2023 Volume 48 Issue 8 Pages 441-456
2023 Volume 48 Issue 8 Pages 441-456
Variability in supply, paucity of donors and cellular instability under in vitro conditions have limited the application of primary human hepatocytes (PHHs) to hepatotoxicity testing. Therefore, alternative sources have been sought for functional liver cells. Many of the earlier in vitro hepatotoxicity studies were carried out using hepatoma-derived cell lines. These cell lines have overcome some of the limitations of PHHs with regard to phenotypic stability and availability; however, they suffer from their own inherent limitations, such as the lack of drug-metabolizing functionality, which renders them inadequate for situations where toxic metabolite formation of the parent drug occurs. In the last decade we have witnessed a burgeoning interest of the research community in using hepatocyte-like cells (HLCs) derived from human induced pluripotent stem cells (iPSCs) as in vitro hepatotoxicity models. HLCs offer the perspective of a defined and renewable supply of functional hepatocytes; more importantly, HLCs maintain their original donor genotype and afford donor diversity, thus opening new avenues to patient-specific toxicity testing. In this review, we first introduce various in vitro hepatotoxicity models, then focus on HLCs and their application in hepatotoxicity studies, and finally offer some perspectives on future developments of the field.
Induced pluripotent stem cells (iPSCs) generated as a result of somatic cell reprogramming are resemblant to embryonic stem cells (ESCs) with respect to both the ability of unlimited self-renewal and the potential to differentiate into almost all cell types in the body, including hepatocytes (Takahashi et al., 2007; Yu et al., 2007). Cells differentiated in vitro from pluripotent stem cells (including ESCs and iPSCs) with a hepatic fate are often called hepatocyte-like cells (HLCs) following some of the early studies (Ghodsizadeh et al., 2010; Rambhatla et al., 2003), as these cells are very similar to hepatocytes in vivo in morphology and, to some extent, in functionality. Therefore, HLCs differentiated from iPSCs could afford a virtually infinite supply of hepatocytes for a wide variety of applications, including cell transplantation and regenerative therapy, patient-specific disease modeling, drug screening and testing, and toxicity testing of other chemicals (Fig. 1).
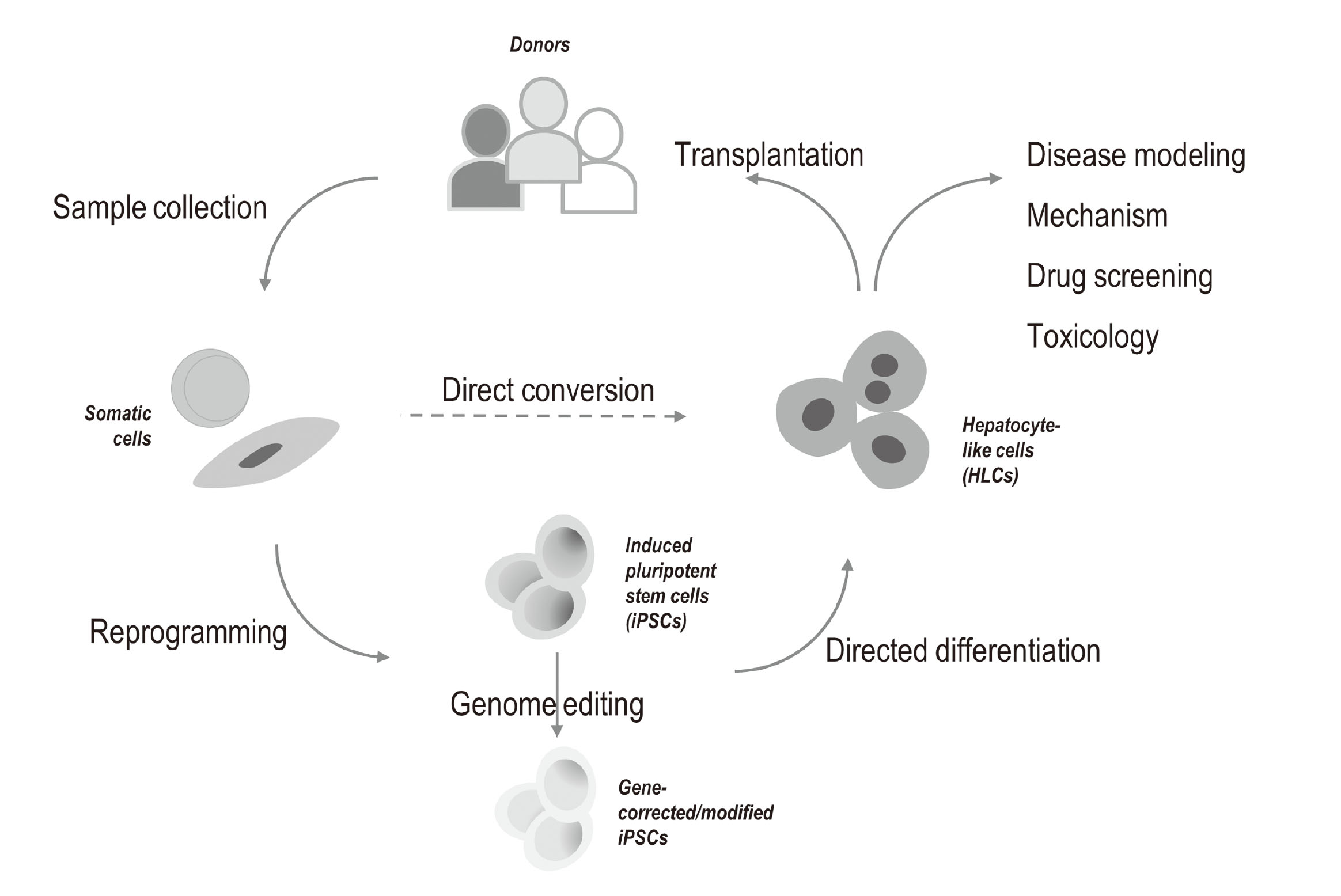
Generation of hepatocyte-like cells (HLCs) and their potential applications. Human induced pluripotent stem cells (iPSCs) can be reprogrammed from a variety of somatic cells collected from donors with diverse genotypic and phenotypic backgrounds. These iPSCs can be further genome-edited to correct or modify certain genes of interest. The wild-type or genome-edited iPSCs can then be differentiated into HLCs, which have promising applications in regenerative medicine, disease modeling, mechanistic studies, drug screening, and toxicity testing. Somatic cells can also be directly converted into HLCs.
