Abstract
Perfluorooctane sulfonate (PFOS), an emerging environmental pollutant, is reported to cause neurotoxicity in animals and humans, but its underlying mechanisms are still unclear. We used in vivo models to investigate the effects of PFOS on cognition-related behaviors and related mechanisms. After 45 days of intragastric administration of PFOS (2 mg/kg or 8 mg/kg) in 7-week-old C57BL/6 mice, muscle strength, cognitive function and anxiety-like behavior were evaluated by a series of behavioral tests. The underling mechanisms of PFOS on impaired behaviors were evaluated by HE/Nissl staining, electron microscopy observation and western blot analysis. The results indicated that PFOS-exposed mice exhibited significant cognitive impairment, anxiety, neuronal degeneration and the abnormities of synaptic ultrastructure in the cortex and hippocampus. Western blot analysis indicated that PFOS exposure increased microtubule-associated protein light chain 3 (LC3) and decreased p62 protein levels, which may be associated with activation of autophagy leading to neuron damage. In summary, our results suggest that chronic exposure to PFOS adversely affects cognitive-related behavior in mice. These findings provide new mechanistic insights into PFOS-induced neurotoxicity.
INTRODUCTION
Perfluorooctane sulfonate (PFOS) is the final product of all kinds of perfluorinated organic compound series products after chemical or biodegradation. The PFOS molecule is a fluorocarbon chain composed of 17 fluorine atoms and 8 carbon atoms, and a sulfonyl group is connected to the carbon atom at the end of the fluorocarbon chain. This chemical structure makes the covalent bond between carbon atoms in the hydrocarbon chain of PFOS molecule not vulnerable to external effects and breaking (Lau, 2012). PFOS has unique and useful chemical properties, including surface activity, thermal, acid resistance and hydrophobic, grease-phobic characteristics (Evich et al., 2022), thus it has been used for many industrial applications such as stain repellents, textile, paints, polishes, lubricants, electronics, adhesives, fire extinguishers and food packaging (Picó et al., 2011).
PFOS is recognized as global emerging organic pollutant of concern due to its ubiquitous distribution in all environmental media, wildlife, and humans, persistency, bioaccumulation, toxicity, and human health-risk potentials (Jian et al., 2018; Wang et al., 2018). A growing number of toxicological studies have indicated that sustained exposure to PFOS may lead to a range of adverse effects, including hepatotoxicity (Li et al., 2021), reproductive toxicity (Yin et al., 2021), genotoxicity (Eke et al., 2017), immunotoxicity (Liang et al., 2022), developmental toxicity (Gaballah et al., 2020) and neurotoxicity (Chen et al., 2014). Among them, neurotoxicity of perfluorooctane sulfonate has been recognized as one of the most serious problems, as it affects a variety of brain functions including cognition and memory (Wang et al., 2015), motor coordination (Harris et al., 2018), social activity (Shin et al., 2020) and anxiety-like behavior (Ninomiya et al., 2022). However, the molecular mechanisms for PFOS-induced neurotoxicity have not been fully elucidated. In regard to toxic mechanisms, PFOS has been revealed to affect the thyroid system, influence the calcium homeostasis, protein kinase C, synaptic plasticity and cellular differentiation (Mariussen, 2012; Wang et al., 2019). Therefore, the adverse effects of PFOS exposure in younger mice and the specific mechanisms of action are highly concerning.
Autophagy is a highly conserved lysosome-dependent process in which eukaryotic cells remove misfolded proteins and damaged organelles in cytoplasm, which is critical for energy metabolism, organelle renewal, and maintenance of intracellular homeostasis (Klionsky et al., 2021). Moderate activation of autophagy is an important cornerstone for maintaining intracellular homeostasis. But autophagy activation also could lead to cell death through lysosomal overactivation, which leads to another pathway of cell death in addition to apoptosis (Taylor et al., 2018). Wen et al. indicate that ROS-mediated pERK1/2 activation is essential to activate autophagy, leading to the apoptosis of PFOS-treated renal tubular cells (Wen et al., 2021). The study found that autophagosome formation was activated in the early stage of PFOS treatment (6 hr). Qiu et al. proposed that PFOS could inhibit the phosphorylation and activation of AKT by inhibiting mTORC2, a key regulator of autophagy. Ultimately, the inhibition of AKT led to insulin resistance in PFOS-treated cells (Qiu et al., 2016). Yao et al. found that autophagy-dependent LMP was induced after treatment with 200 μM PFOS for 12 hr in hepatocytes (Yao et al., 2016). Dong et al. indicated that autophagy and mitochondrial calcium (Ca2+) accumulation were induced after PFOS treatment for 0.5 hr, which might be the mechanism of PFOS-induced insulin resistance (Dong et al., 2022). The above studies support the notion that PFOS stimulation activates autophagy, leading to PFOS-mediated renal and hepatic toxicity. Currently, there are no studies reporting the role of autophagy in PFOS-mediated neurotoxicity.
In the present study, we investigated whether mice exposed to PFOS can affect the autophagy in order to elucidate the relationship between cognitive-related behavioural changes after PFOS exposure and autophagy.
MATERIALS AND METHODS
Animals
Male C57BL/6 mice, specific-pathogen free grade, aged 6 weeks, were purchased from the Provincial Center for Disease Control and Prevention (Hubei, China). They were kept in cages with five mice per cage and maintained at a constant temperature (23 ± 2°C) and humidity (55 ± 5%) and exposed to a 12 hr light/dark cycle. Food and water were supplied ad libitum. All experiments were performed according to procedures approved by the Wuhan University of Science and Technology Experimental Animal Welfare and Ethics Committee.
