2023 Volume 48 Issue 4 Pages 203-210
2023 Volume 48 Issue 4 Pages 203-210
Acetamiprid (ACE), a neonicotinoid chemical, is widely used as a pesticide due to its rapid insecticidal activity. Although neonicotinoids exert very low toxicity in mammals, the effects of early exposure to neonicotinoids on the adult central nervous system are poorly understood. This study investigated the effects of ACE exposure in early life on brain function in adult mice. We exposed male C57BL/6N mice to ACE (10 mg/kg) orally when they were two (postnatal lactation) or 11 weeks old (adult). We examined the effects of ACE on the central nervous system using the mouse behavioral test battery, consisting of the open field test, light/dark transition test, elevated plus-maze test, contextual/cued fear conditioning test, and pre-pulse inhibition test at 12–13 weeks old. In the mouse behavioral test battery, learning memory abnormalities were detected in the mature treatment group. In addition, learning memory and emotional abnormalities were detected in the postnatal lactation treatment group. These results suggest that the behavioral effects of postnatal lactation treatment with ACE were qualitatively different from the behavioral abnormalities in the mature treatment group.
Many chemical substances exist in the environment, and individuals are exposed to these chemicals. Risk assessments are necessary to prevent health hazards induced by chemical exposure and are updated accordingly. However, the harmful delayed effects induced by prenatal chemical exposure during adulthood are yet to be adequately elucidated. In a previous study, we indicated the importance of carefully considering regulatory values for prenatal and postnatal lactating individuals for chemical substances suspected to affect mammals (Saito et al., 2017, 2019).
Neonicotinoids are a new class of insecticides that are chemically similar to nicotine. They are considered less toxic to mammals because they have a much higher affinity for the nicotinic acetylcholine receptors (nAChRs) of insects than mammals (Tomizawa and Casida, 2005). However, their precise effects on mammals are not completely understood. Recently, neonicotinoids have been reported to have nicotine-like excitatory effects on nAChRs in mammals (Kimura-Kuroda et al., 2012). In addition, there is concern that exposure to neonicotinoid pesticides may have adverse effects, on the developing brain of vertebrates causing neurobehavioral effects including anxiety behavior and impairment of cognitive function (Ozdemir et al., 2014; Abreu-Villaça and Levin, 2017; Burke et al., 2018). Acetamiprid (ACE) is one of the neonicotinoid insecticides that is used in many products to control a variety of sucking and chewing insect pests worldwide. ACE has been shown to cross the blood-brain barrier in mice and enter and accumulate in the central nervous system (Terayama et al., 2016). In addition, exposure to ACE during the perinatal period causes neurobehavioral impairments, including socio-sexual and anxiety-related behavior (Sano et al., 2016). Knowledge of the potential neurological effects of neonicotinoids, including ACE, is beginning to accumulate in vitro or in vivo. However, the studies on neonicotinoids conducted to date are limited in number, and more studies are needed to fully understand their effects on human health (Cimino et al., 2017). Therefore, there is still limited information available to discuss the behavioral toxicity of neonicotinoids for mammals, including humans.
Synapses formed immediately after birth are functionally immature. Neural circuits are shaped by a selective strengthening of necessary synapses and the elimination of unnecessary redundant connections during the later postnatal period. Especially, synapse elimination is crucial for the establishment of functionally mature neural circuits (Lichtman and Colman, 2000; Riccomagno and Kolodkin, 2015). In the developing brain, various neuronal signals must be appropriately activated to construct the neural network, and exposure to chemical substances during this period can induce developmental neurotoxicity (Rice and Barone, 2000). Unbalanced specification of excitatory and inhibitory neurons leads to the dysfunction of neural circuits and is a factor in various neurological disorders (Gatto and Broadie, 2010; Nelson and Valakh, 2015; Sawada et al., 2020). Therefore, even transient disruption of neuronal signals with external factors, such as pesticides, may interfere with normal brain development and lead to irreversible functional effects.
Based on the above, the effects of chemical exposure during the postnatal lactation period may be qualitatively different from the effects of administration to mature animals or pregnant animals, and it is important to consider the risk of developmental exposure effects on the brain in later life. However, to our knowledge, very few studies have focused on the developmental exposure effects on the brain in later life of ACE on neurobehavioral toxicity. As neonicotinoids have been regulated in many countries, including the European Union, it is necessary to accumulate data on their toxicity regarding mammalian neurodevelopment from a precautionary viewpoint.
In this study, we focused on the postnatal lactation period, the period that corresponds to synaptic elimination, and evaluated the effects of postnatal lactation and adult ACE exposure on behavior to address these issues using behavioral tests.
The experimental design is illustrated in Fig. 1. C57BL/6N male mice with foster females at postnatal day (P)-10 were purchased from Japan SLC (Shizuoka, Japan). Acetamiprid (ACE; Wako Pure Chemical Industries Ltd., Osaka, Japan) was combined with corn oil (Sigma-Aldrich; Merck, Darmstadt, Germany). The mice were divided into three groups: (1) vehicle control (VC; n = 8), (2) ACE two-week-old exposure (ACE-2w; n = 8), and (3) ACE 11-week-old exposure (ACE-11w; n = 8). We administered a single oral dose of 10 mg/kg ACE, referring to an acute reference dose (ARfD) (EFSA, 2013). The test samples were prepared by dissolving 10 mg samples in 10 mL of corn oil. Thus, the concentration of ACE was 1 mg/mL. Mice received ACE by a single oral dose with a volume of 10 mL/kg to the final doses of 10 mg/kg. The mice in the VC group were administered corn oil only at two and 11 weeks old. The mice in the ACE-2w group were administered ACE at two weeks old and corn oil at 11 weeks old. The mice in the ACE-11w group were administered corn oil at two weeks old and ACE at 11 weeks old.
