2022 Volume 47 Issue 7 Pages 301-308
2022 Volume 47 Issue 7 Pages 301-308
We examined that an estradiol-dominant state against progesterone could affect hematological parameters through hemodilution because estradiol is known to increase plasma volume via oncotic pressure. We performed a 2- and 3-week repeated oral dose study with mifepristone, a progesterone receptor antagonist, in female rats and examined erythrocyte counts, hemoglobin, hematocrit, plasma volume, levels of estradiol and progesterone, water intake, and water loss. Mifepristone treatment decreased some hematological parameters mildly and increased plasma volume. There were no remarkable changes in the balance of water intake and water loss through urination. Both estradiol and progesterone levels and the ratio of estradiol to progesterone increased. Therefore, our findings indicate that repeated mifepristone treatment increases estradiol levels and plasma volume, resulting in lower erythrocyte counts, hemoglobin, and hematocrit. The present study proved the possible contribution of estradiol to understanding the toxicological significance of mifepristone-induced hemodilution.
Water is the largest constituent of the human body and typically accounts for 60%–70% of body weight. Approximately 60% of water is stored intracellularly, and approximately 40% is stored extracellularly. (Jain, 2015; Stachenfeld and Taylor, 2004). Water loss occurs through urination, sweating, salivation, insensible perspiration or defecation. Water loss in urine is regulated by arginine vasopressin (AVP or antidiuretic hormone), which is secreted from the posterior lobe of the pituitary gland and is a potent vasoconstrictor (Sladek and Johnson, 2013; Wenner and Stachenfeld, 2012). Water loss or a deficit in body water can result in lower volumes of extracellular fluid, leading to lower blood volume, higher concentrations of solutes in the extracellular fluid, and increased osmolality. Both thirst and AVP secretion are stimulated by increases in extracellular fluid osmolality and decreases in blood volume and/or blood pressure (Stricker and Sved, 2002).
The renin-angiotensin-aldosterone system (RAAS) regulates blood pressure and body fluid homeostasis (Azushima et al., 2020). RAAS maintains vascular tonicity by regulating extracellular fluid volume and arterial pressure. Renin release occurs through decreases in sodium intake, extracellular fluid, or blood volume. In the RAAS pathway, angiotensinogen is cleaved by angiotensinogen-converting enzyme to produce the main effector of this system, angiotensin II, which modulates vasoconstriction and sodium/water retention. Drugs that antagonize or block RAAS are used to treat hypertension, indicating that RAAS is clinically relevant (Navar, 2014).
The sympathetic nervous system is known regulator of body fluid composition by influencing the renal regulation of arterial pressure (Nishi et al., 2015). Renin secretion is activated by β1-adrenoceptor stimulation, enhanced tubular sodium reabsorption by α1b-adrenoceptors, and reduced renal blood flow (Krum et al., 2013). Drugs that stimulate or inhibit the adrenoceptors can alter extracellular fluid volume (Hettiaratchi and Pickford, 1971). Desmopressin, an antidiuretic agent, is known to induce a reduction in hematocrit and hemoglobin by hemodilution in humans (Sanchis-Gomar et al., 2010). Continued effects on α- or β-adrenoceptor can result in lower erythrocyte counts, hemoglobin, and hematocrit in rats and mice (Gutiérrez et al., 2008; Waghe et al., 1999; Arbo et al., 2009).
Apart from regulation of body fluid homeostasis by antidiuretic hormone, RAAS, or the sympathetic nervous system, sexual hormone levels are known to regulate body fluid in women during the menstrual cycle or menopause (Rosenfeld et al., 2008; Stachenfeld et al., 2001; Stachenfeld, 2014). Plasma volume and hematocrit are distinguished by estrous stage in both humans and rats (Crofton and Share, 1990). Estradiol (E2) and progesterone (P4) regulate body fluid by altering the osmotic action of AVP (Stachenfeld and Keefe, 2002; Stachenfeld and Taylor, 2004; Stachenfeld, 2008). In particular, an E2-dominant state is considered critical to increasing fluid volume because progestin, which contains no E2 but downregulates estrogen receptors, does not alter fluid volume (Speroff et al., 1989). Tamoxifen, a partial agonist of estrogen receptors, has been reported to lower erythrocyte counts, hemoglobin, and hematocrit in normal postmenopausal women, exerting a hemodilutory effect (Grey et al., 1997). These reports suggest that functional E2 dominance against P4 that originates from pharmacological blockade of P4 function or agonistic effects on E2 receptors may induce an increase in plasma volume. Slight or mild decreases in erythrocyte counts, hemoglobin, or hematocrit without histopathologically suggestive suppression of hematopoiesis have been observed in general toxicity studies of mifepristone, a P4 receptor antagonist (Food and Drug Administration [FDA], 2000; Xiao et al., 2016), or contraceptives containing estradiol valerate and norethisterone (Seibert and Günzel, 1994).