Primary human hepatocytes (PHHs) are often regarded as the gold standard for toxicological applications by virtue of their closest resemblance to human liver in vivo. However, PHHs also suffer several critical demerits for such applications, including limited availability of donors, vast variability between different donors or preparations, and rapid deterioration of functionality under in vitro cell culture conditions (Richert et al., 2006). The differentiation of iPSCs to HLCs offers the perspective of a defined yet unlimited supply of cells as a better alternative to PHHs. More importantly, since iPSCs could be derived from donors with different background, iPSC-derived HLCs could therefore afford broad donor diversity. This property makes these cells uniquely suited for toxicological applications where other cell types are unqualified, such as the study of idiosyncratic drug-induced liver injury (iDILI) (Krueger et al., 2014), and toxicity testing and risk assessment of chemicals on susceptible human subpopulations (Dornbos and LaPres, 2018; Wetmore et al., 2014).
In this review, we provide a brief account on various in vitro hepatotoxicity models and discuss the need for iPSC-derived HLCs in toxicological applications. We then present an overview of the current status of hepatocyte differentiation from iPSCs. We focus our discussion on the applications of iPSC-derived HLCs in toxicology, and at the end offer future perspectives on the development of this promising research field.
Human hepatocytes are polygonal in shape, with a diameter of approximately 20-30 µm and a volume of about 3000 µm3. As the most predominant parenchymal cell type in the liver, hepatocytes account for roughly 80% of the adult liver mass. These cells perform a variety of pivotal liver functions, including metabolic homeostasis, plasma protein synthesis and secretion, bile production, detoxification of xenobiotic compounds, and storage of vitamins and minerals. Similar to other epithelial cell types, to perform the vectorial exchange of macromolecules between the external and internal milieu, hepatocytes are polarized with distinct luminal, lateral, and basal domains. As a characteristic feature of mature mammalian hepatocytes, a significant proportion of the cells are polyploid; in human approximately 20-30% of adult hepatocytes are tetraploid and octoploid, whereas in rats and mice, the percentage is 85% (Gentric and Desdouets, 2014).
The liver contains high concentrations of drug-metabolizing enzymes and is very active in the biotransformation and detoxification of xenobiotic compounds; therefore it is often the primary site of exposure to toxicants. Biotransformation of xenobiotics in the liver enables the conversion of lipophilic (fat-soluble) substances, which are readily absorbed from the gastrointestinal tract, accumulate in tissues and cause toxic effects, into hydrophilic (water-soluble) chemicals which are readily excreted to bile or urine (Gómez-Lechón et al., 2003). The process of metabolism and transport of xenobiotics in the liver is often divided into three phases: (I) modification, (II) conjugation, and (III) excretion. In phase I, enzymes consisting mainly of cytochrome P450s (CYPs) introduce reactive and polar groups into the substrate through oxidation, reduction, or hydrolysis and as a result increase the hydrophilicity of the compounds. Subsequently, enzymes in phase II catalyze various biotransformation reactions, including glucuronidation (most common), methylation, acetylation, sulfonation, and conjugation (with glutathione or with amino acids such as glycine, taurine, and glutamic acid), which usually lead to further increased hydrophilicity and elimination. Lastly, in phase III, hepatocyte transporters, many of which belong to the ATP-binding cassette (ABC) family and the solute carrier (SLC) transporters, further metabolize and excrete the newly formed phase II products out of the cell. Therefore, to accurately predict hepatotoxicity of chemicals using in vitro models, the cells used must be able to largely reproduce the entire drug metabolism and transport process.
Various in vitro systems have been used for hepatotoxicity studies, including liver slices, immortalized hepatic cell lines, and PHHs in suspension or in 2D or sandwich cultures (Soldatow et al., 2013). More recently, hepatocytes isolated from chimeric mice with humanized livers (Zerdoug et al., 2022) and HLCs obtained from direct lineage conversion (Rombaut et al., 2021) or pluripotent stem cells (PSCs) differentiation (Tricot et al., 2022) also see increasing applications. Among these models, PHHs are the closest to human liver in vivo and therefore have been recognized as the gold standard for drug clearance and toxicity studies. Primary hepatocytes cultured under in vitro chemically defined conditions possess typical liver-specific functions, and are generally capable of reproducing the response of the liver to pathophysiological factors. More importantly, PHHs largely retain the enzymes and transporters of the liver for drug metabolism and transport (phase I to phase III) in a form that is integral, functional, and inducible (Hewitt et al., 2007). Nevertheless, under standard in vitro culture conditions, PHHs quickly (usually within 24-72 hr) lose their metabolic and transport capacities and other hepatic functions (Richert et al., 2006). Moreover, PHHs also suffer from limited availability and large variability in cell quality between different donors or preparations. These shortcomings have greatly hampered the widespread application of PHHs in hepatotoxicity studies.
A number of hepatic tumor cell lines have also been employed in hepatotoxicity assessments over the last few decades, such as HepG2, its subclone C3A, and Huh7. These cell lines do not have some of the shortcomings of PHHs with regard to phenotypic stability and availability; however, they suffer from their own limitations. Of most importance, these cell lines have very low-level drug-metabolizing functionality (Gerets et al., 2012), which disqualifies them for drug toxicity testing when toxic metabolite forms. The advent of the HepaRG cell line represents a major improvement in terms of hepatocyte metabolism functionality over previous cell line models. HepaRG cells are bipotent progenitor cells which could be further differentiated into hepatic cells expressing major CYP enzymes and nuclear receptors at levels approximating PHHs (Aninat et al., 2006). Unfortunately, this cell line has been reported to suffer low predictivity of hepatotoxicity (Gerets et al., 2012). A plausible explanation is that this cell line was originated from a tumor background and thus display reduced sensitivity to toxic insults. Furthermore, similar to other hepatoma cell lines, HepaRG is unable to represent a broad population for toxicity investigations due to its derivation from a single genotype.