Experimental design
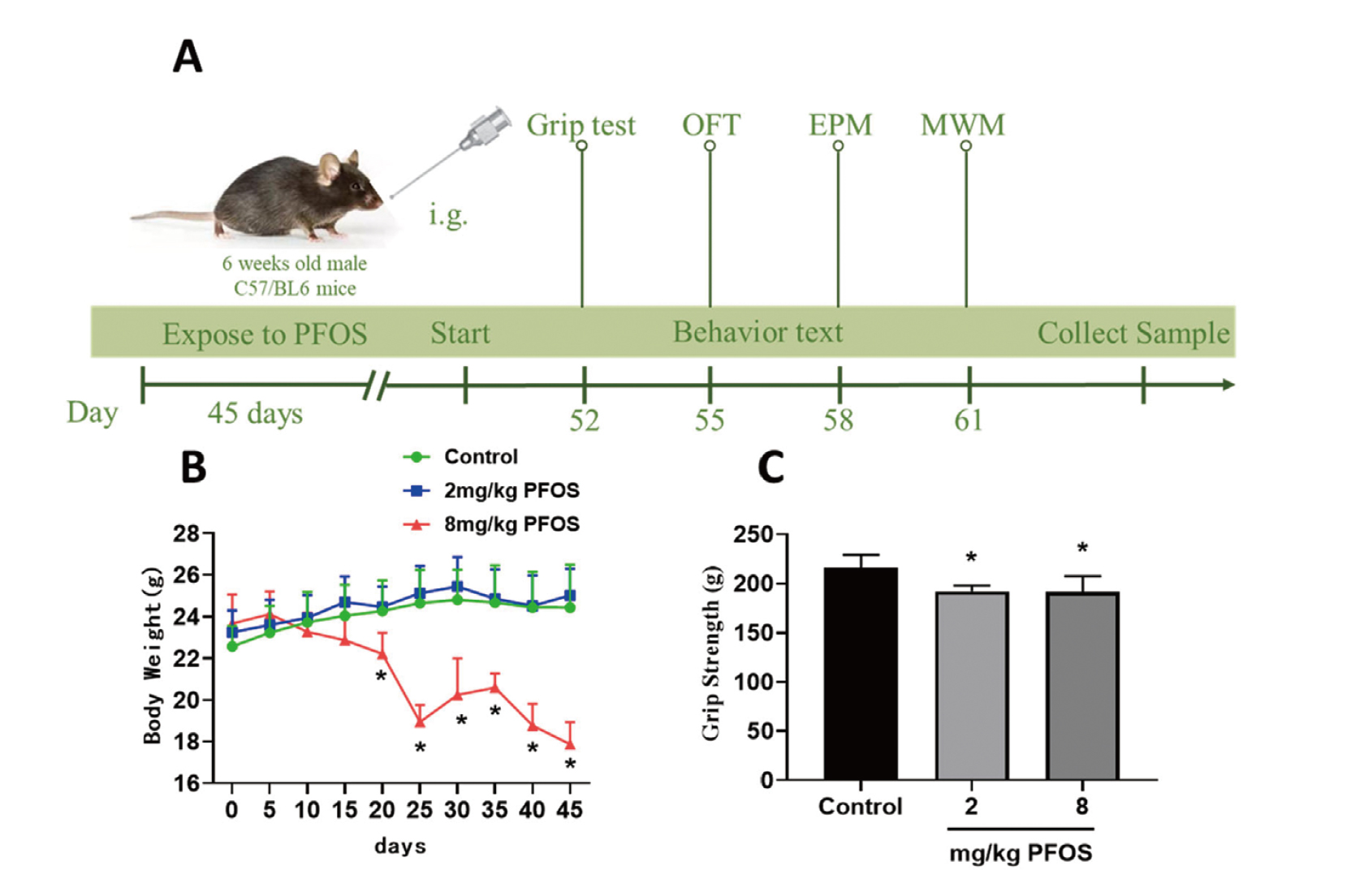
After one-week acclimation period on a regular diet, mice were randomly assigned to three groups. The dosing suspensions of the test substance were prepared with 2% Tween 80 (Xing et al., 2016). After 45 days of continuous exposure, behavioral experiments were conducted to evaluate each index. Animals were weighed every five days. The general condition and mortality of the mice were recorded daily during the intervention. At the end of the behavioral experiments, mice were euthanized by intraperitoneal injection of 20% ethyl carbamate (5 mL/kg) or by perfusion, and fresh tissue from the cerebral cortex and hippocampus was rapidly isolated and stored at -80ºC for subsequent experiments. Potassium perfluorooctanesulfonate (CAS: 2795-39-3, purity > 98%) was purchased from Shanghai Yien Chemical Technology Co., Ltd. (Shanghai, China). The experimental groups were as follows:
1) Control group: mice of 7 weeks old, received equal volume of deionized water with 2% Tween 80 via oral gavage.
2) 2 mg/kg PFOS group: The test substance (2 mg PFOS/kg BW/day) was administered to the mice once daily via oral gavage for 45 days.
3) 8 mg/kg PFOS group: The test substance (8 mg PFOS/kg BW/day) was administered to the mice once daily via oral gavage for 45 days.
Grip Test
The grasping force of the mice was measured with Grip Strength Meter. Place the mice on the dynamometer and gently calm them down. Hold the tail of the mouse and gently pull it backward. After the animal grasps the grasping plate, evenly pull it back, causing the animal to release its claw. At this time, the instrument will automatically record the maximum grasping force of the mouse. After the adaptation, the grip test was carried out and repeated three times for each mouse with an interval of 1 min, and the average value was taken.
Morris Water Maze
Morris Water Maze (MWM) test was performed to assess the learning and spatial memory of mice. The maze consisted of a circular pool divided into four quadrants, with a submerged escape platform placed in the center of one of the quadrants. The whole experiment lasted for six days. For the first five days, the mice were placed into water from 4 entry points facing the maze wall once a day. Escape latency, or the time to the platform hidden under the water surface, was recorded as an index of spatial learning and memory. The platform was removed on the last day. In order to investigate the memory of the mice on the original platform, the mice were placed into the maze at any entry point to record the swimming track of the mice in 60 sec. The mice were monitored by a video tracking system directly above the maze as they swam and parameters were measured using Super-Maze software (Shanghai Xinruan Information Technology Co., Ltd., Shanghai, China).
Elevated Plus Maze Test
The elevated plus maze (35 cm length, 35 cm width, 100 cm height) consisting of two open arms and two closed arms was used to measure anxiety-like behavior in mice. The light source is directly above the maze and is covered with a shade to ensure consistent lighting on each arm. Each mouse was placed individually in the center (5 × 5 cm) of the maze facing one of the open arms. At the same time, the camera monitor was turned on to record the number of open arms and closed arms and the time of entering each arm of experimental animals within 5 min. After each mouse was recorded, the maze was cleaned with 75% alcohol to eliminate the effect of animal odors on subsequent animals. All the data were analyzed by Super-Maze software (Shanghai Xinruan Information Technology Co., Ltd., Shanghai, China). The percentage of time spent and the distance traveled in the maze with open and closed arms were calculated.
Open field test
The open field test assesses anxiety-like behavior in more stressful conditions. The open field area (consisting of a 40 cm × 40 cm × 40 cm box) is divided into 16 small squares, which was used to distinguish the central area from the surrounding area. The time spent in the center of the open field and overall distance traveled were recorded for 5 min with the LimeLight software system (ACTIMETRICS, USA). The test area was cleaned with 75% ethanol after each trial.
Hematoxylin and eosin (HE) staining
Paraffin-embedded brain tissues (including prefrontal cortex and hippocampus) were cut into 4 µm thick sections, dewaxed using xylene I and II for 20 min. Then, the sections were stained with hematoxylin (Wuhan Kerui Biotechnology Co., Ltd., Wuhan, China) for 30 min at 25°C,, washed with double distilled water for 20 min, stained with eosin for 5 min at 25°C, dehydrated with 100, 95, 80 and 70% ethanol for 5 min each, cleared using xylene and fixed with neutral balsam. Histomorphological changes in the cortical and hippocampal areas of mice were observed under the microscope (Olympus, Tokyo, Japan).
Nissl staining
Nissl staining is used to detect the histomorphological changes in the hippocampal neurons. Briefly, the paraffin-embedded sections of the brain tissue were sliced into 4 µm thick sections. Then, the 4-µm sections were incubated with Nissl dye solution (Nanjing Jiancheng Technology Co., Ltd., Nanjing, China) for 10 min at room temperature, rinsed in double distilled water followed by treatment with 70 and 95% ethanol, dehydrated in 100% ethanol, cleared using xylene and coated with neutral resin. Finally, staining changes of cortex and hippocampus were observed under optical microscope. Then the number of neurons was counted by randomly selecting four images from each group (two images per mouse).