We hypothesized that modulating E2 and/or P4 secretion or their function could decrease erythrocyte counts, hemoglobin, or hematocrit by increasing plasma volume (i.e., hemodilution). In the present study, we performed a 2- and 3-week repeated oral dose study with mifepristone to examine the effects on hematological parameters and plasma volume through functional E2 dominance from blockade of P4 function in female rats. The findings indicate that toxicologists should take into consideration a drug’s secondary effects on the estradiol/progesterone (E/P) ratio when studying the mode of action underlying mild decreases in erythrocyte counts, hemoglobin, or hematocrit when the drug does not induce direct pharmacological effects on hematopoiesis or when the findings are not accompanied by histopathological observations suggestive of impaired hematopoiesis.
Mifepristone (11β-[4-(dimethylamino)phenyl]-17β-hydroxy-17-(1-propynyl)-estra-4,9-dien-3-one) was purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Carboxymethylcellulose was purchased from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan). Autoclaved aqueous 0.25% carboxymethylcellulose was used as a vehicle to prepare the test formulation. Mifepristone was suspended in the vehicle in a volume of 10 mL/kg body weight. The test formulation was prepared fresh weekly.
Animals and ethics statementNine-week-old specific pathogen-free female Wistar Hannover rats (RccHan:WIST) were obtained from Japan SLC, Inc. (Shizuoka, Japan) and maintained in a barrier system animal room at a temperature of 23 ± 2°C, relative humidity of 60% ± 10%, and 12-hr light/dark cycle (light on 8:00 a.m. to 8:00 p.m.) with ventilation 10–15 times per hour. The animals were housed in stainless steel cages (260 × 230 × 180 mm) and allowed free access to tap water and a pellet diet (CE-2, CLEA Japan Inc., Tokyo, Japan) during the quarantine and acclimation and study periods. This study was performed in strict accordance with the animal welfare bylaws of the Safety Research Laboratory, Kissei Pharmaceutical Co., Ltd. The Institutional Animal Care and Use Committee in our institution oversaw the animal management of this study. Our institution is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International.
Drug administrationAfter a 10-day quarantine and acclimatization period, a total of 34 animals without any abnormalities in clinical signs were allocated to six groups consisting of five or six animals each using a stratified randomization method. Animals were orally administered mifepristone suspended in the vehicle at a dose of 100 mg/kg once daily for 3 weeks (Experiments 1 and 3) or for 2 weeks (Experiment 2). Animals allocated to the control group were orally administered the vehicle only. Administration was initiated at 10-week-old. The dose was selected from a 4-week toxicity study in rats treated orally with mifepristone in which 100 mg/kg/day was the highest dose (Tamura et al., 2009). Table 1 lists the group designations in detail.
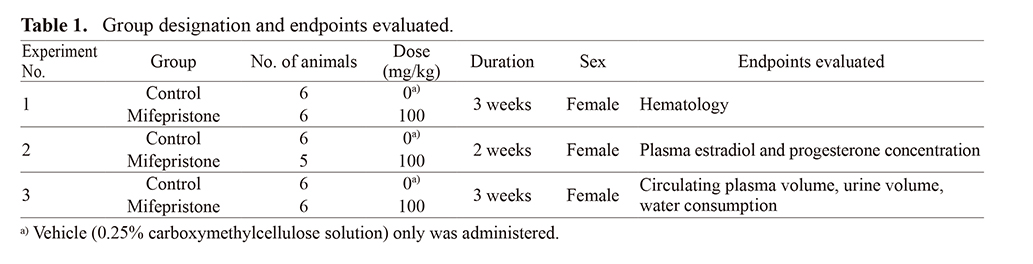 Experiment 1: Effects of mifepristone on hematological parameters
Experiment 1: Effects of mifepristone on hematological parameters
Animals were administered orally the vehicle (n = 6) or mifepristone (100 mg/kg/day, n = 6) daily for 3 weeks in the morning. Approximately 0.2 mL of blood was collected from the jugular vein without anesthesia using EDTA-2K as the anti-coagulant pre-dosing and 2, 4, and 8 hr post-dosing on Day 1 and on the end of Week 2 (Day 15) and Week 3 (Day 22). Hematological examination was performed using an automated hematology analyzer (model XN-1000, Sysmex Corporation, Kobe, Japan). Percent of changes in erythrocytes, hemoglobin, hematocrit, and reticulocytes from pre-dosing on Day 1 in each group were calculated for each measurement time point.