Human iPSCs, similar to ESCs, are capable of replicating indefinitely and differentiating into most cell types in the human body, including hepatocytes. Compared to PHHs, iPSC-derived HLCs could be obtained with unlimited and consistent supply and are sustainable of functionality in long-term culture; while compared to hepatoma cell lines, HLCs often display improved hepatic phenotypes without features of neoplastic transformation that may bias the interpretation of the results. More importantly, iPSC-derived HLCs afford donor diversity and the feasibility of genome modification (Tricot et al., 2022). Therefore, it has been anticipated that HLCs derived from human iPSCs could serve as a promising and attractive alternative to PHHs and hepatoma cell lines as in vitro models for hepatotoxicity applications.
Hepatocyte differentiation from human ESCs was first attempted by Rambhatla et al. (2003). The authors reported the generation of cells with features similar to hepatocytes, hence the term hepatocyte-like cells (HLCs). In the years that followed, a variety of protocols appeared in the literature that are capable of differentiating ESCs into HLCs. These protocols usually involve step-wise addition of growth factors (GFs) to recapitulate in vivo developmental signals under in vitro conditions. In many of these protocols, activin A and Wnt3A were first added for endoderm induction, fibroblast growth factor (FGF) and bone morphogenetic protein (BMP) were added subsequently for hepatic specification, and hepatocyte growth factor (HGF) and oncostatin M (OSM) were added lastly for hepatocyte maturation (Asgari et al., 2010). With the advent of human iPSCs in 2007, studies on hepatocyte differentiation have thereafter shifted from using ESCs as the starting material to using iPSCs, with the first attempt reported by Song et al. (2009). It was demonstrated that using an almost identical differentiation process that had been used for human ESCs, human iPSCs were also able to differentiate into HLCs. Independent studies from other groups published in the subsequent year further established that human iPSCs could be differentiated with high efficiency into HLCs that exhibit key features of human hepatocytes (Si-Tayeb et al., 2010; Sullivan et al., 2010; Touboul et al., 2010). Together, these pioneering studies laid the foundation for future work on hepatocyte differentiation from human iPSCs or ESCs (Schwartz et al., 2014; Takayama and Mizuguchi, 2017). Currently, a three-step strategy recapitulating ontogenetic liver development is shared by the most commonly employed protocols for hepatocyte differentiation (Song et al., 2009; Si-Tayeb et al., 2010; Touboul et al., 2010): (1) definitive endoderm (DE) induction, (2) hepatic specification, and (3) hepatocyte maturation (Fig. 2). However, specific culture conditions may vary between different protocols.
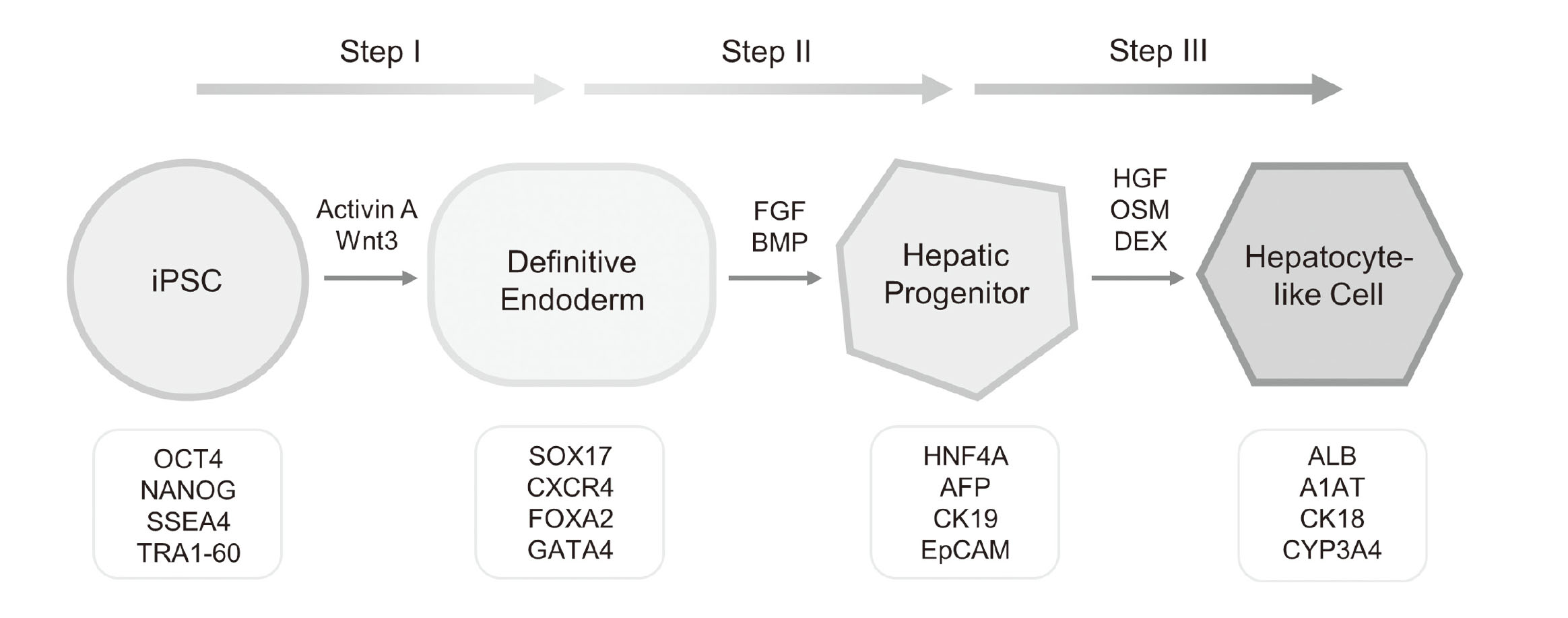
Schematic diagram of the three-step hepatocyte differentiation strategy. Growth factors that drive hepatocyte differentiation are shown above the arrows between the cell states, and specific markers at each stage are indicated in the boxes underneath. A1AT, α1-antitrypsin; AFP, α-fetoprotein; ALB, albumin; BMP, bone morphogenetic protein; CK, cytokeratin; CXCR4, C-X-C chemokine receptor type 4; CYP, cytochrome P450; DEX, dexamethasone; EpCAM, epithelial cell adhesion molecule; FGF, fibroblast growth factor; FOXA2, forkhead box protein A2; HGF, hepatocyte growth factor; HNF4A, hepatocyte nuclear factor 4 alpha; OCT4, octamer-binding transcription factor 4; OSM, oncostatin M; SOX17, SRY-box transcription factor 17; SSEA4, stage-specific embryonic antigen; TRA1-60, tumor resistance antigen 1-60.