Electron microscopy observation
After infusion with 4% paraformaldehyde, 0.5~1 mm3 of tissue blocks from the hippocampus were dissected and rinsed in phosphate buffered saline (PBS), post-fixed flat in 1% osmium tetroxide for 60 min and dehydrated in ascending concentrations of ethanol. Tissues were then treated with propylene oxide and impregnated with resin overnight. Each sample was placed on a Teflon support and covered with a capsule containing pure epoxy resin for 1 hr at 60°C and polymerized overnight at 80°C. A series of consecutive ultrathin sections was prepared from each sampling field and stained with uranyl acetate-lead citrate. Finally, the ultrastructure of neurons, synapses, autophagosomes was observed by transmission electron microscope (Tecnai-200, FEI, USA).
Western blotting
Cortex or hippocampus were homogenized in RIPA buffer (Applygen, Beijing, China) and centrifuged at 12,000 x g for 10 min at 4°C. The total protein concentration was determined using a BCA protein assay kit (Applygen, Beijing, China) according to the manufacturer's protocol. Subsequent to mixing with the loading buffer and boiling at 95°C for 5 min, proteins were electrophoretically transferred onto polyvinylidene difluoride membranes and then incubated with the corresponding primary antibodies overnight at 4°C subsequent to blocking using 5% non-fat milk in PBS-Tween 20 for 1 hr at room temperature. Blots were processed using the following primary antibodies; β-actin (Servicebio, GB11001, 1:5000), brain-derived neurotrophic factor (BDNF, Wanleibio, WL0168, 1:1000), PSD-95 (Proteintech, 20665-1-AP, 1:5000), synaptophysin (Proteintech, 17785-1-AP, 1:50000), LC3 (Proteintech, 14600-1-AP, 1:3000), p62 (Proteintech, 18420-1-AP, 1:5000), Beclin1 (Wanleibio, WL02508, 1:1000), Atg5 (Wanleibio, WL02411, 1:1000), Atg12 (Wanleibio, WL03144, 1:1500). The blots were developed using ultrasignal western ECL substrate (Wuhan Kerui Biotechnology Co., Ltd., Wuhan, China). The relative expression levels of the protein bands were quantified and normalized to β-actin using Image J software (NIH, USA).
Statistical analysis
All experimental data were presented as the mean ± standard error of the mean (SEM). One-way ANOVA was used to compare multiple groups of data. If the total variance was uniform, it was further compared by LSD-t test. If the overall variance was uneven, multiple comparisons were analyzed directly with Dunnett’s T3. Repeated measurement analysis of variance was used for the changes of escape latency in water maze and body weight. Statistical analysis was performed using SPSS 26.0 statistical software (SPSS Inc., Chicago, IL, USA). Values of P < 0.05 were considered statistically significant.
RESULTS
Effects of PFOS exposure on general condition of young mice into adulthood
We found that PFOS exposure worsened the general state such as slow movement, dark coat and vertical hair, especially 8 mg/kg PFOS exposure. With the extension of exposure time, PFOS-exposed mice showed different degrees of body weight loss. Compared with the control group and the 2 mg/kg PFOS group, the body weight of the 8 mg/kg PFOS group decreased from day 20 to day 45 (P < 0.05), while there was no significant change in the 2 mg/kg PFOS group compared with the control group (Fig. 1B). Additionally, grip strength was different between control group (216.09 ± 13.22 g) and 8 mg/kg PFOS (191.40 ± 16.05 g) exposure group, indicating that PFOS exposure altered grasping force in this study (P < 0.05, Fig. 1C).
Effects of PFOS exposure on adult cognitive function of young mice
To investigate the effects of PFOS exposure on learning and cognitive ability of mice, we performed MWM text. Results from MWM test showed that 2 mg/kg PFOS group and 8 mg/kg PFOS group need more latency time to reach the hidden platform whereas the control group took less latency time (Fig. 2A), implying that PFOS induced memory impairment. In the training stage of the MWM test, the total swimming distance and average swimming speed of mice in 8 mg/kg PFOS group were significantly reduced compared with the control mice (P < 0.05, Fig. 2B and 2C). On day 6 of the space exploration stage, compared with the control group, the number of stays in the target quadrant of 8 mg/kg PFOS exposure group was reduced (F = 7.023, P < 0.05; Fig. 2D) and the total movement distance across the target quadrant platform decreased significantly (F = 7.406, P < 0.05; Fig. 2E).
Effects of PFOS exposure on anxiety-like behaviors and movement ability of mice
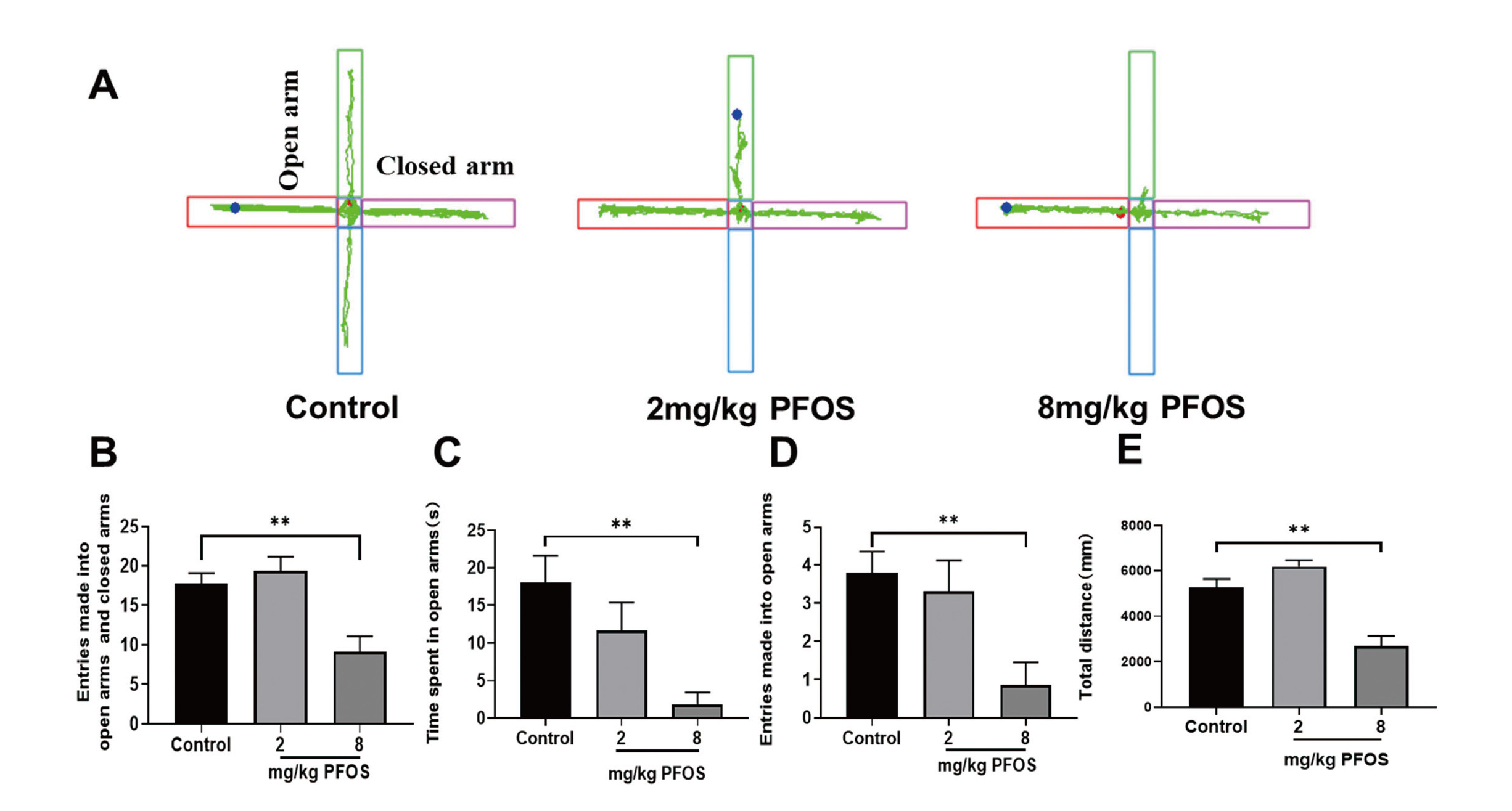
To evaluate the effects of PFOS exposure on anxiety-like behaviors and movement ability, mice performed the open field test and the elevated plus maze test. In the elevated plus maze test, the 8 mg/kg PFOS group appeared to exhibit more anxiety-like behaviors than the control group, as indicated by less total time spent in the open arms of the maze (F = 4.483, P < 0.05; Fig. 3C) and the number of entries into the open arms (F = 4.437, P < 0.05; Fig. 3D). Mice in the 2 mg/kg PFOS group also showed the same effect, with fewer entries into the open arms compared to the control group (P < 0.05).
