2024 Volume 49 Issue 12 Pages 531-541
2024 Volume 49 Issue 12 Pages 531-541
Numerous studies have confirmed that the apoptosis induced by the methacrylate resin monomers triethyleneglycol-dimethacrylate (TEGDMA), 2-hydroxy ethyl methacrylate (HEMA), etc., in pulp cells and odontoblast-like cells is caused mainly by oxidative stress (OS). Reactive oxygen species (ROS), recognized as the most important risk factor for apoptosis in cells of the pulp–dentin complex, are produced mainly via the mitochondrial respiratory chain. When the free resin monomers in the oral cavity and pulp reach a toxic level, the monomers induce oxidative DNA damage, activate ATM-p53 in the nucleus, and mediate the intrinsic apoptotic pathway in the presence of Bcl-2 family proteins. A vicious cycle is established between OS cellular responses and abnormalities in mitochondrial dynamics that accelerate apoptosis. Despite numerous products generated via iteration, complete polymerization of resin monomers is not currently possible. The cytotoxicity of free monomers may lead to adverse reactions, such as pulp sensitivity. This review is based on the most important papers describing the roles of resin monomers in mediating apoptosis in the pulp–dentin complex and provides an overview of the precise mechanisms related to mitochondrion-mediated cytotoxicity, suggesting ways to reduce or eliminate their cytotoxicity in the future through advancements in material technology.
Commercial dental resin composites (DRCs) typically consist of 70~80 wt% inorganic particles and 20~30 wt% resin monomers (Barszczewska-Rybarek et al., 2021; Moharamzadeh et al., 2007; Szczesio-Wlodarczyk et al., 2021; Y. Wang et al., 2021). Since the beginning of the twenty-first century, the dominant resin monomers have consisted of methacrylates; more specifically, the following four main groups have been dominant: bisphenol A-glycidyl methacrylate (BisGMA), triethyleneglycol-dimethacrylate (TEGDMA), 2-hydroxy ethyl methacrylate (HEMA) and urethane dimethacrylate (UDMA) (Mulligan et al., 2022). Scholars have identified the prevalence of TEGDMA and HEMA by characterizing the composition of the resin monomers in different composite resin eluates (De Angelis et al., 2022; Jiang et al., 2023; Liu et al., 2019; Lovász et al., 2021). These resin monomers reach the pulp, affecting reparative dentin production and inducing chronic pulpitis (Galler et al., 2011; Schweikl et al., 2006; Spagnuolo et al., 2004). Since the pulp‒dentin complex consists of odontoblasts, odontoblast-like cells, and pulp stem cells, an increasing number of studies have focused on the toxicity of resin monomers to the pulp‒dentin complex. Recent studies on free monomers in composite resins are based largely on TEGDMA and HEMA (Aalto-Korte et al., 2007; Baldion et al., 2020; Jiang et al., 2023; Sloan and Smith, 2007).
In the oral environment, none of the currently developed resin monomers can be fully polymerized. In addition, the monomers in polymerized resins can free up under the influence of tooth wear (Drummond, 2008), the enzymatic action of saliva (Ferracane, 2006; Sideridou et al., 2004), ion release at the interface between the restorative material and dental tissue (Stanislawski et al., 2003), and bacterial proliferation (Bakopoulou et al., 2009; Schweikl et al., 2006). Approximately 1.5–5% of the methacrylate acid group of monomers remain free (Lempel et al., 2016; Stanislawski et al., 1999). Free monomers can penetrate through residual dentin of insufficient thickness, leading to potentially irritating and cytotoxic effects (Hadjichristou et al., 2021). Second, unbound monomers favor bacterial proliferation and contribute to the development of secondary caries (Bergenholtz, 2000; Khalichi et al., 2004). In addition, fillings may cause more than local hypersensitivity, resulting in rapid diffusion via interactions with odontoblast cell membrane phospholipids, as the released monomers may exert corresponding toxic effects on surrounding tissues (Geurtsen- and Leyhausen, 2001; Krifka et al., 2013; Martinez-Gonzalez et al., 2023; Salehi et al., 2015). In addition, systemic reactions involving these monomers have been reported (Goldberg, 2008). This paper focuses on the first point, the potentially irritating and cytotoxic effects of monomers on cells.