Experiment 2: Effects of mifepristone on plasma hormone concentrations and clinical chemistry parametersAnimals were administered the vehicle (n = 6) or mifepristone (100 mg/kg/day, n = 5) daily for approximately 2 weeks in the morning. The treatment was continued for 13 days or more until the appearance of the estrus stage. Four hours after the final dosing on the day of estrus in Week 2, blood samples were collected by decapitation and treated with heparin sodium as an anti-coagulant. Plasma concentrations of E2 and P4 were measured simultaneously by Nexera/QTRAP 4500 liquid chromatography-tandem mass spectrometry (Nexera: Shimadzu, Kyoto, Japan, QTRAP 4500: AB Sciex, Toronto, ON, Canada). Before the measurement, E2 and its internal standard, d4-E2, were derivatized by the reagent 1,2-dimethylimidazole-5-sulfonyl chloride (Keski-Rahkonen et al., 2015). Column temperature was 50°C with a XBridge BEH C18 column (100 × 2.1 mm; 2.5 μm) and flow rate 0.20 mL/min with mobile phase [mixture of 0.01 mol/L ammonium formate buffer (pH3.0) and methanol (37:63)]. Injection volume was 10 μL and the autosampler temperature was 4°C. A QTRAP 4500 mass spectrometer was used with electrospray ionization (ESI) ion source in positive polarity. Two mass transitions were monitored for each analyte as follows (first product ion quantitative, second confirmatory): E2: 431 → 96, d4-E2: 435 → 96, P4: 315 → 97, d9-P4 (internal standard of P4): 324 → 100. The E/P ratio was calculated for each animal to determine balance between E2 and P4. The quantification range of E2 was 0.625–100 pg/mL and that of P4 was 0.625–20 ng/mL. Values below the lower limit of quantification were regarded as a half of the lower limit of quantification (E2: 0.3 pg/mL; P4: 0.3 ng/mL) to calculate the E/P ratio. Clinical chemistry parameters were measured by an automatic analyzer (model 7180, Hitachi High-Tech Corporation, Tokyo, Japan).
Experiment 3: Effects of mifepristone on circulating plasma volume, water consumption, and urine volumeAnimals were administered the vehicle (n = 6) or mifepristone (n = 6) daily for 3 weeks in the morning. Measurement of circulating plasma volume was performed 4 hr post-dosing on the day of estrus stage in Weeks 2 and 3. Blood samples were collected 5 min after an intravenous injection of Evans blue dye (FUJIFILM Wako Pure Chemical Corporation) at 2.5 mg/kg through the tail vein and treated with heparin sodium as an anti-coagulant. Plasma concentration of the dye was determined by measuring absorbance at 620 nm using a multi-mode reader (Cytation5 MFV, BioTek Instruments, Inc., Winooski, VT, USA). Both 24-hr water consumption and urine volume were measured in Weeks 1 (Day 7) and 3 (Day 21) using metabolic cages.
Statistical analysisComparisons between the control group and mifepristone-treated group were calculated for all three experiments. The statistical significance of a difference in the variance between the two groups was examined by F-test. When the F-test variance was homoscedastic, the difference was assessed by Student’s t-test (two-sided test). When the variance was not homoscedastic, the difference was tested by an approximation of the t-test according to the Aspin-Welch method (two-sided test). For each test, differences were considered statistically significant at P < 0.05.