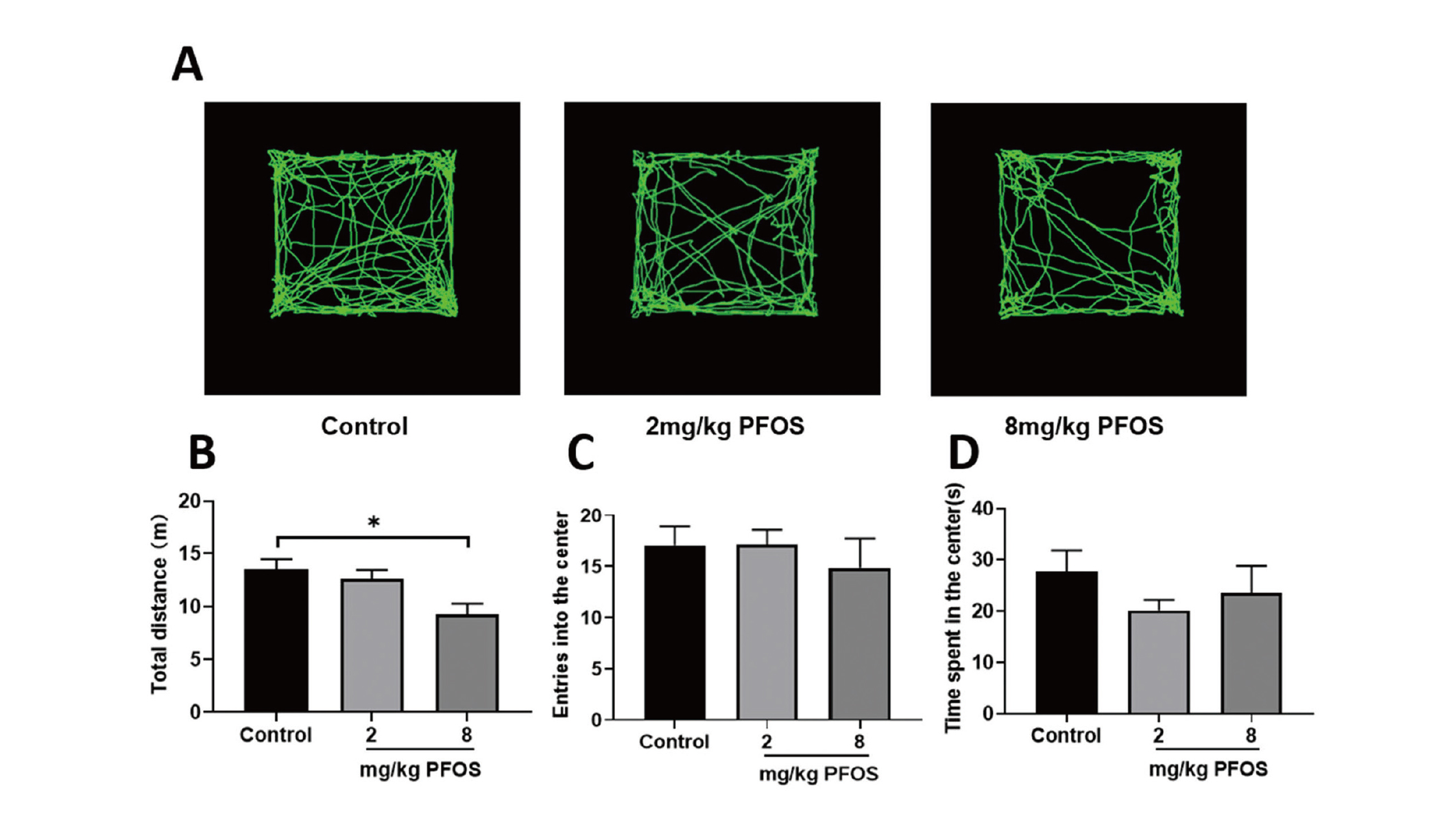
In the open field test, no difference was found for number of times entering the central area between the PFOS-exposed and control groups (P > 0.05, Fig. 4C). Similarly, no differences were found in time spent in the center of the open field between the PFOS-exposed and control groups (P > 0.05, Fig. 4D). The only difference found was that the total distance of horizontal movement in the open field decreased in the 8 mg/kg PFOS group (F = 4.437, P < 0.05; Fig. 4B), suggesting that there was anxiety in the mice.