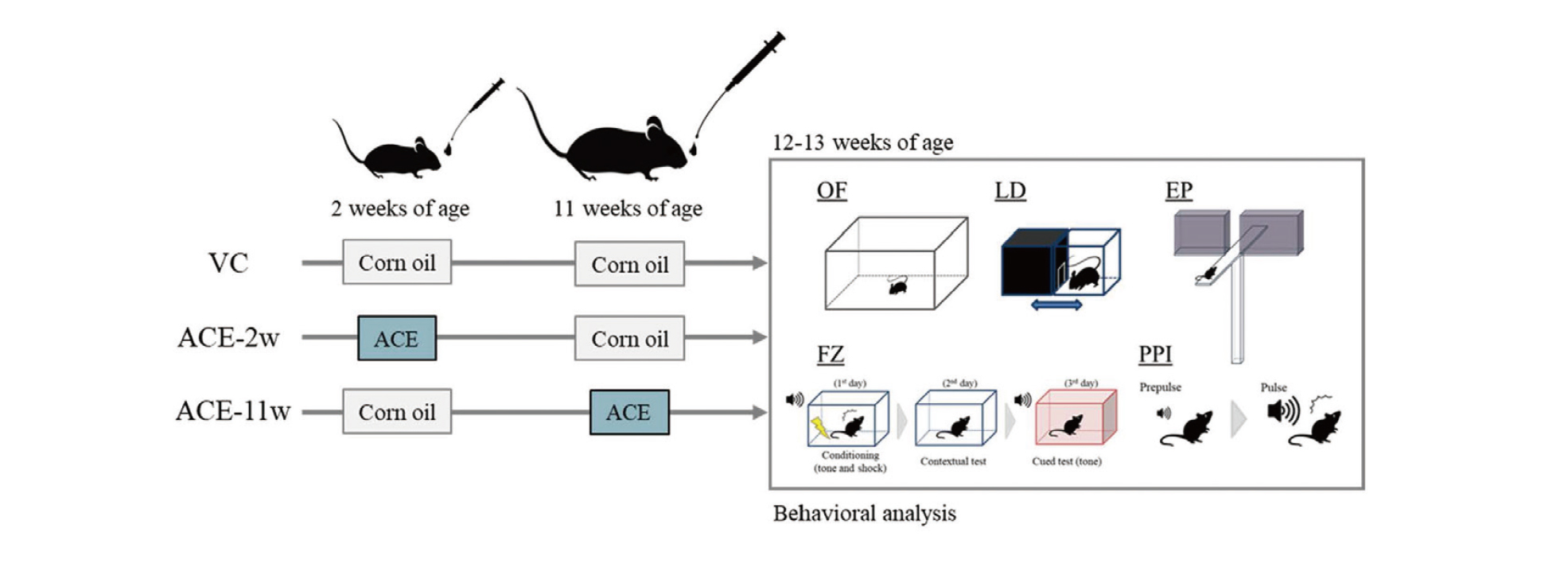
Schematic of the acetamiprid administration protocol. VC: vehicle control group, ACE-2w: acetamiprid two-week-old exposure group, ACE-11w: acetamiprid 11-week-old exposure group. OF: open field test, LD: light/dark transition test, EP: elevated plus-maze test, FZ: contextual/cued fear conditioning test, PPI: pre-pulse inhibition test.
Subsequently, we conducted a mouse behavioral test battery at 12–13 weeks old. We carefully controlled the noise levels in the animal room and adjacent areas to minimize animal stress. All animals were handled daily for 1 min for one week before commencing the mouse behavioral test battery. Mice were maintained in a room at a constant temperature (24 ± 1°C) and humidity (60% ± 10%) with a 12 hr light/dark cycle and had free access to food and water. After the mouse behavioral test battery, the mice were euthanized by exsanguination under deep anesthesia by inhalation of isoflurane. The brains were surgically removed, and fixed with methacarn solution (methanol/chloroform/acetic acid at 6:3:1) for future research. All animal care and experimental procedures were conducted in accordance with the Regulations for Animal Experiments and Related Activities at the National Institute of Health Sciences. This study was approved by the Animal Ethics Committee of the National Institute of Health Sciences (Permission No. 65-1).
Mouse behavioral test batteryThe mouse behavioral test battery was conducted at 12–13 weeks old. We performed a behavioral test battery that consisted of the open field (OF) test, light/dark transition (LD) test, elevated plus-maze (EP) test, contextual/cued fear conditioning (FZ) test, and pre-pulse inhibition (PPI) test. We used a previous study as a reference (Tanemura et al., 2009; Juliandi et al., 2015; Furukawa et al., 2016; Saito et al., 2019) with some modifications. The experimental apparatus and image analysis software (ImageJ OF4, ImageJ LD2, ImageJ EP2, ImageJ FZ2, and SR-9040) were obtained from O’Hara & Co., Ltd. (Tokyo, Japan) ImageJ OF4, LD2, EP2, and FZ2 were developed using ImageJ. All experiments were performed with 7–8 mice per group and performed sequentially using the same set of male mice. The background noise level during behavioral testing was approximately 50 dB. After each trial, the apparatus was cleaned with water and wiped off.
OF test: Locomotor activity was measured for 10 min using an OF apparatus made of white plastic (50 cm × 50 cm × 40 [H] cm). The LED light system was positioned approximately 60 cm above the center of the field (50 lx at the center of the field). Behavior was measured using a charge-coupled device (CCD) camera positioned 50 cm above the center of the OF apparatus. Behavioral parameters were measured using ImageJ OF4.