Encouraging results have been seen in HLCs generated by GF-based protocols with regard to specific markers expression and certain functional assays; however, in all cases these cells manifested a rather immature hepatic phenotype that resembles fetal hepatocytes more closely than adult ones (Palakkan et al., 2017). Furthermore, due to the use of GFs, such protocols are often costly and have low reproducibility, and for GFs produced from animal sources, there are potential safety concerns for clinical applications. More recently, increasing numbers of studies have shown that it is feasible and advantageous to use small molecules (SMs) for cell fate conversion (Li et al., 2018; Ma et al., 2017). SMs are easier to apply and remove compared to GFs, and due to their relatively low cost are more efficient and amenable to scale up. Efforts to drive the conversion of pluripotent stem cells towards a hepatic fate using SMs were first attempted only for the first stage of hepatocyte differentiation, i.e., DE induction, using ESCs as the starting materials (Borowiak et al., 2009; Bone et al., 2011; Tahamtani et al., 2013). Complete endodermal differentiation towards HLCs using only SMs was first reported by Sullivan and colleagues (Siller et al., 2015; Mathapati et al., 2016). Using a novel 3-step protocol, the authors were able to differentiate iPSCs to functional HLCs without the use of any GFs. Later, Du et al. (2018) reported a similar 3-step SM-based protocol. Both groups demonstrated that HLCs generated using SM-based differentiation protocols display key hepatic functionality at levels similar to those derived using GF-based approaches (Siller et al., 2015; Du et al., 2018). Transcriptomic characterization revealed that the quality of the HLCs derived using a SM protocol was on par with those differentiated using GFs, with both overall gene expression and the expression of liver-specific genes such as metabolic enzymes, transporters, and nuclear receptors being very similar between cells generated using GFs and SMs (Gao et al., 2020).
Several groups attempted iPSC differentiation into liver organoids, in which tissue-like architecture forms and interactions/crosstalk exist between different cell types. Therefore, compared with 2D cell culture, liver organoids offer the advantage of more closely mimicking in vivo systems. Ultimately, liver organoid models can potentially replace animal models for hepatotoxicity studies (Harrison et al., 2021; Caiazza et al., 2021). The first such study employed iPSC-derived endoderm (iPSC-HE) co-cultured with human mesenchymal stem cells (MSCs) and human umbilical vein endothelial cells (HUVECs), and the cells spontaneously assemble and form a 3-dimension (3D) iPSC-derived liver bud (iPSC-LB) (Takebe et al., 2013). Transferring the production of iPSC-HE into a good manufacturing practice (GMP)-grade system further improved the technique (Takebe et al., 2017). In a later study, it was shown that paracrine signaling is involved in liver organoid maturation and that stromal cells not only played a structural role but also a functional one. Angiotensin, α-2 macroglobulin and plasminogen were identified through proteomics analysis as the paracrine signals that promote hepatocyte differentiation and maturation (Asai et al., 2017). A giant step forward towards the generation of functional liver organoids from iPSCs was made by Guan et al. (2017). The authors developed a protocol in which sequential changes are applied to the culture medium in terms of growth factors and chemical composition, which drives iPSC differentiation gradually through multiple stages, resembling human liver development in the embryo. In the resultant liver organoids, 2 types of cells are organized into a complex structure which contains sheets of hepatocytes and epithelia comprised of cholangiocytes that surround the lumina of bile duct–like structures. Aside from applications in regenerative medicine, such hepatic organoids also afford remarkably improved in vitro liver models for toxicity testing and prediction (Kim et al., 2022).
HLCs differentiated from human iPSCs afford the advantages of a phenotype similar to primary hepatocytes and virtually unlimited and fairly consistent availability; more importantly, these cells are genotype-specific representing the different individuals from which they are derived. These attributes make iPSC-derived HLCs a promising in vitro hepatotoxicity model for toxicological applications. We have conducted a comprehensive transcriptomic study where a commercially available HLC line was compared to several hepatoma cell lines (including HepaRG, Huh7, HepG2 and its clonal variant C3A) and 6 PHH lines. The results showed that, with the exception for HepaRG, iPSC-derived HLCs are more closely correlated to PHHs than hepatoma cell lines, highlighting the potential usefulness of HLCs for in vitro hepatotoxicity applications (Gao and Liu, 2017). It is expected that a panel of HLCs derived from a multitude of patients with diverse xenobiotic metabolism phenotypes would improve the predictability of the in vitro model (Chun et al., 2010; Anson et al., 2011). Moreover, by utilizing iPSCs originated from specific human populations with certain genotypical or phenotypical characteristics, such as ethnicity, health or disease status, sex, and age, toxicity testing and risk assessment for specific subpopulations become feasible (Welsh et al., 2009).