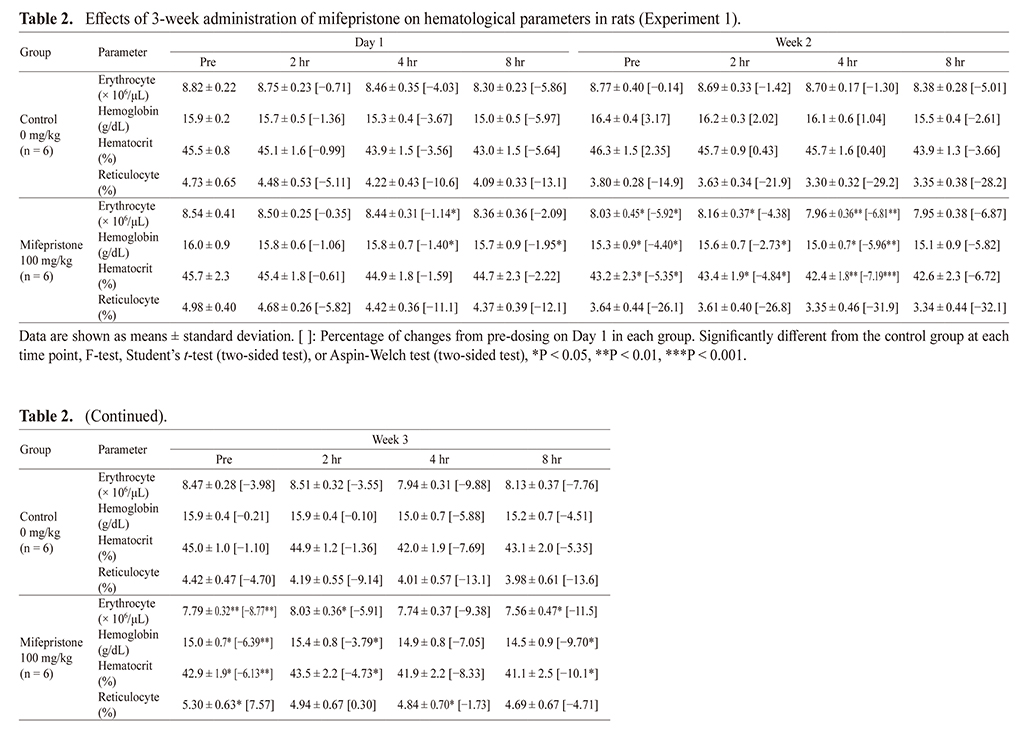
Hematology parameters at each time point in the control and mifepristone-treated groups are shown in Table 2. In addition, as hematology parameters exhibited a circadian rhythm, percent of changes from pre-dosing on Day 1 in each group were calculated and are shown in Table 2 and Fig. 1. Statistical analysis was performed between the control and mifepristone-treated group at each time point. Erythrocyte counts, hemoglobin, and hematocrit decreased with or without statistical significance at pre-dosing in Week 2, and these decreases continued until termination in Week 3 in mifepristone-treated rats. The decreases in these parameters were within 10% of control levels throughout the treatment period without deterioration by repeated administration. Reticulocyte (%) decreased slightly in Week 2 and then increased in Week 3; however, that in Week 3 were comparable to those measured on Day 1 in mifepristone-treated rats.