LD test: The apparatus used for the LD test consisted of a cage (21 cm × 42 cm × 25 [H] cm) divided into two chambers by a partition with an opening. One chamber was made of white plastic and brightly illuminated (250 lx, light area), whereas the other chamber, made of black plastic, was dark (5 lx, dark area). Behavior was measured using a CCD camera positioned above each chamber. A mouse was placed in the dark and allowed to move freely between the two chambers through the opening for 5 min. Behavioral parameters were measured using ImageJ LD2 and were used as indices of anxiety-related behavior.
EP test: The plus-shaped apparatus consisted of four arms (25 cm × 5 cm) connected to a central square area (5 cm × 5 cm). Two opposing arms were enclosed with 20 cm high transparent walls, and the other two were left open. The maze floor was made of white plastic and elevated 50 cm above the room floor (10 lx at the center of the apparatus). Behavior was measured using a CCD camera positioned above each chamber. A mouse was placed in the central square area of the maze, facing one of the arms, and its behavior was recorded for 10 min. Behavioral parameters were measured using ImageJ EP2 to analyze anxiety-related behavior.
FZ test: The apparatus consisted of a conditioning chamber (test chamber; 17 cm × 10 cm × 10 (H) cm) made of clear plastic with a ceiling. The chamber floor had stainless steel rods (2 mm diameter) spaced 5 mm apart for the electric foot shock to the mouse. The LED light system was positioned 50 cm above the chamber floor (100 lx at the center of the floor). Behavior was measured using a CCD camera positioned 20 cm above the ceiling of the chamber. The white background noise level was set to approximately 50 dB in a soundproof box of white-colored wood. During the conditioning trial (day one), mice were placed individually in the conditioning chamber, and, after 90 sec, they were given three tone shock pairings (30 sec of tone at 65 dB, 10 kHz directly followed by 3 sec of 0.1 mA electric shock), each separated by 120 sec. Mice were then returned to their home cage. The next day (day two), as a contextual fear test, they were returned to the conditioning chamber for 6 min without tone or shock. As a cued fear test on the third day (day three), they were placed in a novel chamber (with a different design, lacking stainless steel rods, 50 lx at the center of the floor). After 3 min, a conditioning tone (with no shock) was presented for 3 min. ImageJ FZ2 measured the freezing response of the mice as a consecutive 2 sec period of immobility. The freezing rate (%) was calculated as (freezing/session time) × 100.
PPI test: The apparatus consisted of a light source, a sound system, and a startle measurement load cell. They were set in a soundproof box. The software for the operation of the apparatus and data analysis was SR-9040 (O’Hara & Co., Ltd.). The white background noise level in the soundproof box was set to 70 dB. The mouse was placed in a plastic cylinder and kept there for 90 sec before testing. The test schedule consisted of three blocks, and the total trial duration was 30 min. The breakdown of each block was as follows: 80, 85, 90, 95, 100, 105, and 110 dB pulse × 3 (acclimation block) and 120 dB pulse × 10 (acoustic startle response block). The combinations of pre-pulse were 80–120, 85–120, 90–120, 95–120, 100–120, and 105–120 dB, with a delay of 100 msec × 6 (PPI measurement block). These combinations were presented in a pseudorandom order, such that each trial type was presented once within a block. The inhibition ratio (%) of the startle response was calculated as follows: (1-pre-pulse [80, 85, 90, 95, 100, or 105 dB] startle response value/acoustic startle response value) × 100.
Statistical analysisStatistical analysis was performed using Dunnett’s multiple comparisons with GraphPad Prism version 9.3.1 for Windows (GraphPad Software, San Diego, California, USA). P-values < 0.05 were considered statistically significant (**p < 0.01, *p < 0.05).
The total distance and number of movement episodes did not significantly change in the ACE-2w and ACE-11w groups compared to those in the VC group (Fig. 2A, C). The total center time was significantly higher in the ACE-2w group than in the VC group, whereas no significant differences were observed in the ACE-11w group (Fig. 2B).