Effects of PFOS exposure on morphology and structure of the cortical and hippocampal neurons of mice
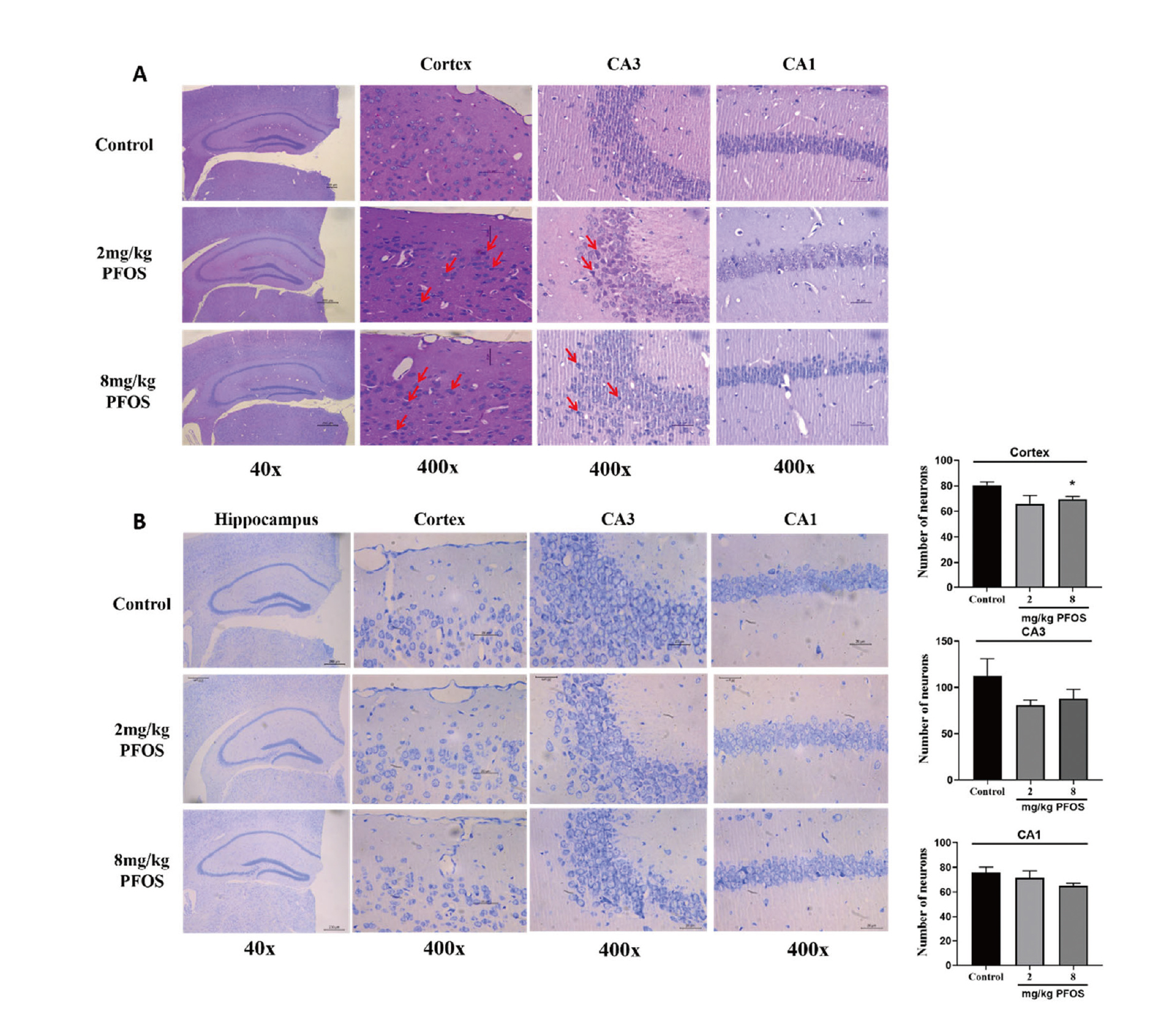
Firstly, HE staining was performed to evaluate the effects of PFOS exposure on the morphology and structure of the cortical and hippocampal neurons in mice. HE staining revealed that the neurons in both cortex and hippocampus CA1 and CA3 regions of the control group had regular morphology, dense arrangement, clear nucleus cytoplasm and rare degeneration. Conversely, compared with control group, histological changes such as degenerative neurons with darkly stained pyknotic nuclei, cellular swelling and pyknotic nuclei were observed in both cortex and hippocampus of the PFOS-exposed group (Fig. 5A). Additionally, the number of survival neurons stained by Nissl in the CA1, CA3 and cortex regions was reduced in the 8 mg/kg PFOS group compared with that in the control group (Fig. 5B), suggesting that PFOS exposure led to neuronal damage.
Effects of PFOS exposure on ultrastructural changes of neuron morphology, synapses and autophagosome in the cortex and hippocampus of mice
Results from electron microscopy observation showed that the nuclear membrane of hippocampal neurons in the control group was round and complete (Fig. 6A). Whereas they were shrunken slightly in the 2 mg/kg PFOS group (Fig. 6C, blue arrow), they were obviously shrunken and sunken in 8 mg/kg PFOS group (Fig. 6E, blue arrow). In the control group, the number of mitochondria was higher and the structure of internal crest was clear (Fig. 6A and 6B, yellow arrow), while in the 2 mg/kg PFOS and 8 mg/kg PFOS-exposed groups, the mitochondrial cristae decreased or even disappeared (Fig. 6C-6F, yellow arrow). Early autophagosomes were seen in control group (Fig. 6A, red arrow), and autophagy lysosomes were seen in the 2 mg/kg PFOS- and 8 mg/kg PFOS-exposed groups, with lipofuscin deposition (Fig. 6D and 6F, orange arrow).
Effects of PFOS exposure on the protein levels of BDNF, PSD95 and synaptophysin in the cortex and hippocampus
The BDNF is a secretory polypeptide that is distributed in the brain and is involved in many cellular processes that regulate behavior (Cutuli et al., 2022). Substantial studies demonstrate that BDNF is released at the synapse, where it affects synaptic plasticity, producing critical changes in cognitive functions (Nieto et al., 2013). The postsynaptic density protein PSD-95 is a major element of synapses (Savioz et al., 2014). And synaptophysin is one of the most abundant membrane proteins of small synaptic vesicles (Cousin, 2021). Results showed that protein levels of BDNF and PSD-95 were significantly reduced in the cortex and hippocampus of 8 mg/kg PFOS group in comparison with the control group (P < 0.05, Fig. 7A and 7B). The synaptophysin protein level was decreased in the cortex and hippocampus of the 8 mg/kg PFOS group in comparison with the control group (P < 0.05, Fig. 7C and 7D).

To further explore whether the autophagy pathway involved in PFOS exposure leads to impaired behavioral changes of young mice, we examined the levels of autophagy-related proteins such as LC3, P62 and Beclin1. Compared with the control group, LC3B protein level in the cortex and hippocampus of 8 mg/kg PFOS group was significantly increased, and p62 protein level was significantly decreased (P < 0.05, Fig. 8A and 8B). However, the levels of Beclin1 proteins showed no significant difference among all groups (P > 0.05, Fig. 8C).
DISCUSSION
The present study demonstrated that mice exposed to PFOS (2 or 8 mg/kg body weight) exhibited cognitive impairment and anxiety-like behaviors as well as histopathological changes. Further studies found that PFOS exposure could activate autophagy with increased LC3 protein level and decreased p62 level, suggesting that the autophagy signaling pathway may be involved in the behavioral deficits resulted from PFOS exposure.