Cytotoxicity studies of resin monomers have shown that the extent of their cytotoxicity is based on the number of monomers released into the pulp. Due to the lack of standardized quantitative methods and the inconsistent presentation of results, accurately determining the amount of diffusible monomers released into the pulp is difficult (Van Landuyt et al., 2011). Notably, even low concentrations can exert a considerable influence on the pulp–dentin complex, such as interference with dentin regeneration or severe pulp inflammation (Bouillaguet et al., 2000, 1996; Noda et al., 2002b, 2002a).
The signaling pathways that mediate apoptosis can be divided into three main categories: the mitochondrial pathway (also called the intrinsic pathway or caspase-dependent pathway) (Bano and Prehn, 2018; Yeh et al., 2015), the death receptor pathway (also called the extrinsic pathway or caspase-independent apoptotic pathway) (Salehi et al., 2015), and the endoplasmic reticulum pathway (Batarseh et al., 2014; Szegezdi et al., 2003). These pathways engage in crosstalk with each other to coregulate the apoptotic process. Mitochondria, as the most important organelles for cellular energy production, are considered the core components of the apoptosis mechanism mediated through signaling and regulating cellular energy metabolism to determine the survival and death of cells. Through the mitochondrial pathway, resin monomers can induce oxidative damage in the pulp–dentin complex, leading to apoptosis.
When commercially available light-curing resins for dentistry undergo a polymerization reaction, the double bonds of most of the monomers are opened, and a three-dimensional crosslinked polymer is formed from the methacrylates connected by covalent bonds. However, this polymerization is not complete. Resin monomers in the free state retain their original chemical structures. They usually have lipophilic functional groups that easily penetrate cell membranes. They are able to act directly on membrane lipids and mitochondria, inducing the overproduction of ROS and leading to oxidative stress by affecting the redox balance of cells. For example, the structure of a common resin monomer such as bisphenol A-glycidyl methacrylate (BisGMA) contains two aromatic rings, two ester groups, and two acrylate double bonds.
Aromatic rings, through their hydrophobicity and π-electron off-domain properties, are able to interact with the lipid layer in the cell membrane, thereby altering the fluidity and structure of the membrane, and this interaction may disrupt the integrity of the cell membrane (Vom Saal and Vandenberg, 2021). The ester groups (C=O) contain a carbonyl carbon atom and have nucleophilic attack properties. When the ester groups react with cellular proteins and enzymes (e.g., antioxidant enzymes), they may alter the normal function of the enzyme by reacting with its active site. Such reactions typically involve covalent binding of the ester groups to nucleophilic groups of specific amino acid residues (e.g., serine or cysteine) to form stable reaction intermediates (Behrens et al., 2011). This interaction may interfere with the activity of antioxidant enzymes and indirectly increase ROS levels, which in turn can trigger cellular damage (Feral et al., 2023). Acrylate double bonds (C=C) are capable of reacting with free radicals in the body, especially in the case of incomplete polymerization. The double bonds in the resin monomer not only act as free radical trapping agents but also may initiate free radical chain reactions, which can further generate reactive oxygen species such as peroxides, superoxide anions and hydroxyl radicals. These reactive oxygen species have strong oxidizing properties and can react with other chemicals, thus affecting the performance and stability of the resin (Fukumoto et al., 2013).
The mitochondrial ETC, also known as the respiratory chain, is an exergonic oxidation process of nutrients. Cellular oxidative damage is triggered when the balance of the mitochondrial respiratory complex, which is in the inner mitochondrial membrane, is disrupted. Mitochondrial ROS (MtROS), which are produced via aerobic respiration and oxidizing free radicals, have been recognized as major sources of intracellular ROS. MtROS are produced mainly via oxidative phosphorylation (OXPHOS), with the notable involvement of mitochondrial respiratory chain complexes I and III (Sb et al., 2019).