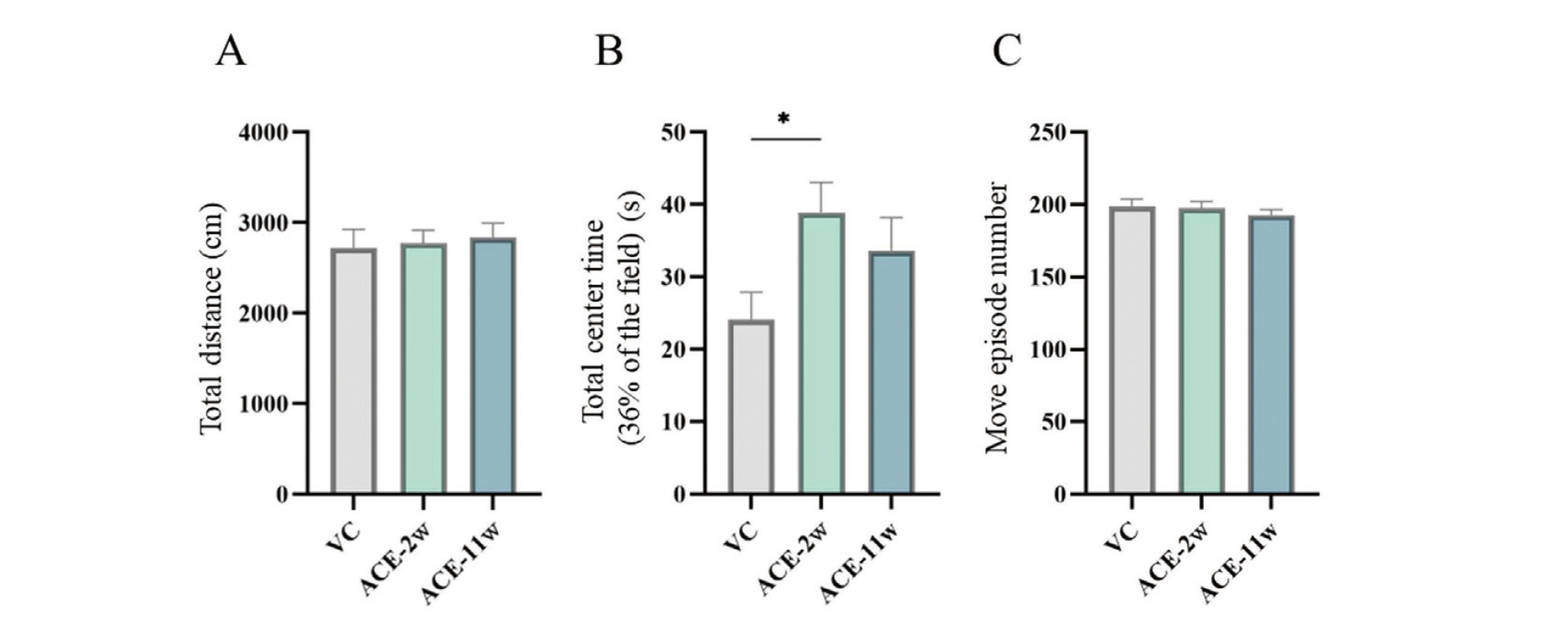
Results of the open field (OF) test. Scores of the OF test (total test time, 600 sec) are shown. (A) Distance traveled (cm) during the test period. (B) Time spent in the center area (sec). (C) Number of movements during the test period. Data are expressed as the mean ± S.E. Data were tested statistically using Dunnett’s test. *p < 0.05 versus the VC group. VC: vehicle control group (n = 8), ACE-2w: acetamiprid two-week-old exposure group (n = 8), ACE-11w: acetamiprid 11-week-old exposure group (n = 8).
No significant differences were found between the ACE-2w and ACE-11w groups and the VC group (Fig. 3).
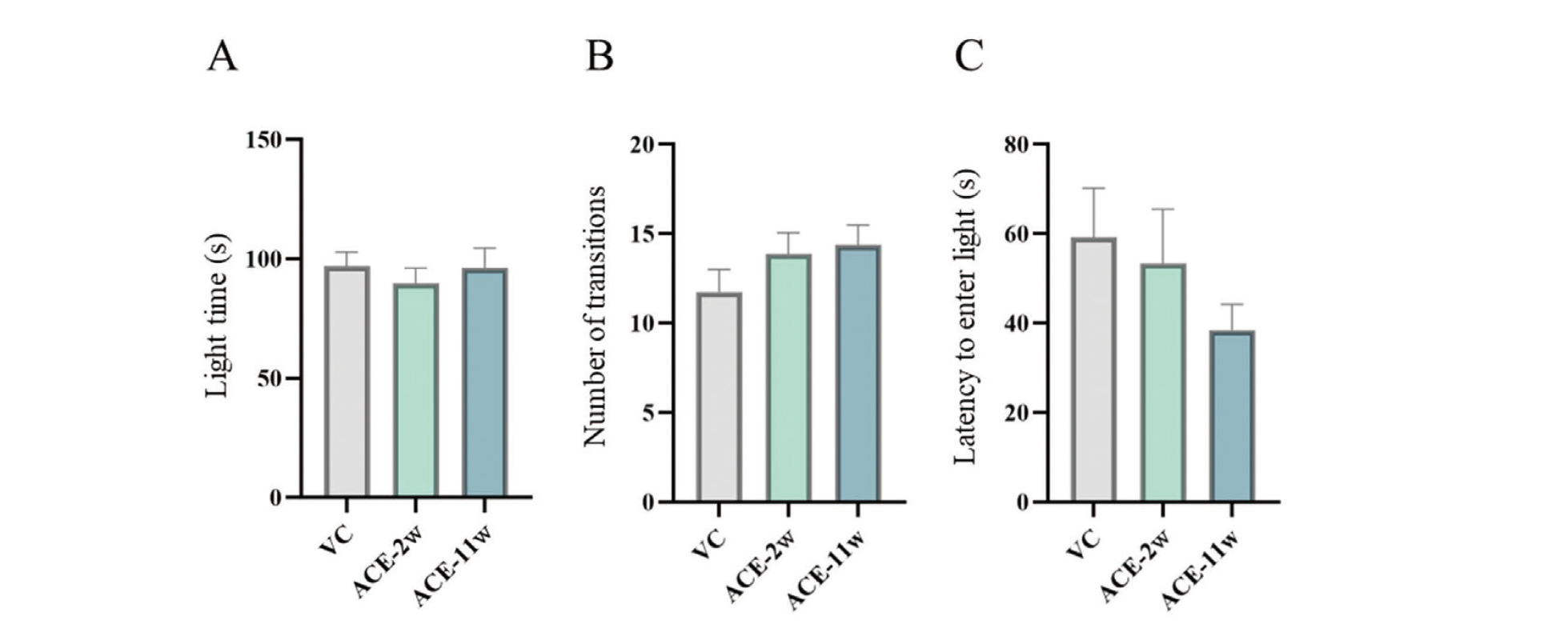
Results of the light/dark transition (LD) test. Scores of the LD test (total test time, 300 sec) are shown. (A) Time spent in the light area (sec). (B) Number of transitions between the dark and light areas. (C) Latency to enter the light area (sec) for the first time. Data are expressed as the mean ± S.E. Data were tested statistically using Dunnett’s test. VC: vehicle control group (n = 8), ACE-2w: acetamiprid two-week-old exposure group (n = 8), ACE-11w: acetamiprid 11-week-old exposure group (n = 8).