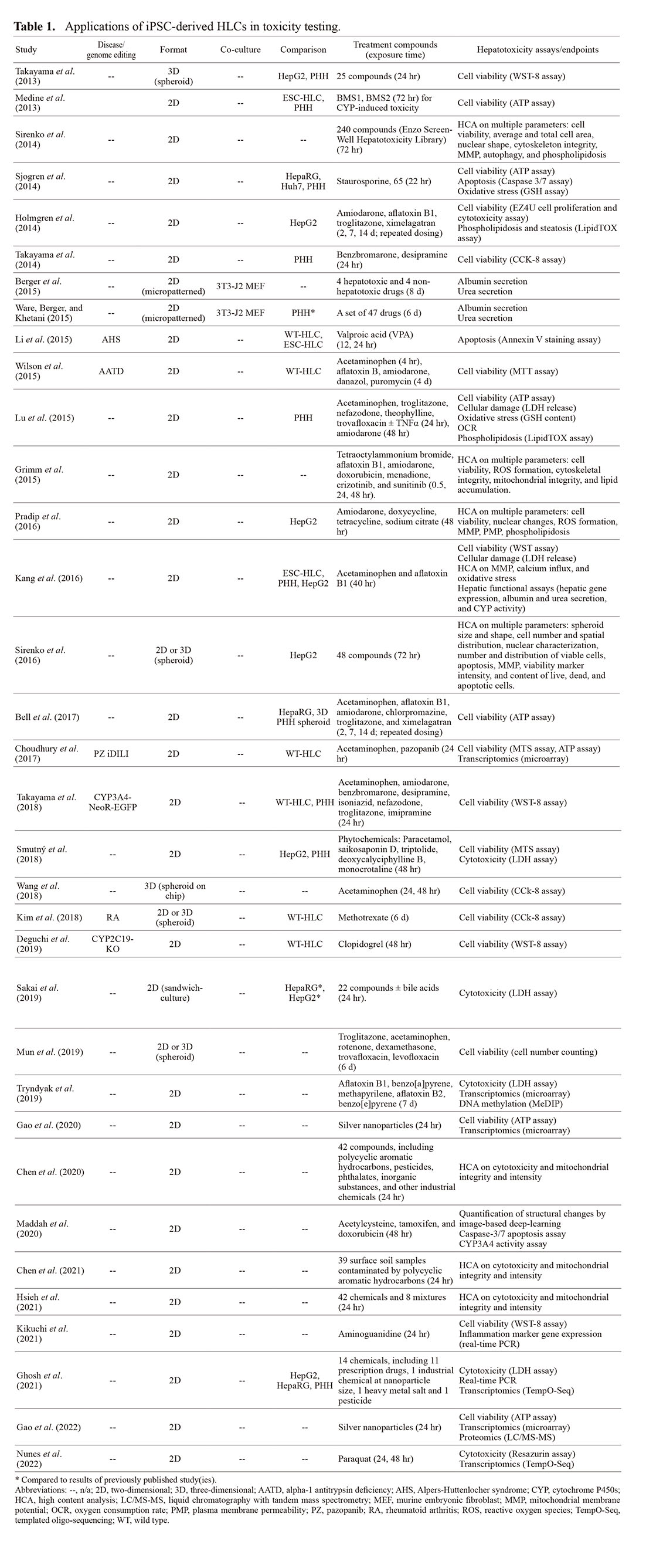
There is consensus in the literature that the maturity of HLCs derived from iPSCs usually does not match that of PHHs; nevertheless, these cells were reported to display certain degrees of hepatic phenotype that might be useful in hepatotoxicity studies. Therefore, the potential for toxicity application of iPSC-derived HLCs has been explored extensively in the last decade (Table 1). In addition, several excellent reviews covering the application of HLCs in drug testing with an emphasis on drug-induced liver injury have also appeared in the literature during the past few years (Mann, 2015; Csöbönyeiová et al., 2016; Donato and Tolosa, 2019; Wills and Rajagopalan, 2019; Tricot et al., 2022).
 Patient-specific HLCs for hepatotoxicity prediction
Patient-specific HLCs for hepatotoxicity prediction
Compared with other in vitro hepatotoxicity models, iPSC-derived HLCs afford donor diversity since iPSCs could be derived from different donors. Therefore, on the individual level these cells are particularly useful for studying iDILI (Krueger et al., 2014); and on the population level, they are well suited for toxicity testing and risk assessment in susceptible ethnic groups (Dornbos and LaPres, 2018; Wetmore et al., 2014).
A pioneering work along this line was reported by Takayama et al. (2014). In this well-designed study, the authors demonstrated that HLCs were highly correlated with their corresponding parental PHHs from the same donor with regard to drug metabolism capacity and drug responsiveness. Moreover, the interindividual differences among parental PHHs in metabolism capacity and drug responsiveness as a result of single nucleotide polymorphisms (SNPs) in the CYPs were largely reproduced in the derivative HLCs. These findings provide convincing evidence that it is feasible to predict interindividual differences in drug metabolism capacity and drug response via the use of HLCs. This seminal study made it possible to use HLCs as an in vitro model for iDILI prediction.
Several proof-of-concept studies appeared later in the literature demonstrating the feasibility of iDILI prediction using patient-specific HLCs (Li et al., 2015; Wilson et al., 2015; Choudhury et al., 2017; Kim et al., 2018; Deguchi et al., 2019). Valproic acid (VPA) is a drug widely used for the treatment of epilepsy, migraine, chronic headache and bipolar disorder, and as adjuvant chemotherapy; however, patients with Alpers-Huttenlocher syndrome (AHS), a neurometabolic disorder, have increased risk of developing fatal hepatotoxicity to VPA (Schwabe et al., 1997). Li et al. (2015) showed that compared to normal HLCs derived from healthy individuals, HLCs developed from patients with AHS were more sensitive to mitochondrial-dependent apoptosis induced by VPA. The authors further discovered that in HLCs derived from AHS patients, the mitochondrial permeability transition pore (mPTP) opens more frequently than normal HLCs due to the genetic mutations in mitochondrial DNA, which results in increased apoptosis of the cells. Severe alpha 1-antitrypsin deficiency (PiZZ) is a hereditary disorder caused by a single gene mutation. Wilson et al. (2015) generated iPSCs from a cohort of patients carrying PiZZ mutations and differentiated the iPSCs into HLCs. It was found that compared to normal control HLCs, PiZZ HLCs are more sensitive to some known hepatotoxic drugs. In addition, PiZZ HLCs display intracellular accumulation of the mutant protein leading to increased autophagic flux, and exposure to the drug carbamazepine further augmented autophagic flux. Another similar study was conducted on iDILI of pazopanib. Pazopanib is a tyrosine kinase inhibitor and has been commonly prescribed as a drug to treat advanced renal cell carcinoma. Pazopanib has been reported to cause hepatotoxicity in patients with high incidence rate but the mechanism remains unknown. Using a panel of HLCs derived from patients with differential clinical hepatotoxicity to pazopanib, Choudhury et al. (2017) demonstrated that pazopanib caused higher cytotoxicity in cells from patients with clinically identified hepatotoxicity than cells from other individuals; for comparison, a prototype hepatotoxicant acetaminophen showed similarly toxicity to all HLCs in the study. Moreover, as shown by a transcriptomic analysis, although pazopanib induces oxidative stress in all the HLCs, the burden of oxidative stress caused by pazopanib is higher in HLCs from susceptible individuals due to disruption of iron metabolism. In a more recent study, Kim et al. (2018) found that HLCs derived from rheumatoid arthritis (RA) patients show higher sensitivity to methotrexate than control cells derived from healthy individuals. Methotrexate is an immunosuppressive agent widely used in RA therapy. However, long-term use of methotrexate causes hepatotoxicity due to increased aminotransferases. Along the same line, CYP2C19-knockout (KO) HLCs were generated via CRISPR/Cas9 gene editing by Deguchi et al. (2019) as a novel CYP2C19 poor metabolizer model, and the authors demonstrated that CYP2C19-KO HLCs can predict liver toxicity caused by clopidogrel. Therefore, these cells are valuable for evaluating the safety and effectiveness of candidate drugs for patients of CYP2C19 poor metabolizers. Collectively, the studies discussed above support the potential utility of iPSC-derived HLCs as valuable in vitro models to emulate the genotypic and phenotypic variability found in the human population. Such models would be highly valuable for the prediction of individual-specific or subpopulation-specific toxicities. Moreover, the findings from these studies add further evidence to the notion that patient-specific HLCs are potentially useful in predicting specific therapeutic or toxic responses to drugs and in providing new mechanistic insights into the pathogenesis of genetic liver diseases.