TEGDMA inhibits Complex I-dependent (CI-dependent) O2 depletion in pulp cells, leading to the depolarization of the mitochondrial membrane and a reduction in ATP synthesis. This depolarization causes mitochondrial dysfunction and is an early marker of toxicity mediated by caspase-induced apoptosis (Spinelli and Haigis, 2018). In addition, the inhibition of CI-induced O2 depletion by TEGDMA increases mitochondrial hydrogen peroxide production and hinders ROS removal (Mikulás et al., 2020, 2018). TEGDMA stimulation of primary odontoblasts inhibits Complex III (CIII) activity, leading to an increase in ROS levels in response to reduced CIII activity and their involvement in mitochondrial oxidative stress. Additionally, TEGDMA reduces the protein expression of CV and inhibits the activation of citrate synthase. Deficiencies in these two enzymes, which are essential factors for ATP synthesis, affect ATP production and increase superoxide formation to promote apoptosis (Sb et al., 2019).
OS is a key mechanism by which TEGDMA induces apoptosis in pulp cells and preodontoblasts (Krifka et al., 2013; Schweikl et al., 2006; Wang et al., 2021b). Recently, SB H et al. discovered that TEGDMA significantly contributed to mtROS production. They also showed that the use of the mitochondrion-targeted antioxidant MitoQ to remove mtROS significantly increased cell viability (Sb et al., 2019). Yu Q et al. reported that melatonin protects against TEGDMA-induced preodontoblast mitochondrial apoptosis via the JNK/MAPK signaling pathway (Yu et al., 2024). Wang K et al. identified that notoginsenoside R1 alleviates TEGDMA-induced mitochondrial apoptosis in preodontoblasts through the activation of Akt/Nrf2 pathway-dependent mitophagy (Wang et al., 2021a). These findings suggest that mtROS play key roles in the apoptosis induced by the resin monomer TEGDMA in preodontoblasts under oxidative stress conditions. Notably, although insufficient evidence has been found in the dentin‒pulp complex, in other types of cells, resin monomers stimulate excess mtROS production by mitochondria, resulting in cellular stress and apoptosis, which are due to the imbalance of cell redox homeostasis. It is, at least to some extent, the result of the depletion of the intracellular antioxidant system (Eckhardt et al., 2009; Engelmann et al., 2004; Krifka et al., 2013; Morisbak et al., 2020; Paranjpe et al., 2009; Schweikl et al., 2007; Stanislawski et al., 2003). The complex mechanism of interaction between these antioxidant molecules, such as glutathione (GSH), N-acetyl cysteine (NAC), catalase (CAT), superoxide dismutase (SOD) and glutathione peroxidase (GPx), remains to be further explored.
MtROS production depends not only on the redox state of the ETC but also on the proton motive force (pmf). The pmf is generated in the mitochondrial membrane and consists of both an electrical gradient called Δψ and a chemical gradient called pH. Notably, the electrical gradient is also known as the mitochondrial membrane potential (MMP). As electrons are transferred through the ETC, complexes I, III, and IV push protons out of the mitochondrial matrix into the intermembrane space (IMS), thereby changing the pmf. When the pmf is increased, the production of mtROS is increased, creating positive feedback (Scialo and Sanz, 2021).