The total distance was significantly decreased in the ACE-2w group compared to that in the VC group, whereas no significant differences were observed in the ACE-11w group (Fig. 4A). The open area time and total arm entry number did not significantly change in the ACE-2w and ACE-11w groups compared to the VC group (Fig. 4B, C).
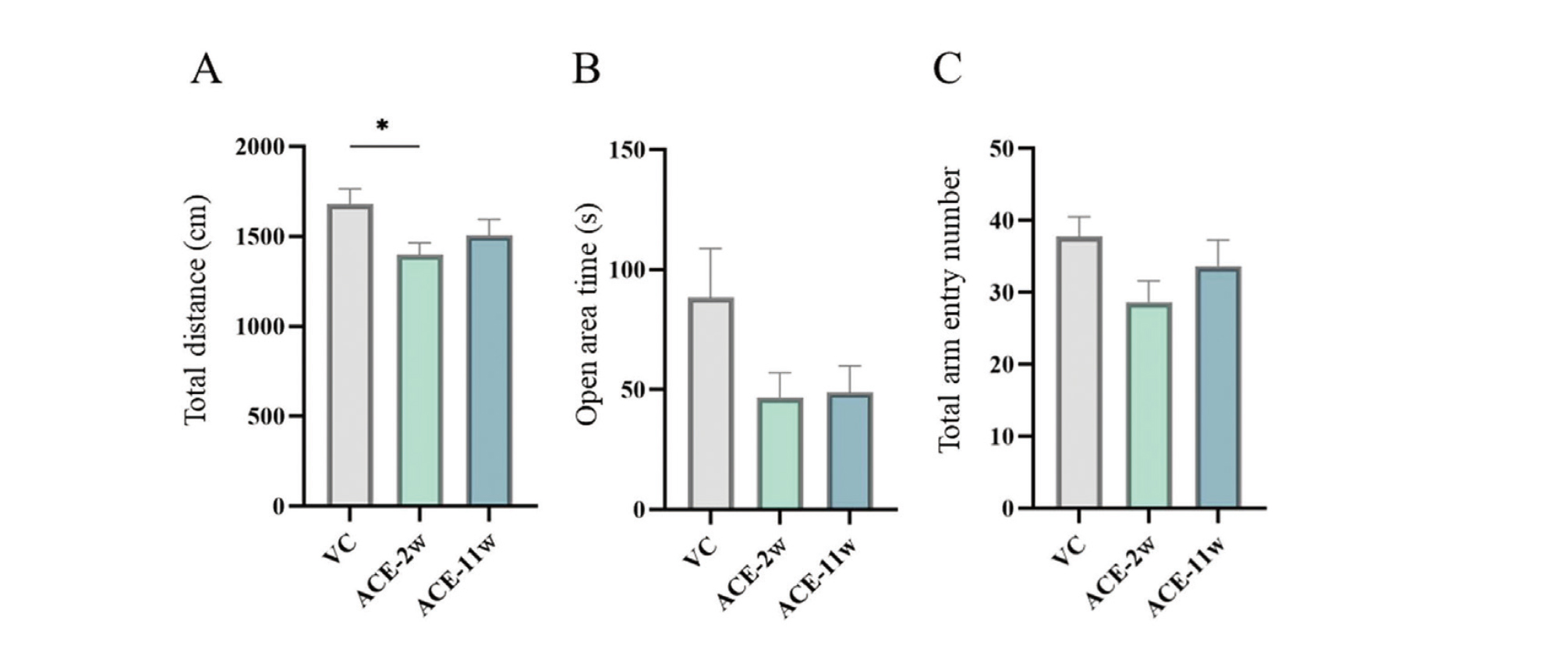
Results of the elevated plus-maze (EP) test. Scores of the EP test (total test time, 600 sec) are shown. (A) Distance traveled (cm) during the test period. (B) Time spent in the open area (sec). (C) Total entry number in arms. Data are expressed as the mean ± S.E. Data were tested statistically using Dunnett’s test. *p < 0.05 versus the VC group. VC: vehicle control group (n = 8), ACE-2w: acetamiprid two-week-old exposure group (n = 8), ACE-11w: acetamiprid 11-week-old exposure group (n = 8).
Significant differences were observed in the FZ test between the ACE-2w and VC groups in terms of the freezing rate during conditioning (Fig. 5A). In the contextual fear test, the freezing rate was significantly lower in the ACE-2w and ACE-11w groups than in the VC group (Fig. 5B). In the cued fear test, there was no significant difference in the freezing rate (after the cued tone) between the ACE-2w and ACE-11w groups and the VC group (Fig. 5C).