The neurotoxicity of PFOS exposure has been extensively studied around the world. Behavioral studies have preliminarily demonstrated that PFOS can damage neural development, which is characterized by increased autonomic activity and decreased cognitive abilities. In in vitro studies, PFOS exposure has been shown to induce neuroinflammation, oxidative damage, and apoptosis in nerve cells (Sun et al., 2018; Chen et al., 2018). Yang et al. observed that the dysfunction of hippocampus which finally account for the impairments of spatial learning and memory in adult mice after exposure to 10.75 mg/kg PFOS for three months (Long et al., 2013). One previous study showing the disruption of learning and memory by lactational PFOS exposure in male offspring mice confirmed such tendency. The effects of PFOS on short-term memory have been reported in related studies. Discrimination index was dose-dependently reduced in 1 mg/kg PFOS-exposed mice during OLTs and ORTs (Mshaty et al., 2020). On the other hand, some studies demonstrate the effects of PFOS exposure on motion-related behaviors. A population-based case-control study found that maternal serum high levels of PFOS increase the incidence of autism spectrum disorder in children (Shin et al., 2020). It is reported that PFOS has a mild effect on social activity and anxiety, which is consistent with the human reports (Ninomiya et al., 2022). In this study, we found that mice exposed to PFOS for 45 days showed significant cognitive impairment and increased anxiety in the 8 mg/kg PFOS group, accompanied by significant weight loss and neuronal degeneration in the cortex and hippocampus. The 2 mg/kg PFOS group showed a similar tendency, though less so. Although food intake was not counted in this study, it has been demonstrated that there is no correlation between body weight and food intake in PFOS-treated mice (Xing et al., 2016). Therefore, the decrease in body weight of PFOS-treated mice may be due to the toxic effects of PFOS. In the present study, a significant reduction in grip strength was observed in the 8 mg/kg dose group, but the relationship between the grip strength and body weight loss is not clear and requires further investigation. Brain-derived neurotrophic factor (BDNF) is a neurotrophin with well-established properties of promoting neuronal survival and synaptic integrity, which also correlated with improved spatial memory (Wang and Holsinger, 2018). Reduced BDNF may underlie age-related synaptic loss, in turn contributing to cerebral atrophy, cognitive decline, and increased risk for psychiatric disorders (Oh et al., 2016). Synapse is the place where neurons contact with each other and is an important structure for transmitting information. The nervous system dominates various behaviors and physiological activities such as learning and memory through synaptic synthesis, release and information transmission (Batool et al., 2019). Postsynaptic density protein 95 and synaptophysin are the main synaptic markers, whose decreased expression indicates synaptic loss or damage (Pan et al., 2016; Xu et al., 2016). Similarly, our study showed that the expressions of BDNF, PSD95 and Syn proteins in the cerebral cortex and hippocampus of mice exposed to 8 mg/kg PFOS for 45 days were reduced.
As so far, the toxic mechanism of PFOS in causing behavioral injury is under investigation. Previously studies have shown that PFOS exposure to neonatal induced irreversible neurotoxic effects, which partially by altering neuronal calcium homeostasis and calcium signaling pathways (Harada et al., 2006; Liao et al., 2008; Liu et al., 2011). One idea suggests that neurotransmitters bind to receptors in the dominant neuron or cell membrane to complete the information transfer, which leads to increased excitability and neurotoxicity (Hallgren et al., 2015; Ishida et al., 2017). Another idea suggests that PFOS might cause neurotoxicity by affecting synaptic plasticity (Pinkas et al., 2010; Dong et al., 2016). Moreover, both in vivo and in vitro studies have reported apoptosis of neuronal cells induced by PFOS (Chen et al., 2014). In recent years, autophagy has attracted extensive attention. Yao et al. observed that perfluorooctane sulfonate increased the number of autophagosomes in HepG2 cells by transmission electron microscopy, which further supported the notion that autophagic cell death contributed to PFOS-induced hepatotoxicity (Yao et al., 2014). It was reported that exposure of rat primary hippocampal neurons to PFOS has led to oxidation-antioxidation imbalance, increased apoptosis and abnormal autophagy (Li et al., 2017). In addition, the research results of Liu et al. showed that PFOS induced mitochondrial dysfunction via blocking autophagy-lysosome degradation, leading to cardiomyocyte toxicity from ES cells (Liu et al., 2020). Autophagy is a core molecular pathway for the preservation of cellular and organismal homeostasis. Autophagy results from the formation of a phagophore, initiated by the ULK1 and the ATG5-ATG12-ATG16L complexes. They then close around ubiquitinized proteins bound to p60 and LC3II. The phagophore then closes to form an autophagosome, which then fuses with a lysosome, resulting in the degradation of the contents (D’Arcy, 2019). Among them, Beclin1 is a protein known for its key role in regulating autophagy and other membrane trafficking processes as an essential member of the Class III PI3K complexes, which can mediate the localization of autophagy-related proteins in autophagy vesicles and react with a variety of proteins to regulate the formation and maturation of autophagosomes (Tran et al., 2021). In the process of autophagosome formation, a cytosolic form of LC3 (LC3-I) is conjugated to phosphatidylethanolamine to form LC3-phosphatidylethanolamine conjugate (LC3-II), which is recruited to autophagosomal membranes. Autophagosomes fuse with lysosomes to form autolysosomes, and intra-autophagosomal components are degraded by lysosomal hydrolases. Concomitantly, LC3-II in autolysosomal lumen is degraded. Thus, lysosomal turnover of the autophagosomal marker LC3-II reflects autophagic activity (Streeter et al., 2016). p62 is the bridge between LC3 and the substrate to be degraded, which can specifically enrich the substrate to autophagosome and finally fuse with lysosome to form autophagy lysosome, which can be cleared. At the same time, p62 itself is also degraded as autophagy substrate. Therefore, the increase of p62 level is generally regarded as a sign that autophagy activity is inhibited or autophagy pathway is not smooth. Data on the relationship between PFOS and autophagy are very limited. More studies have been conducted based on in vitro experiments to demonstrate that the mechanism of PFOS toxicity may be related to autophagy. In the present study, the effect of PFOS exposure on autophagy was demonstrated in an in vivo experiment. In fact, our results showed that the expression of LC3 protein increased and the expression of p62 protein decreased in the hippocampus and cortex of mice exposed to PFOS for 45 days, suggesting that autophagy pathway was activated in mice exposed to PFOS.Transmission electron microscopy showed that a small amount of autophagic lysosomes appeared in PFOS-exposed hippocampal neurons. Therefore, it is not clear how the autophagy pathway is regulated in PFOS toxicity, which needs further attention and exploration.
The limitations of current studies indicate that the following should be explored in the future. First, dose-dependent effects of PFOS were not shown in behavioral tests, pointing to a complex dose-dependent effect of PFOS. The dose of PFOS used in animal models is often much higher than doses in the environment, and the relatively short exposure time cannot reflect the actual physiological and pathological change in animals. Therefore, low-dose and long-term chronic toxicity studies are needed to appropriately reflect the toxic effects of PFOS in humans, which is essential for evaluating the health risk of PFOS. Another potential limitation is that the present results show that a decrease in locomotion has been observed in EPM and OFT, it is uncertain if this has an effect on the determination of subsequent results, which suggests that a more complete and accurate investigation is needed. Additionally, the effect of PFOS exposure on autophagy also needs to be explored to elucidate the mechanism of PFOS-induced learning and memory decline. We have not yet determined whether the possible cause of these behavioral changes is related to the autophagy overactivation caused by PFOS exposure or the blocked autophagic lysosomal degradation pathway. So we need to use autophagy inhibitors or observe autophagy flow to further explore in future.
In summary, exposure to PFOS for 45 days causes cognitive impairment, anxiety, neuronal degeneration and decreased synaptic density in mice accompanied by the expression alteration of autophagy-related proteins. This further suggests that the autophagy activation might be an important aspect in PFOS-induced neurotoxicity.