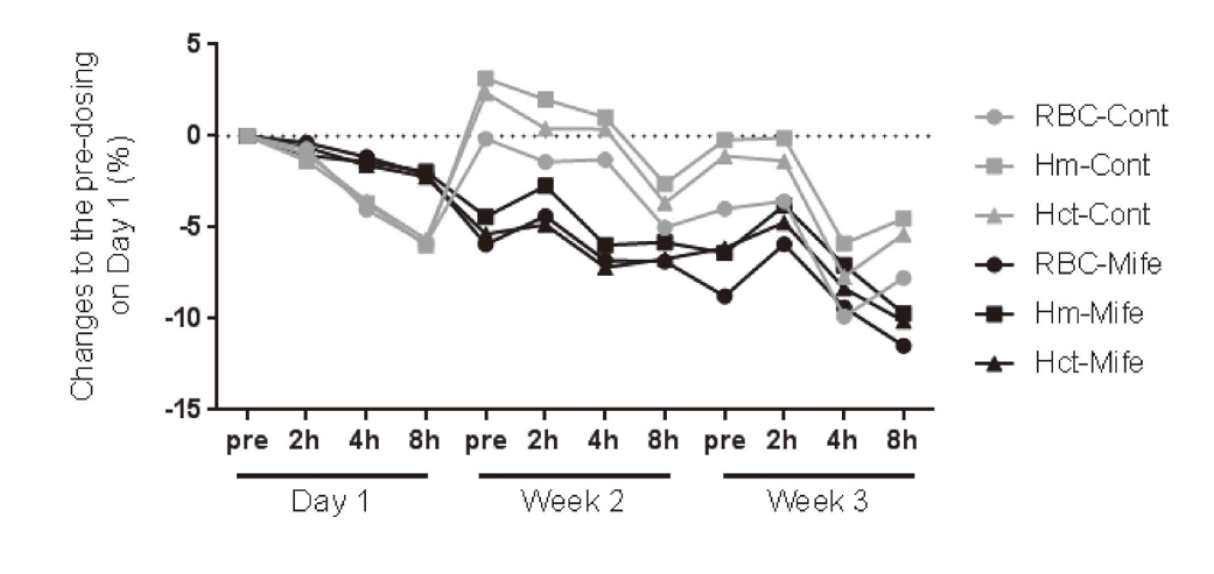
Effects of 3-week administration of mifepristone on hematological parameters in rats (Experiment 1). Percentages of change from pre-dosing on Day 1 in erythrocyte count, hemoglobin, and hematocrit during 3-week treatment period. Values are represented as percent of changes from pre-dosing on Day 1. Hematological testing was performed pre-dosing and 2, 4, and 8 hr post-dosing on Day 1, week 2, and week 3. RBC: erythrocyte, Hm: hemoglobin, Hct: hematocrit, Cont: control, Mife: mifepristone
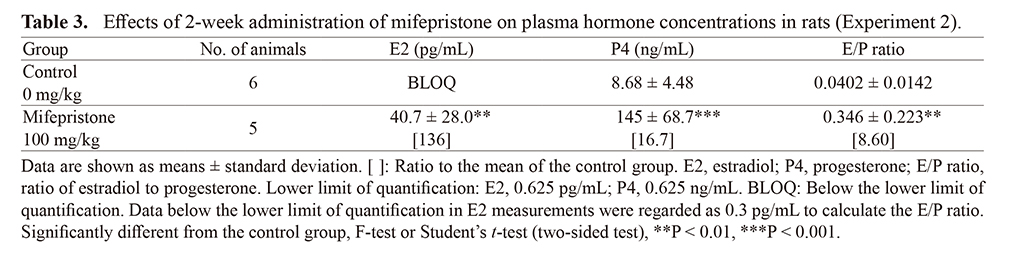
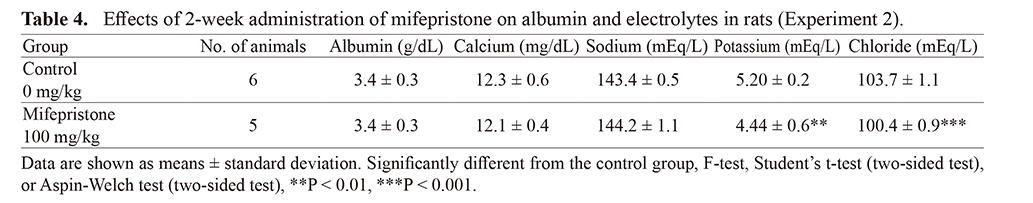
As plasma hormone concentrations are influenced by various factors or processes, daily oral dosing was performed for 2 weeks to evaluate E2 and P4 concentrations and clinical chemistry parameters. In the control group, E2 levels were below the lower limit of quantification for all animals and the E/P ratio was low (0.0402). By contrast, in mifepristone-treated rats, E2 and P4 levels increased 136-fold and 16.7-fold from control levels, respectively. The E/P ratio was elevated 8.6-fold (P < 0.05). Levels of plasma albumin, calcium and sodium did not change; however, potassium and chloride decreased.