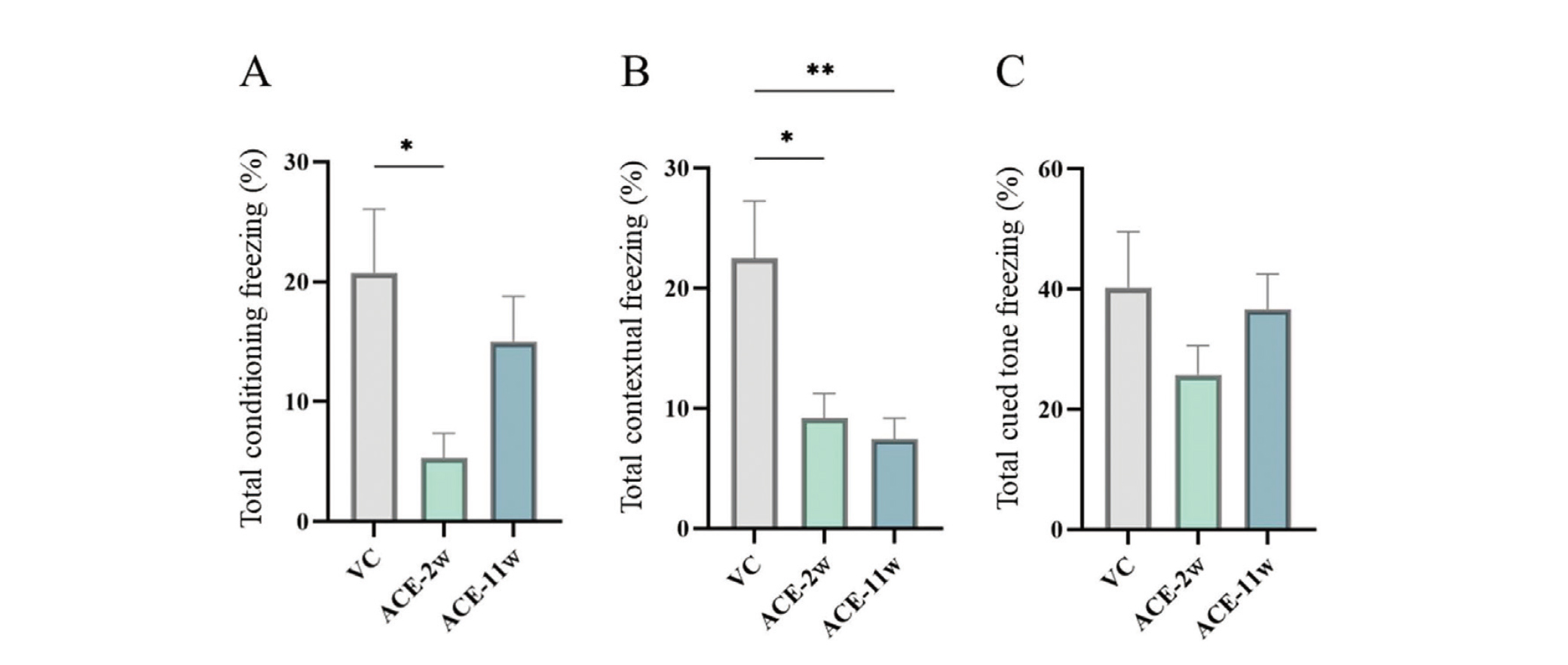
Results of the contextual/cued fear conditioning (FZ) test. The total time of the test was 360 sec. (A) The FZ was conducted to analyze the effects of acetamiprid on learning and memory, i.e., place (context) and sound (cued tone). The average total freezing scores (%) of the VC and acetamiprid exposure groups in the conditioning test are shown. (B) The contextual test was conducted to analyze the effects of acetamiprid on place memory function. The average total freezing scores (%) of the VC and acetamiprid exposure groups in the contextual test are shown. (C) The cued test was used to analyze the effects of acetamiprid on cued memory function. The average freezing scores (%) of the VC and acetamiprid exposure groups after the tone are presented. Data are expressed as the mean ± S.E. Data were tested statistically using Dunnett’s test. *p < 0.05, **p < 0.01 versus the VC group. VC: vehicle control group (n = 8), ACE-2w: acetamiprid two-week-old exposure group (n = 8), ACE-11w: acetamiprid 11-week-old exposure group (n = 8).
None of the groups showed any changes in the PPI test results compared to the VC group (Fig. 6).
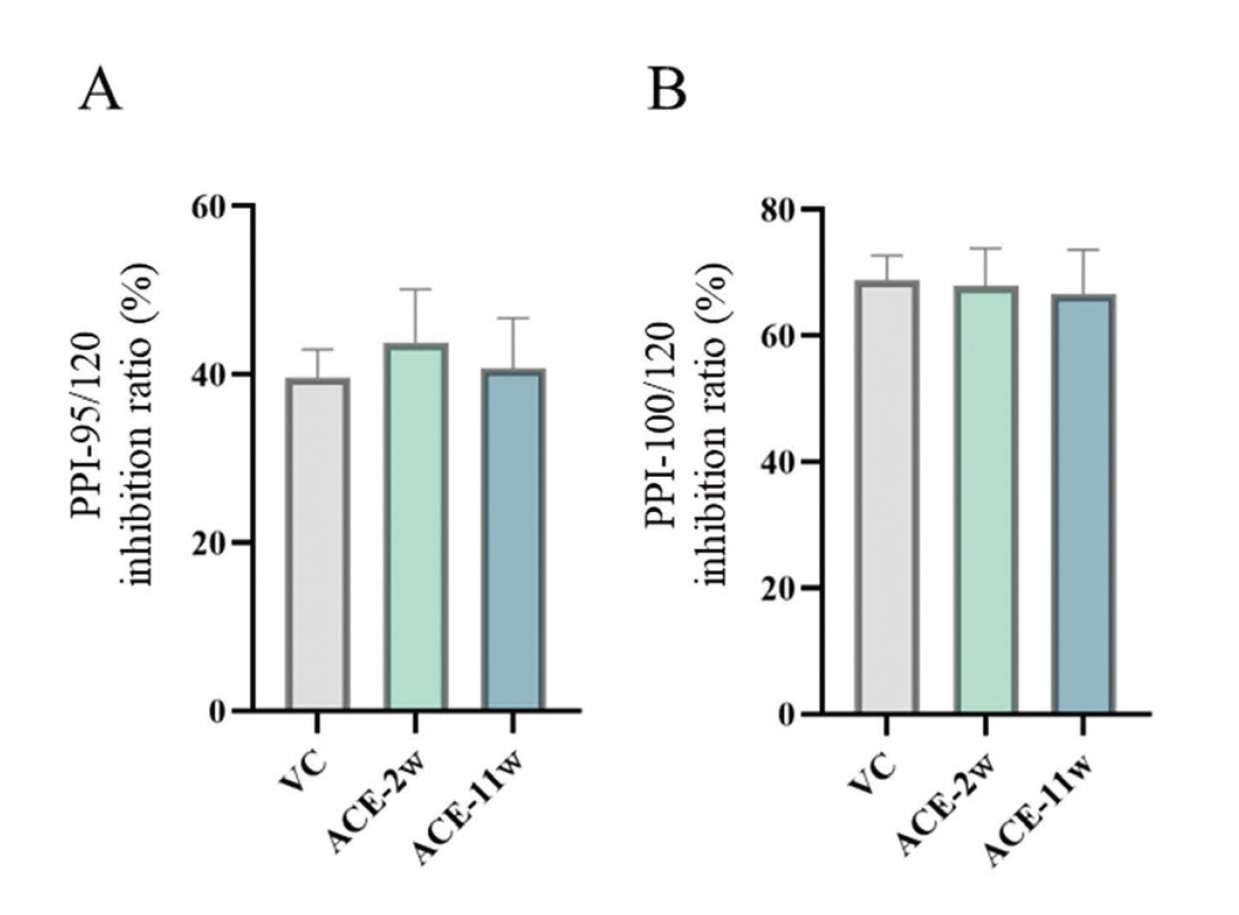