ACKNOWLEDGMENTS
This study was supported by Research Fund for Hubei Province Key Laboratory of Occupational Hazard Identification and Control, Wuhan University of Science and Technology (OHIC2020K01).
Conflict of interest
The authors declare that there is no conflict of interest.
REFERENCES
- Batool, S., Raza, H., Zaidi, J., Riaz, S., Hasan, S. and Syed, N.I. (2019): Synapse formation: from cellular and molecular mechanisms to neurodevelopmental and neurodegenerative disorders. J. Neurophysiol., 121, 1381-1397.
- Chen, N., Li, J., Li, D., Yang, Y. and He, D. (2014): Chronic exposure to perfluorooctane sulfonate induces behavior defects and neurotoxicity through oxidative damages, in vivo and in vitro. PLoS One, 9, e113453.
- Chen, X., Nie, X., Mao, J., Zhang, Y., Yin, K. and Jiang, S. (2018): Perfluorooctanesulfonate induces neuroinflammation through the secretion of TNF-α mediated by the JAK2/STAT3 pathway. Neurotoxicology, 66, 32-42.
- Cousin, M.A. (2021): Synaptophysin-dependent synaptobrevin-2 trafficking at the presynapse-Mechanism and function. J. Neurochem., 159, 78-89.
- Cutuli, D., Landolfo, E., Petrosini, L. and Gelfo, F. (2022): Environmental Enrichment Effects on the Brain-Derived Neurotrophic Factor Expression in Healthy Condition, Alzheimer’s Disease, and Other Neurodegenerative Disorders. J. Alzheimers Dis., 85, 975-992.
- D’Arcy, M.S. (2019): Cell death: a review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int., 43, 582-592.
- Dong, X., Yang, J., Nie, X., Xiao, J. and Jiang, S. (2016): Perfluorooctane sulfonate (PFOS) impairs the proliferation of C17.2 neural stem cells via the downregulation of GSK-3β/β-catenin signaling. J. Appl. Toxicol., 36, 1591-1598.
- Dong, Z., Wang, J., Qiu, T., Wu, J., An, Y., Shi, X., Sun, X., Jiang, L., Liu, X., Yang, G., Cao, J. and Yao, X. (2022): Perfluorooctane sulfonate induces mitochondrial calcium overload and early hepatic insulin resistance via autophagy/detyrosinated alpha-tubulin-regulated IP3R2-VDAC1-MICU1 interaction. Sci. Total Environ., 825, 153933.
- Eke, D., Çelik, A., Yilmaz, M.B., Aras, N., Kocatürk Sel, S. and Alptekin, D. (2017): Apoptotic gene expression profiles and DNA damage levels in rat liver treated with perfluorooctane sulfonate and protective role of curcumin. Int. J. Biol. Macromol., 104 (Pt A), 515-520.
- Evich, M.G., Davis, M.J., McCord, J.P., Acrey, B., Awkerman, J.A., Knappe, D.R., Lindstrom, A.B., Speth, T.F., Tebes-Stevens, C., Strynar, M.J., Wang, Z., Weber, E.J., Henderson, W.M. and Washington, J.W. (2022): Per- and polyfluoroalkyl substances in the environment. Science, 375, eabg9065.
- Gaballah, S., Swank, A., Sobus, J.R., Howey, X.M., Schmid, J., Catron, T., McCord, J., Hines, E., Strynar, M. and Tal, T. (2020): Evaluation of Developmental Toxicity, Developmental Neurotoxicity, and Tissue Dose in Zebrafish Exposed to GenX and Other PFAS. Environ. Health Perspect., 128, 47005.
- Hallgren, S., Fredriksson, A. and Viberg, H. (2015): More signs of neurotoxicity of surfactants and flame retardants - Neonatal PFOS and PBDE 99 cause transcriptional alterations in cholinergic genes in the mouse CNS. Environ. Toxicol. Pharmacol., 40, 409-416.
- Harada, K.H., Ishii, T.M., Takatsuka, K., Koizumi, A. and Ohmori, H. (2006): Effects of perfluorooctane sulfonate on action potentials and currents in cultured rat cerebellar Purkinje cells. Biochem. Biophys. Res. Commun., 351, 240-245.
- Harris, M.H., Oken, E., Rifas-Shiman, S.L., Calafat, A.M., Ye, X., Bellinger, D.C., Webster, T.F., White, R.F. and Sagiv, S.K. (2018): Prenatal and childhood exposure to per- and polyfluoroalkyl substances (PFASs) and child cognition. Environ. Int., 115, 358-369.
- Ishida, K., Tsuyama, Y., Sanoh, S., Ohta, S. and Kotake, Y. (2017): Perfluorooctane sulfonate induces neuronal vulnerability by decreasing GluR2 expression. Arch. Toxicol., 91, 885-895.
- Jian, J.M., Chen, D., Han, F.J., Guo, Y., Zeng, L., Lu, X. and Wang, F. (2018): A short review on human exposure to and tissue distribution of per- and polyfluoroalkyl substances (PFASs). Sci. Total Environ., 636, 1058-1069.
- Klionsky, D.J., Petroni, G., Amaravadi, R.K., Baehrecke, E.H., Ballabio, A., et al. (2021): Autophagy in major human diseases. EMBO J., 40, e108863.
- Lau, C. (2012): Perfluorinated compounds. Experientia Suppl., 101 (Suppl), 47-86.
- Li, D., Jiang, L., Hong, Y. and Cai, Z. (2021): Multilayered glycoproteomic analysis reveals the hepatotoxic mechanism in perfluorooctane sulfonate (PFOS) exposure mice. Environ. Pollut., 268 (Pt A), 115774.
- Li, Z., Liu, Q., Liu, C., Li, C., Li, Y., Li, S., Liu, X. and Shao, J. (2017): Evaluation of PFOS-mediated neurotoxicity in rat primary neurons and astrocytes cultured separately or in co-culture. Toxicol. In Vitro, 38, 77-90.
- Liang, L., Pan, Y., Bin, L., Liu, Y., Huang, W., Li, R. and Lai, K.P. (2022): Immunotoxicity mechanisms of perfluorinated compounds PFOA and PFOS. Chemosphere, 291, 132892.
- Liao, C.Y., Li, X.Y., Wu, B., Duan, S. and Jiang, G.B. (2008): Acute enhancement of synaptic transmission and chronic inhibition of synaptogenesis induced by perfluorooctane sulfonate through mediation of voltage-dependent calcium channel. Environ. Sci. Technol., 42, 5335-5341.
- Liu, D., Liu, N.Y., Chen, L.T., Shao, Y., Shi, X.M. and Zhu, D.Y. (2020): Perfluorooctane sulfonate induced toxicity in embryonic stem cell-derived cardiomyocytes via inhibiting autophagy-lysosome pathway. Toxicol. In Vitro, 69, 104988.
- Liu, X., Jin, Y., Liu, W., Wang, F. and Hao, S. (2011): Possible mechanism of perfluorooctane sulfonate and perfluorooctanoate on the release of calcium ion from calcium stores in primary cultures of rat hippocampal neurons. Toxicol. In Vitro, 25, 1294-1301.