Hepatotoxicity studies using HLCs in 3D format and on microfluidic chipsA variety of strategies have been explored over the past years in order to enhance the maturity of HLCs thus improving the accuracy of toxicity prediction of chemicals. Several studies have demonstrated that 3D culture recapitulates the complex cellular interactions and cell- matrix contacts in the liver, resulting in augmented HLC function and improved prediction performance of the in vitro model (Takayama et al., 2013; Sirenko et al., 2016; Wang et al., 2018; Kim et al., 2018; Mun et al., 2019; Holmgren et al., 2020). It has been shown that 3D cultures display higher sensitivity to toxic compounds in comparison to two-dimensional (2D) cultures (Sirenko et al., 2016; Mun et al., 2019). In addition, cells in 3D culture could maintain functionality for prolonged culture period (Kim et al., 2018), affording long-term repeated-dose studies.
The commonly used 3D formats in toxicity studies have been spheroids formed by cells (usually of a single cell type) and attached onto the surface of the culture vessel (Takayama et al., 2013); several very recent studies reported the use of liver organoids generated from iPSCs for improved drug toxicity testing and safety assessment (Shinozawa et al., 2021; Lee et al., 2021; Kim et al., 2022). Different than spheroids formed by a single cell type, organoids are self-organized multicellular 3D in vitro tissue constructs that mimic the corresponding in vivo organ with regard to cell types, structure, and function. Organoids are generated from pluripotent stem cells (embryonic or induced) or progenitor cells in a process that recapitulates complex molecular and cellular changes of early organ development. Mun et al. have recently reported a novel method for the generation of liver organoids from human iPSCs that are self-renewable and functionally mature (Mun et al., 2019). During the differentiation process, the cellular identity and cell-cell interaction keep evolving, and the organoid internal structure and self‐organization changes accordingly, which ultimately result in specialized mature cells. Organoids thus become a powerful in vitro model for toxicological applications by virtue of the formation of organ-specific structures with highly organized multicellular architecture (Augustyniak et al., 2019). Compared with other existing in vitro models, the unique properties and advantages of organoids for toxicity testing include co-culture of diverse organ-specific cell types, improved internal dynamic flow of fluids, complex cell-cell and cell-matrix interactions, and moreover, a tissue-like morphology. For more detailed reviews on the use of liver organoids as tools for toxicity assessment, please see Harrison et al. (2021) and Brooks et al. (2021).
More interestingly, Wang et al. developed a new platform where functional liver organoids were generated through differentiation of iPSCs in situ on a 3D perfusable chip system, or microphysiological system (MPS) (Wang et al., 2018). Markedly enhanced liver-specific functions and metabolic capabilities were displayed by the HLC organoids in this platform. In addition, these organoids also showed dose- and time-dependent hepatotoxicity to acetaminophen. Although only one compound was tested, this study highlights the important contribution of mechanical fluid flow in the MPS to the maturation and function of HLC-derived liver organoids. With the advancement in microfluidic device fabrication technology in recent years, it is anticipated that more systematic and comprehensive studies using HLC organoids on the MPS platform will come out in the near future. Several recent studies on the MPS platform involving HLCs co-cultured with other cell types will be discussed in the next section (Section “Hepatotoxicity studies using HLC co-cultures’’).
Hepatotoxicity studies using HLC co-culturesPrimary hepatocytes showed stable phenotype when cultured in vitro together with liver- and non-liver-derived stromal cells from the same or different species as a result of cell-cell interactions in the co-culture (Bhatia et al., 1999). To improve the maturity and functionality of iPSC-derived HLCs for use in DILI prediction, similar strategies have been employed (Berger et al., 2015; Ware et al., 2015). Controlled presentation of cell-cell interactions with murine embryonic fibroblasts on a micropatterned co-culture (MPCC) system substantially enhanced the functional maturity of human iPSC-derived HLCs; moreover, the HLCs were able to stay alive and functional in culture for an extended period of more than 4 weeks (Berger et al., 2015). The system was able to correctly classify 37 hepatotoxic drugs and 10 non-toxic drugs with 65% sensitivity and 100% specificity; for comparison, PHHs showed a marginally higher sensitivity (70%) for the same set of compounds (Ware et al., 2015).
Co-culture of HLCs with liver non-parenchymal cells of human origin provides a model with better physiological relevance (Holmgren et al., 2020). Co-culture with activated human primary stellate cells was achieved both in 2D culture and in 3D spheroids after TGF-β treatment, which could serve as a valuable model to study non-alcoholic steatohepatitis (NASH). However, the beneficial effects of the co-culture on HLC maturation and toxicity prediction of chemicals were not shown in the study. In a later study, co-culture of HLCs with liver non-parenchymal cells such as liver sinusoidal endothelial cells and hepatic stellate cells to improve HLC function has been attempted (Kido and Koui, 2019), yet their utility in toxicity prediction awaits further demonstration. In another study, Tasnim et al. (2019) reported the generation of Kupffer cells (KCs) from human iPSCs and demonstrated that co-culture of hepatocyte with KCs was more sensitive than hepatocytes alone in detecting hepatotoxicity induced by two inflammation-associated drugs acetaminophen and trovafloxacin, and in detecting cholestasis induced by chlorpromazine. Furthermore, minimal non-specific background response was detected in co-culture of KCs with HLCs derived from the same iPSC line (donor-matched) in comparison to donor-mismatched counterpart.