Results of the pre-pulse inhibition (PPI) test. Representative scores are shown. (A and B) The inhibition (%) of the startle response to a 120 dB sound with pre-pulse sounds of 95 and 100 dB compared to the response to a 120 dB sound without a pre-pulse sound. Data are expressed as the mean ± S.E. Data were tested statistically using Dunnett’s test. VC: vehicle control group (n = 7), ACE-2w: acetamiprid two-week-old exposure group (n = 8), ACE-11w: acetamiprid 11-week-old exposure group (n = 8).
The results of the behavioral test battery are summarized in Fig. 7.
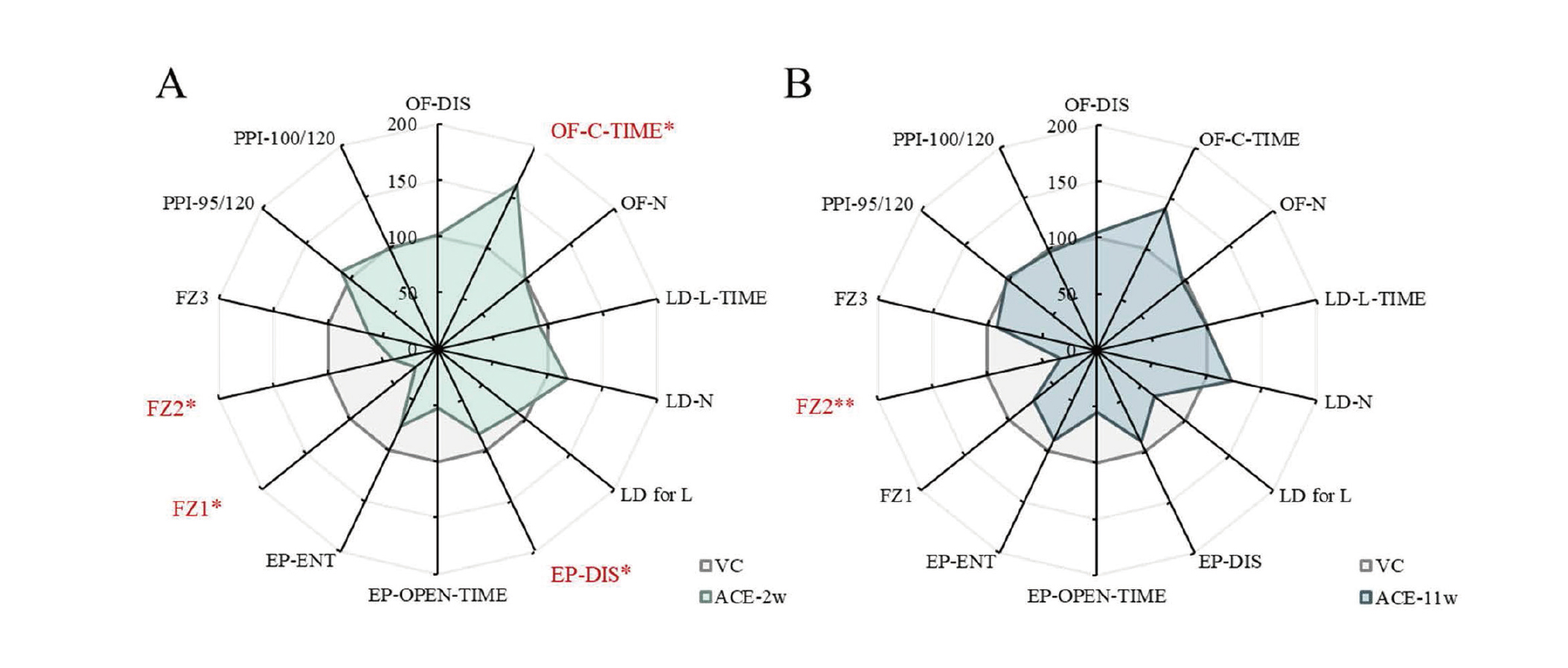
A summary of the mouse behavioral test battery. The main items of the mouse behavioral test battery were standardized by the average scores of the VC mice and shown by a radar chart as the difference between the ACE-2w (A) and ACE-11w (B) against the VC group (the average scores of VC mice = 100%). Data are expressed as the mean ± S.E. Data were tested statistically using Dunnett’s test. *p < 0.05, **p < 0.01 versus the VC group. n = 7–8 per group. OF; OF-DIS: total distance, OF-C-TIME: total center time, OF-N: move episode number. LD; LD-L-TIME: light time, LD-N: number of transitions, LD for L: latency to enter light. EP; EP-DIS: total distance, EP-OPEN-TIME: open area time, EP-ENT: total arm entry number. FZ; FZ1: total conditioning freezing, FZ2: total contextual freezing, FZ3: total cued tone freezing. PPI; PPI-95/120: pre-pulse 95 dB, PPI-100/120: pre-pulse 100 dB. VC: vehicle control group (n = 7–8), ACE-2w: acetamiprid two-week-old exposure group (n = 8), ACE-11w: acetamiprid 11-week-old exposure group (n = 8).