- Long, Y., Wang, Y., Ji, G., Yan, L., Hu, F. and Gu, A. (2013): Neurotoxicity of perfluorooctane sulfonate to hippocampal cells in adult mice. PLoS One, 8, e54176.
- Mariussen, E. (2012): Neurotoxic effects of perfluoroalkylated compounds: mechanisms of action and environmental relevance. Arch. Toxicol., 86, 1349-1367.
- Mshaty, A., Haijima, A., Takatsuru, Y., Ninomiya, A., Yajima, H., Kokubo, M., Khairinisa, M.A., Miyazaki, W., Amano, I. and Koibuchi, N. (2020): Neurotoxic effects of lactational exposure to perfluorooctane sulfonate on learning and memory in adult male mouse. Food Chem. Toxicol., 145, 111710.
- Nieto, R., Kukuljan, M. and Silva, H. (2013): BDNF and schizophrenia: from neurodevelopment to neuronal plasticity, learning, and memory. Front. Psychiatry, 4, 45.
- Ninomiya, A., Mshaty, A., Haijima, A., Yajima, H., Kokubo, M., Khairinisa, M.A., Ariyani, W., Fujiwara, Y., Ishii, S., Hosoi, N., Hirai, H., Amano, I. and Koibuchi, N. (2022): The neurotoxic effect of lactational PFOS exposure on cerebellar functional development in male mice. Food Chem. Toxicol., 159, 112751.
- Oh, H., Lewis, D.A. and Sibille, E. (2016): The Role of BDNF in Age-Dependent Changes of Excitatory and Inhibitory Synaptic Markers in the Human Prefrontal Cortex. Neuropsychopharmacology, 41, 3080-3091.
- Pan, W., Han, S., Kang, L., Li, S., Du, J. and Cui, H. (2016): Effects of dihydrotestosterone on synaptic plasticity of the hippocampus in mild cognitive impairment male SAMP8 mice. Exp. Ther. Med., 12, 1455-1463.
- Picó, Y., Farré, M., Llorca, M. and Barceló, D. (2011): Perfluorinated compounds in food: a global perspective. Crit. Rev. Food Sci. Nutr., 51, 605-625.
- Pinkas, A., Slotkin, T.A., Brick-Turin, Y., Van der Zee, E.A. and Yanai, J. (2010): Neurobehavioral teratogenicity of perfluorinated alkyls in an avian model. Neurotoxicol. Teratol., 32, 182-186.
- Qiu, T., Chen, M., Sun, X., Cao, J., Feng, C., Li, D., Wu, W., Jiang, L. and Yao, X. (2016): Perfluorooctane sulfonate-induced insulin resistance is mediated by protein kinase B pathway. Biochem. Biophys. Res. Commun., 477, 781-785.
- Savioz, A., Leuba, G. and Vallet, P.G. (2014): A framework to understand the variations of PSD-95 expression in brain aging and in Alzheimer’s disease. Ageing Res. Rev., 18, 86-94.
- Shin, H.M., Bennett, D.H., Calafat, A.M., Tancredi, D. and Hertz-Picciotto, I. (2020): Modeled prenatal exposure to per- and polyfluoroalkyl substances in association with child autism spectrum disorder: A case-control study. Environ. Res., 186, 109514.
- Streeter, A., Menzies, F.M. and Rubinsztein, D.C. (2016): LC3-II Tagging and Western Blotting for Monitoring Autophagic Activity in Mammalian Cells. Methods Mol. Biol., 1303, 161-170.
- Sun, P., Nie, X., Chen, X., Yin, L., Luo, J., Sun, L., Wan, C. and Jiang, S. (2018): Nrf2 Signaling Elicits a Neuroprotective Role Against PFOS-mediated Oxidative Damage and Apoptosis. Neurochem. Res., 43, 2446-2459.
- Taylor, M.A., Das, B.C. and Ray, S.K. (2018): Targeting autophagy for combating chemoresistance and radioresistance in glioblastoma. Apoptosis, 23, 563-575.
- Tran, S., Fairlie, W.D. and Lee, E.F. (2021): BECLIN1: Protein Structure, Function and Regulation. Cells, 10.
- Wang, R. and Holsinger, R.M. (2018): Exercise-induced brain-derived neurotrophic factor expression: therapeutic implications for Alzheimer’s dementia. Ageing Res. Rev., 48, 109-121.
- Wang, Y., Liu, W., Zhang, Q., Zhao, H. and Quan, X. (2015): Effects of developmental perfluorooctane sulfonate exposure on spatial learning and memory ability of rats and mechanism associated with synaptic plasticity. Food Chem. Toxicol., 76, 70-76.
- Wang, Y., Wang, L., Chang, W., Zhang, Y., Zhang, Y. and Liu, W. (2019): Neurotoxic effects of perfluoroalkyl acids: neurobehavioral deficit and its molecular mechanism. Toxicol. Lett., 305, 65-72.
- Wang, Y., Zhong, Y., Li, J., Zhang, J., Lyu, B., Zhao, Y. and Wu, Y. (2018): Occurrence of perfluoroalkyl substances in matched human serum, urine, hair and nail. J. Environ. Sci. (China), 67, 191-197.
- Wen, L.L., Chen, Y.T., Lee, Y.G., Ko, T.L., Chou, H.C. and Juan, S.H. (2021): Perfluorooctane sulfonate induces autophagy-associated apoptosis through oxidative stress and the activation of extracellular signal-regulated kinases in renal tubular cells. PLoS One, 16, e0245442.
- Xing, J., Wang, G., Zhao, J., Wang, E., Yin, B., Fang, D., Zhao, J., Zhang, H., Chen, Y.Q. and Chen, W. (2016): Toxicity assessment of perfluorooctane sulfonate using acute and subchronic male C57BL/6J mouse models. Environ. Pollut., 210, 388-396.
- Xu, J., de Winter, F., Farrokhi, C., Rockenstein, E., Mante, M., Adame, A., Cook, J., Jin, X., Masliah, E. and Lee, K.F. (2016): Neuregulin 1 improves cognitive deficits and neuropathology in an Alzheimer’s disease model. Sci. Rep., 6, 31692.
- Yao, X., Sha, S., Wang, Y., Sun, X., Cao, J., Kang, J., Jiang, L., Chen, M. and Ma, Y. (2016): Perfluorooctane Sulfonate Induces Autophagy-Dependent Apoptosis through Spinster 1-Mediated lysosomal-Mitochondrial Axis and Impaired Mitophagy. Toxicol. Sci., 153, 198-211.
- Yao, X.F., Cao, J., Xu, L.M., Sun, X.C., Kang, J., Yang, G., Jiang, L.P., Geng, C.Y., Gao, C.Z., Zhong, L.F. and Ma, Y.F. (2014): Perfluorooctane sulfonate blocked autophagy flux and induced lysosome membrane permeabilization in HepG2 cells. Food Chem. Toxicol., 67, 96-104.
- Yin, J., Jian, Z., Zhu, G., Yu, X., Pu, Y., Yin, L., Wang, D., Bu, Y. and Liu, R. (2021): Male reproductive toxicity involved in spermatogenesis induced by perfluorooctane sulfonate and perfluorooctanoic acid in Caenorhabditis elegans. Environ. Sci. Pollut. Res. Int., 28, 1443-1453.