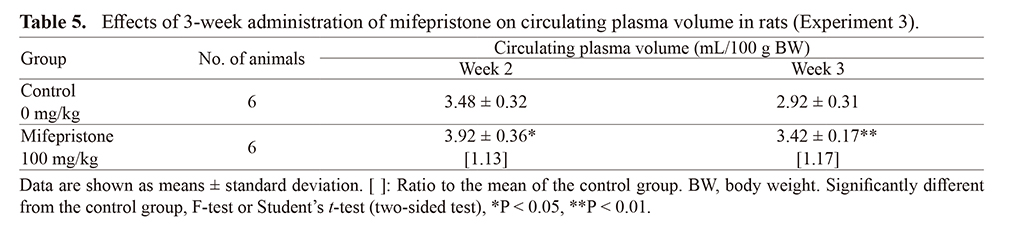
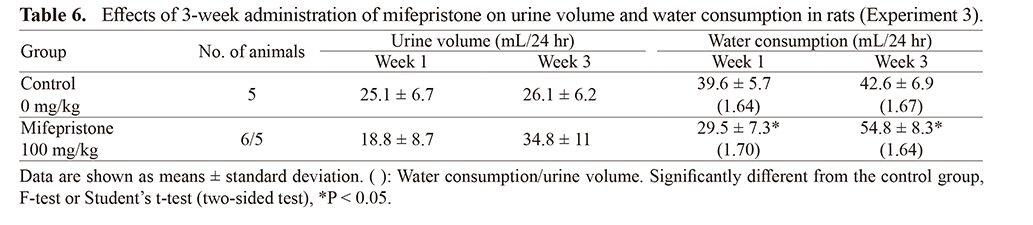
Experiment 3: Effects of 3-week repeated administration of mifepristone on circulating plasma volume, urine volume, and water consumption (Tables 5 and 6)Circulating plasma volume in the mifepristone-treated group increased slightly but significantly in both Weeks 2 and 3 (Table 4). In the mifepristone-treated group, no significant changes in urine volume were observed, although water consumption changed significantly (Table 5). However, the ratios of water consumption to urine volume were comparable (approximately 1.6) in both Week 1 and Week 3 in the control and mifepristone-treated groups.
The present study demonstrated that repeated oral administration of mifepristone, a selective progesterone receptor antagonist, resulted in mild decreases in erythrocyte counts, hemoglobin, and hematocrit and in a significant elevation in both E2 and P4 levels and the E/P ratio. Moreover, mifepristone increased circulating plasma volume.
Mild decreases in hematological parameters have also been reported for hemoglobin and hematocrit in female rats orally administered with 200 mg/kg/day mifepristone for 30 days (Xiao et al., 2016). Although the changes were not statistically significant, hemoglobin and hematocrit decreased from control levels by 7.5% and 1.0%, respectively. The authors considered these changes as varied but not adverse. Thus, repeated administration of mifepristone can induce mild but not adverse decreases in erythrocyte counts, hemoglobin, and hematocrit in rats. No findings suggestive of impairment in hematopoiesis in mifepristone-treated rats were observed in the present study or in previous studies (Tamura et al., 2009; Xiao et al., 2016). Reticulocyte (%) in the mifepristone-treated group increased in Week 3 up to the pre-dosing level after a decrease in Week 2. This increase was considered a compensatory response to lower erythrocyte counts; therefore, hematopoiesis was not impaired by repeated mifepristone treatment.
Circulating plasma volume increased slightly but significantly by 13% and 17% in Weeks 2 and 3, respectively. Systemic fluid homeostasis involves both water intake and water loss through urine. In the present study, the ratio of water consumption to urine volume was comparable in both the control and mifepristone-treated group in Weeks 1 and 3. A previous study in rats reported no changes in the posterior lobe of the pituitary gland, where AVP is secreted, following repeated mifepristone treatment (Tamura et al., 2009); therefore, we consider that mifepristone treatment does not alter plasma volume regulation by AVP.
Plasma volume is known to be regulated by endocrine systems such as RAAS. Mifepristone has been reported to suppress aldosterone-related increases in plasma volume (Salyer et al., 2013; Agarwai, 1996); thus, an incremental effect of aldosterone on plasma volume may be inhibited in the rats treated with mifepristone in the present study. Mifepristone has also been reported to exert no influence on renin activity in rats (Clapham and Turner, 1997). Sodium content in plasma is indicative of renin secretion, but we did not observe changes in sodium concentrations in the present study. Therefore, we consider that repeated dosing with mifepristone did not affect RAAS under our experimental conditions. Moreover, the sympathetic nervous system can regulate plasma volume, but there are no reports that suggest that mifepristone modulates the function of the sympathetic nervous system. Thus, repeated dosing with mifepristone is unlikely to alter body fluid regulation by a major regulatory system such as AVP, RAAS, or the sympathetic nervous system.