In this study, we focused on the effects of chemical exposure during the postnatal lactation period, administered a single oral dose of ACE to male mice, and examined its developmental exposure effects on the brain in later life on behavior during adulthood. In the OF test, the total time in the center was significantly longer in the ACE-2w group than in the VC group. This result is attributed to less anxiety in a novel environment in the ACE-2w group. In addition, in the EP test, the total distance traveled was significantly lower in the ACE-2w group than in the VC group. This finding may be attributed to mice experiencing high anxiety in the open area and entering it less frequently in the ACE-2w group. These results suggest that anxiety-related behavioral deviations occur in the postnatal lactation exposure group. However, these results are not consistent in terms of their impact on anxiety-related behaviors. In this regard, different anxiogenic properties of the OF and EP tests were reported in a previous study (Carola et al., 2002). Factor analysis studies using rodents also revealed that anxiety-related behaviors evaluated in the OF and EP tests do not load on a common factor (Trullas and Skolnick, 1993; Ramos et al., 1998; Nakano et al., 2016). Therefore, it is inferred that these results are based on different control mechanisms for anxiety-related behavior. In the FZ test, significant differences were observed in the freezing rates of the ACE-2w group during conditioning. In the contextual fear test, the freezing rates of the ACE-2w and ACE-11w groups were significantly lower than that of the VC group. In these results, short-term memory formation and spatial memory abnormalities were observed in the ACE-2w group. Spatial memory abnormalities were also observed in the ACE-11w group. Cholinergic neurons in the brain are widely distributed throughout the central nervous system (CNS). The basal forebrain contains the nuclei of cholinergic neurons in the CNS, projecting fibers to various regions, such as the neocortex, hippocampus, and amygdala (Mesulam et al., 1983; Woolf, 1991; Ballinger et al., 2016). This acetylcholine-mediated neurotransmission is closely related to cognitive functions, such as learning and memory (Conner et al., 2003; Mitsushima et al., 2013), and nAChRs modulate the neurobiological processes that underlie hippocampal learning and memory (Kutlu and Gould, 2015). Since fear contextual conditioning is highly hippocampal-dependent (Phillips and LeDoux, 1992), the administration of ACE may have induced abnormalities in hippocampal signaling.
As shown in Fig. 7, there were significant differences in the OF, EP, and FZ tests in the ACE-2w group, whereas there were significant differences in the FZ test only in the ACE-11w group. These results may reflect a qualitative difference in the behavioral effects between ACE-2w and ACE-11w groups. In addition, the behavioral effects of ACE exposure may be greater during postnatal lactation than in adulthood from the number of behavioral test items with significant differences. Furthermore, in the ACE-2w and ACE-11w groups, many items in the behavioral test battery deviated from the mean values of the VC group, although no significant differences were found. Thus, its behavioral toxicological significance should also be examined in future studies.
This study found that long-lasting neurobehavioral deficits occurred due to the disruption of normal brain development during the postnatal lactation period. In addition, the behavioral effects in the postnatal lactation treatment group were qualitatively different from the behavioral abnormalities in the mature treatment group, suggesting that they were memory and emotional abnormalities. In examining the toxic effects of any chemical, dose-response and metabolite effects provide more reliable data. However, diurnal variation exists in anxiety-related behavior and learning and memory in mice (Nakano et al., 2016; Shimizu et al., 2016). In this study, behavioral tests were conducted with the smallest number of groups to minimize the effects of diurnal variation on the behavior of mice. Furthermore, it is limited to outputting behavioral data. Therefore, there are limitations to the results obtained with this experimental design. Including the background molecular mechanisms of these behavioral effects, the dose-dependent and metabolite effects of ACE also need to be examined. Thus, it is important to accumulate data on an ongoing basis and carefully consider the potential effects on individuals during the early postnatal stages.
This study was supported in part by the Health Sciences Research Grants from the Ministry of Health, Labour, and Welfare, Japan (H30-KAGAKU-IPPAN-003) and JSPS KAKENHI (Grant Number 19H01142). We would like to thank Editage (www.editage.com) for English language editing.
Conflict of interestThe authors declare that there is no conflict of interest.