vascular endothelial cells were transfected with the control or p65 siRNA for 24 hr. Then were incubated in the presence or absence of Pb (10 or 20 μM) for further 24 hr, and the expression levels of p65 and ZIP8 mRNAs were determined by real-time RT-PCR. Each value represents the mean ± S.E. of three technical replicates; **p < 0.01 compared with the corresponding siControl.
Although Pb increased the expression of phosphorylated p38 MAPK and JNK proteins in vascular endothelial cells, the increased expression of ZIP8 mRNA was not reduced by pretreatment with U0126, SB203580, or SP600125, inhibitors of ERK, p38 MAPK, and JNK signaling, respectively (Supplemental Fig. 1). We previously showed that Pb-induced endoplasmic reticulum stress activates IRE1α and PERK, but not ATF6, in vascular endothelial cells (Shinkai et al., 2010; Shinkai and Kaji, 2012). However, knockdown of IRE1α, PERK, or ATF6 failed to suppress the Pb-induced increase in ZIP8 mRNA expression (Fig. S2), suggesting that IRE1α, PERK, and ATF6 are not associated with the induction of ZIP8 expression by Pb. Moreover, MAPK pathway and endoplasmic reticulum stress are also not involved in Pb-induced ZIP8 expression.
Since ZIP8 is a metal transporter that transports essential metals such as Zn, Fe, and Mn, the abnormal expression of ZIP8 may result in the abnormal function of various cells. Consequently, this would result in the initiation and progression of various diseases due to the metabolic disturbance of these metals (Fujishiro and Himeno, 2019). Additionally, ZIP8 contributes to the uptake of Cd into cells and is involved in Cd toxicity (Himeno et al., 2009; Liu et al., 2013). Previously, we have shown that Cd induces ZIP8 in vascular endothelial cells, and that this induction is crucial for determining the susceptibility of the cells to Cd cytotoxicity. (Fujie et al., 2022). However, the present study revealed that Pb-induced cytotoxicity was suppressed by the knockdown of ZIP8, and the suppression was independent of the intracellular accumulation of Pb in vascular endothelial cells, although Pb induced the expression of ZIP8. These results suggest that Pb induces ZIP8 expression by activating the NF-κB signaling via phosphorylating and decreasing the expression of IκBα, and suggests that the induced ZIP8 functions as a protective molecule against Pb cytotoxicity without decreasing the intracellular accumulation of Pb in vascular endothelial cells.
A cohort study in the United States of America suggested an association between the blood concentration of Pb and cardiovascular disease mortality, including ischemic heart disease mortality (Lanphear et al., 2018). Additionally, a previous epidemiological study reported that mutations in the ZIP8 gene increase mortality due to cardiovascular disease mortality (Johansson et al., 2016). The present data indicate that ZIP8 is involved in the protection of vascular endothelial cells against Pb cytotoxicity without altering the intracellular accumulation of Pb, although the detailed mechanisms remain to be elucidated. Taken together, these results suggest that endothelial ZIP8 may prevent the initiation and progression of cardiovascular diseases such as atherosclerosis caused by Pb. However, since we had previously revealed that TGF-β1 induces ZIP8 and potentiates Cd cytotoxicity in vascular endothelial cells (Ito et al., 2021), it is likely that endothelial ZIP8 may be a contributing factor for the progression of atherosclerosis caused by Cd. Therefore, whether ZIP8 provides protection or contributes towards the initiation and progression of atherosclerosis may depend on the type of risk factor.
NF-κB usually binds to IκBα to form an inactive complex in the cytoplasm. When IκBα is phosphorylated by IκB kinase, NF-κB is released from IκBα, translocated into the nucleus, and recruited to the NF-κB-binding sites of the promoter region of target genes to activate the transcription of downstream genes. Phosphorylated IκBα is marked for ubiquitination and degradation via the ubiquitin-proteasome system (Hayden and Ghosh, 2008). In this study we observed increased expression levels of phosphorylated IκBα protein and decreased expression of total IκBα, suggesting that Pb activates NF-κB signaling by promoting both the phosphorylation and degradation of IκBα by the ubiquitin-proteasome system. We recently reported that Cd activates NF-κB signaling by phosphorylation of IκBα and stabilization of NF-κB via activation of JNK signaling, suggesting that the mechanism underlying the activation of NF-κB signaling is different between Pb and Cd. In other words, the activating mechanism of NF-κB signaling by Pb does not include the stabilization of NF-κB mediated by JNK signaling. Moreover, Cd induced a transient increase in ZIP8 expression (Fujie et al., 2022), but Pb induced a continuous increase (Fig. 2B). This difference in inducibility may also be due to differences in their induction mechanisms. It has been suggested that the mechanisms by which heavy metals activate NF-κB signaling may be diverse, and signaling is important in the initiation and progression of atherosclerosis through the regulation and abnormality of vascular endothelial cell functions.
In summary, the present study revealed that Pb cytotoxicity was suppressed by ZIP8 via intracellular Pb accumulation independent mechanisms, and that the expression of ZIP8 was induced by the NF-κB signaling pathway in vascular endothelial cells after exposure to Pb (Fig. 4). Vascular endothelial ZIP8 may prevent the initiation and progression of cardiovascular diseases such as atherosclerosis caused by Pb. Atherosclerosis is an inflammatory disease (Ross, 1999), and Pb is a heavy metal known to be associated with the promotion of inflammatory responses (Metryka et al., 2018). ZIP8 may contribute to the suppression of Pb-induced inflammatory responses during atherosclerosis progression. However, there are reports suggesting that ZIP8 is a precipitating factor in inflammatory changes (Melia et al., 2019; Cheng et al., 2018). Thus, further studies are required to clarify the physiological and pathological roles of ZIP8 in the initiation and progression of atherosclerosis induced by heavy metals.

Possible mechanisms underlying the induction of ZIP8 by Pb in vascular endothelial cells. Pb activates the NF-κB signaling pathway by increasing phosphorylation of IκBα to induce the ZIP8 expression in vascular endothelial cells. Pb may promote the degradation of IκBα by the ubiquitin-proteasome system and this promotion may contribute to the activation of the NF-κB signaling pathway. It is suggested that the induction of ZIP8 is involved in the protection against Pb cytotoxicity by intracellular Pb accumulation independent mechanisms.
The authors would like to thank Ms. Aki Nakamura for her technical assistance. This research was funded by the Japan Society for the Promotion of Science (JSPS) KAKENHI (Grant Numbers JP19K07089 to T.K. and JP19K16361 to T.F.) and the Study Group of the Health Effects of Heavy Metals organized by the Ministry of the Environment, Japan.
Conflict of interestThe authors declare that there is no conflict of interest.