Interestingly, repeated dosing with mifepristone has been reported to lead to persistent estrus from both E2 increases and absence of the counteracting action of P4 (Sánchez-Criado et al., 1993). Moreover, higher levels of E2 without the balancing actions of P4 induce elevations in prolactin levels, which stimulate luteinized granulosa cells to produce P4 (van der Schoot et al., 1987). In line with these reports, we found increases in both E2 and P4 levels and the E/P ratio following mifepristone treatment. Repeated dosing with mifepristone has been reported to increase luteinized cysts and unruptured luteinized follicles partially lined by luteinized granulosa cell layers in the ovaries (Tamura et al., 2009), probably predictive of alterations in female reproductive endpoints such as persistent estrus. Increases in luteinized cysts and plasma E2 concentrations indicate that the probable source of E2 is luteal cells because these possess aromatase activity and may transform ovarian androgens to E2 (Keyes and Wiltbank, 1988). Repeated dosing with mifepristone can therefore alter follicle development in the ovary and increase E2 and P4 levels with a functional E2-dominant state because mifepristone inhibits the counteracting action of P4.
E2 is also known to increase plasma volume (Stachenfeld and Taylor, 2004) by lowering the transcapillary escape of albumin, resulting in increases in plasma albumin content and plasma oncotic pressure (Oian et al., 1987; Stachenfeld and Taylor, 2004). Increased plasma oncotic pressure can direct interstitial fluid into capillaries and lead to hemodilution. However, elevated plasma albumin concentrations in mifepristone-treated rats were not observed in the present study. Temporal elevation of plasma albumin concentrations might occur due to E2 increase in plasma in mifepristone-treated rats; however, that elevation would be normalized concurrently by increasing plasma volume because homeostasis of oncotic pressure is highly maintained. Of decreases in plasma potassium and chloride concentration in mifepristone-treated rats, the mode of action is unclear, but these are considered incidental because 30-day or 26-week repeated dose toxicity study with mifepristone in rats reported no changes in these parameters (FDA, 2000). E2 supplement resulted in approximately 3.3% increase in plasma volume and then approximately 1.5% decrease in hematocrit in humans (Stachenfeld and Taylor, 2004). In addition, in 30-day repeated dose with mifepristone in rats, approximately 1.0% and 7.5% decreases were noted in hematocrit and hemoglobin, respectively (Xiao et al., 2016). Thus, hemodilution with E2 supplement or increase can result in these mild decreases in hematological parameters. Therefore, the mild decreases in erythrocyte counts, hemoglobin, and hematocrit observed in the present study are probably attributable to hemodilution by a functional E2-dominant state in mifepristone-treated rats.
The findings of the present study demonstrate that mifepristone can alter secondary systemic fluid regulation and lead to hemodilution, resulting in apparent decreases in hematological parameters. Mifepristone is a P4 receptor antagonist and is not expected to exert direct effects on systems that regulate body fluids, such as AVP, RAAS, and the sympathetic nervous system. Because erythrocyte counts, hemoglobin, and hematocrit are key endpoints in non-clinical toxicity studies, changes in these parameters should be evaluated for toxicological significance with relevance to the risk of anemia in clinical use. Appropriate consideration of the drug’s mode of action is required to ensure patient safety when unexpected decreases in hematological parameters are observed in toxicity studies of drug candidates. In such a case, toxicologists should determine whether the compound exerts pharmacological effects on hematopoiesis or whether findings in related organs or tissues may suggest an influence on hematopoiesis. When there are no pharmacological effects or suggestive findings, the E/P ratio can indicate hemodilution by E2 dominance.
The authors wish to thank Shohei Kobayashi, Masaoki Kajino, Takaya Kutsukake, Keigo Miyazawa, Yoshimasa Maruyama, Tetsuaki Takahashi, Chizuru Nishiyama, and Yamato Miyajima for dedicated support for this work. We also thank Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Conflict of interestThe authors declare that there is no conflict of interest.