Co-culture of iPSC-derived HLCs with other cell types on MPS platforms offer the combined benefits of cell-cell interactions, 3D microenvironment, and mechanical fluid flow (discussed in Section “Hepatotoxicity studies using HLCs in 3D format and on microfluidic chips”). Sakolish et al. (2021b) showed that co-culture of HLCs with 3 other human cell types (endothelial, Kupffer and stellate cells) on a human microfluidic liver acinus microphysiology system (LAMPS) afford a robust and reproducible in vitro liver model that is more physiologically and clinically relevant than 2D monolayer cultures. When tested with compounds of known liver toxicity for intrinsic hepatic clearance over a 10-day period, the LAMPS model demonstrated higher accuracy and precision compared to PHHs in suspension or 2D culture; however, as with other MPS models, the system tends to underestimate in vivo clearance (Sakolish et al., 2021a). Bircsak et al. (2021) developed an automated MPS for high-throughput hepatotoxicity screening, in which iPSC-derived HLCs were co-cultured, in separate channels, with endothelial cells and macrophages differentiated from THP-1 monocytes. The system was demonstrated to be stable for 15 days based on assay results of cell viability and albumin and urea secretion. A library of 159 compounds with known liver effects was first screened for 72 hr at 50 µM, and the resultant 21 hits were further evaluated by a follow up dose-response study. It was found that among the multiple endpoint assays (albumin, urea, hepatocyte nuclear size and viability staining), the albumin assay is the most sensitive in assessing compound toxicity. The studies discussed above highlight that co-culture of HLCs with other cell types on MPS platforms offers new perspectives for toxicity testing. Along this line, it is interesting to note that recently a novel system to study drug-drug interaction (DDI) was reported (Lee-Montiel et al., 2021). The system integrates a liver MPS with a cardiac MPS – both created with the same iPSC line. A well-known example of clinically relevant DDI is the interaction of cisapride with ketoconazole, the former being a gastroprokinetic drug and the latter a fungicide. CYP3A4 catalyzes the conversion of arrhythmogenic cisapride to non-arrhythmogenic norcisapride. The isogenic system was able to show that ketoconazole inhibited the metabolic conversion of cisapride to norcisapride in the liver MPS, leading to arrhythmia in the cardiac MPS. This study established the feasibility of integrating iPSC-derived isogenic multi-organ MPSs to facilitate screening for DDI-mediated inter-organ toxicity in drug efficacy and toxicity evaluation.
Multi-parameter toxicity assessment using high-content analysis (HCA) and omics technologiesTypically, only a few endpoints (e.g., cell viability, ATP content, LDH release) were assessed for toxicity studies involving iPSC-derived HLCs. Development of assays allowing for simultaneous evaluation of a wide variety of toxicity-related parameters can not only provide more detailed information but also distinguish between different mechanisms of toxicity, thus broadening the application of HLCs. Using high-content imaging assays, several studies achieved multi-parameter hepatotoxicity assessment in iPSC-HLCs (Sirenko et al., 2014; Grimm et al., 2015; Sirenko et al., 2016; Pradip et al., 2016; Chen et al., 2020; Chen et al., 2021; Hsieh et al., 2021; Maddah et al., 2020). The endpoints employed by these studies are diverse in nature and represent different mechanisms of hepatotoxicity, including cell viability, nuclear shape, mitochondrial membrane potential (MMP), phospholipid accumulation, cytoskeleton integrity, reactive oxygen species (ROS) formation, and apoptosis (Sirenko et al., 2014; Grimm et al., 2015; Maddah et al., 2020; Chen et al., 2020). By nature, these assays are high-throughput and therefore capable of analyzing hundreds of compounds (Sirenko et al., 2014). Moreover, HCA could be conducted on both 2D HLC monolayer cultures and on 3D spheroids of HLCs as well (Sirenko et al., 2016).
Omics technologies, such as transcriptomics and proteomics that evaluate genome-wide gene and protein expressions, make it possible to measure tens of thousands of endpoints in a single assay. Transcriptomic profiling in toxicity studies, or toxicogenomics, have been used to explore and delineate mechanisms of action of potential toxicants to human and the environment. Many studies have also used toxicogenomics to identify biomarkers that may improve the detection or prediction of specific toxic effects; such markers are also useful for categorizing or discriminating different classes of compounds. Moreover, toxicogenomic assays are more sensitive than conventional biological endpoints such as morphological changes and cytotoxicity assays, as gene or protein expression changes could be detected earlier after exposure or at lower concentrations. In the past years, several groups used omics to study hepatotoxicity in iPSC-derived HLCs, and demonstrated the significance of transcriptomic alterations as an informative endpoint in in vitro toxicity testing and for mechanistic study of chemicals (Choudhury et al., 2017; Tryndyak et al., 2019; Gao et al., 2021, 2022; Ghosh et al., 2021; Nunes et al., 2022). As discussed in section “Patient-specific HLCs for hepatotoxicity prediction”, Choudhury et al. (2017) used multiple lines of patient-specific HLCs to model idiosyncratic hepatotoxicity to pazopanib. Using microarray for transcriptional analyses, the authors showed that pazopanib induces oxidative stress in HLCs in all groups, but in HLCs from susceptible individuals, pazopanib causes more disruption of iron metabolism and higher level of oxidative stress. This study shed light on the mechanism behind pazopanib-induced hepatotoxicity. Our group used microarrays to characterize transcriptional changes in iPSC-derived HLCs to study the toxicity of silver nanoparticles (AgNPs) (Gao et al., 2021), and later on confirmed the transcriptomic findings using proteomics analysis (Gao et al., 2022). The results of these studies are concordant with those reported previously on hepatoma cell lines and primary hepatocytes, highlight the contribution of oxidative stress to AgNP-induced toxicity, and point out the potential risk of cancer after long-term AgNP exposure (Gao et al., 2021, 2022).
HLCs derived from iPSCs genome-edited with CRISPR/Cas9Genome editing with CRISPR/Cas9 has fueled a wave of scientific discoveries over the past decade and has been bringing in revolutions to many areas of biomedical research. Short for “clustered regularly interspaced short palindromic repeats”, CRISPR uses a small RNA fragment to guide and target a non-sequence-specific DNA endonuclease to the site of interest, and make modifications (substitution, deletion or insertion) on the gene with high efficiency and accuracy (Ben Jehuda et al., 2018). With the CRISPR/Cas9 system, isogenic cell lines with desired mutations could be generated for a variety of biomedical applications. In several studies that appeared in the last few years, iPSCs were modified by CRISPR/Cas9 and the genetically modified cells were further differentiated into HLCs for toxicity applications (Takayama et al., 2018; Deguchi et al., 2019; Kim et al., 2020; Deguchi et al., 2021). Using the CRISPR/Cas9 system, a CYP3A4-NeoR-EGFP transgenic reporter human iPSC line was established by inserting a neomycin resistant gene (NeoR) and an enhanced green fluorescent protein (EGFP) into the CYP3A4 locus (Takayama et al., 2018). The mutant iPSC line was subsequently differentiated into HLCs, and cells with strong CYP3A4 expression were then selectively concentrated following neomycin treatment. The results showed that neomycin treatment increased the percentage of CYP3A4-positive cells from 21% to 81%; in addition, other than CYP3A4, gene expression and activity of some other major CYPs, such as CYP1A2, CYP2C19, and CYP2D6, were also increased significantly. Of more importance, using a panel of 8 test compounds, it was demonstrated that the detection sensitivity of drug-induced hepatotoxicity of the CRISPR-edited iPSCs was enhanced after neomycin treatment. In a separate study by the same group, a CYP2C19-knockout (KO) human iPSC line was constructed using CRISPR/Cas9 technology and subsequently differentiated into HLCs (Deguchi et al., 2019). The gene expression of major hepatocyte markers in CYP2C19-KO HLCs was at similar levels to wild-type iPSC-derived HLCs; however, the CYP2C19 metabolic activity in CYP2C19-KO HLCs was almost eliminated. Using the CYP2C19 substrate clopidogrel as an example, the authors demonstrated that CYP2C19-KO HLCs could serve as an in vitro model of CYP2C19 poor metabolizers (PMs), which will contribute substantially to drug development and toxicity evaluation (Deguchi et al., 2019). The same strategy could be applied to model other CYP PMs, such as CYP2C9-PMs or CYP2D6-PMs. A panel of hepatocyte lines with diverse metabolic profiles could be established from these cells, which is expected to make a significant contribution to the field of personalized drug testing. It has to be pointed out that there was a flaw in the experimental design of the study. CYP2C19 was completely deleted as a result of its exon 1 being biallelically targeted by the transgene (Deguchi et al., 2019). A better design would be to only introduce SNPs into the gene locus, such as CYP2C19*2 or *3, which would result in mutant HLCs to mimic the pharmacokinetics in CYP2C19 PMs more precisely. More recently, using the same technology, the group generated a CYP3A4-KO iPSC line, and from which generated HLCs to evaluate CYP3A4-mediated drug metabolism and drug-induced toxicity. Using a small group of model drugs, including acetaminophen, amiodarone, desipramine, leflunomide, tacrine, and tolcapone, the authors confirmed that the CYP3A4-KO HLCs were able to correctly predict CYP3A4-mediated toxicity (Deguchi et al., 2021).
Hepatocytes with reporter genes enabling live cell imaging would be extremely useful for toxicity screening applications. To this end, Kim et al. (Kim et al., 2020) generated an iPSC reporter line expressing mCherry-tagged CYP1A1 via CRISPR/Cas9-mediated knock-in, and HLCs derived from this iPSC line were used for live-cell screening of aryl hydrocarbon receptor (AHR) modulators. A total of 23 hits modulating CYP1A1 expression were obtained from high-content screening of 241 hepatotoxicity chemicals and nuclear receptor ligands, among which 3 upregulating chemicals and 2 downregulating compounds were identified. The authors also demonstrated that responses of the iPSC-HLCs against an AHR agonist were more similar to PHHs than HepG2 cells.
A variety of methods have been established in the past decade to differentiate iPSCs into HLCs, which have so far been tested for a wide range of applications, including tissue engineering, disease modeling, drug discovery, and toxicity screening. For toxicological applications, the appealing feature of maintaining the genetic make-up of their source cell makes iPSC-derived HLCs uniquely suited for iDILI study and hazard assessment on susceptible subpopulations. In this regard, it is encouraging to note that efforts have been made to characterize iPSC-derived HLCs as models of hepatocyte function and toxicity testing in the regulatory context (Qosa et al., 2021). However, progress in toxicological applications has been impeded with difficulties and complexities in obtaining functionally mature HLCs. It has been more than a decade since the first reports of directed hepatocyte differentiation; however, currently HLCs differentiated from iPSCs still display heterogeneous phenotypes and incompetent functionalities (Baxter et al., 2015). Although the heterogeneity of HLCs may be a reflection of the heterogeneous hepatocyte population in the liver (Stanger, 2015); however, as has been shown in some recent studies, enormous differences still exist between HLCs and primary hepatocytes (Gao and Liu, 2017; Gao et al., 2020). Therefore, further effort is needed to substantially improve and standardize protocols for the differentiation of iPSCs into HLCs. However, attaining maturation to a level on par with primary hepatocytes has proven to be challenging for hepatocyte differentiation. A wide variety of approaches have been explored over the past years to enhance the maturity of HLCs, such as the formation of tissue-like 3D structures, coculture of HLCs with endothelial cells or other cell types, the addition of SMs to modulate differentiation, and overexpression of certain transcription factors (Chen et al., 2018). Nonetheless, improvements on HLC maturation attained by these methods have been limited and unsatisfying. A better understanding of the molecular and cellular basis of liver development coupled with emerging innovative technologies, such as genome modification, 3D biofabrication, and more physiologically relevant multi-organ MPS, is hoped to bring forth breakthroughs in the field.
The authors thank Dr. Marianne Miliotis-Solomotis for critical review of the manuscript.
This work was supported by internal funding from the U.S. Food and Drug Administration.
Conflict of interestThe authors declare that there is no conflict of interest.