Resin monomers induce oxidative damage to nuclear DNAResin monomers have been reported to cause cellular DNA damage and apoptosis in various cell types (Ansteinsson et al., 2011; Shehata et al., 2013; Yang et al., 2019). 8-oxo-7,8-dihydroguanine (8-oxoGua) is commonly regarded as a biomarker of oxidative DNA damage (Eckhardt et al., 2009; Gedik et al., 2005; Styllou et al., 2017), and as a result, Harry S found that methacrylate monomers induced double-strand breaks (DSBs) in human gingival fibroblasts (Styllou et al., 2015). Helmut Schweikl revealed indirect HEMA-induced DNA damage for the first time by analyzing the cell cycle (Schweikl et al., 2014). Exposure to resin monomers led to a significant increase in the number of cells in G1 phase, and the number of these cells further increased under the action of the GSH synthesis inhibitor BSO, whereas the accompanying delay in the cell cycle was abolished by the action of the antioxidant NAC, which indicates that DNA damage is induced at least in part by monomer-induced ROS (Eckhardt et al., 2010; Styllou et al., 2017). Interestingly, however, the antioxidant Trolox was unable to offset the HEMA-induced cell cycle blockade, suggesting that DNA damage may not be related to increased oxidation (Ansteinsson et al., 2011). Researchers have speculated that, as previously discussed, the carbonyl portion of methacrylate can interact with nucleophilic centers in molecules such as DNA in a manner similar to glutathione undergoing the Michael addition reaction; namely, methacrylate may create, e.g., DNA adducts (monomeric-DNA adducts), and then intrastrain DNA cross-links may be formed in a process analogous to Michael addition reactions involving glutathione (Ansteinsson et al., 2013; Geurtsen- and Leyhausen, 2001; Samuelsen et al., 2011; Wang et al., 2021b).
The ataxia-telangiectasia mutated (ATM) protein is a serine–threonine protein kinase that is involved in the first step of the cellular response to a DSB (Eckhardt et al., 2009) and plays a central role in HEMA-induced apoptosis. Resin monomers have been shown to act on ATM downstream targets, such as phosphorylation of histone H2AX and activation of the cellular checkpoint kinase Chk2 (Styllou et al., 2017; Szczepanska et al., 2012; Yang et al., 2018).
In summary, resin monomers cause DNA oxidative damage largely indirectly through the generation of ROS or possibly directly through covalent binding to the nucleophilic center of double-stranded DNA. DSBs, one of the most serious consequences of DNA damage, exert an inhibitory effect on the downstream effector molecule ATM, blocking or delaying the cell cycle and activating programmed cell death (Jones and Petermann, 2012; Roos and Kaina, 2013).
The ATM-dependent signaling pathway triggered by DNA oxidative damage induces nuclear p53 expression (Krifka et al., 2011; Morisbak et al., 2015; Samuelsen et al., 2008; Schweikl et al., 2014). It has been shown that p53 can promote the transcription and synthesis of DNA repair proteins. For example, in TEGDMA-treated cells, p53 promotes the expression of the downstream target gene p21 and activates the defense mechanism, causing the cells to remain in G1 phase and enabling DNA damage repair, thereby preventing further replication of faulty DNA (Krifka et al., 2011; Wang et al., 2022). However, the extent to which this mechanism of DNA repair can inhibit the toxicity caused by resin monomers to the pulp–dentin complex still needs to be further explored.
In unstressed cells, p53 is conjugated to Bcl-xl or BCL-2 to form inactivating complexes that inhibit the action of the apoptosis antagonists Bcl-2 and Bcl-xl (Wei et al., 2021). When resin monomers are sufficiently stimulated to reach a toxic concentration, p53 in the nucleus activates the transcription of the apoptosis-promoting proteins Bax, Bak, Bad, Bid, Puma, Noxa, etc., and downregulates the expression of antiapoptotic proteins, such as Bcl-2, Bcl-xl, and Mcl1 (Schweikl et al., 2014). Although antiapoptotic proteins in the cytoplasm are sufficient to inhibit p53 activity in the cytoplasm, proapoptotic proteins transcribed due to the action of p53 in the nucleus, e.g., PUMA, can directly antagonize apoptotic Bcl-2 family members to form, e.g., the Puma/Bcl-xl complex or Puma/BCL-2 complex, releasing p53 from the bound state and leading mainly to the expression of proapoptotic p53 (Yu and Zhang, 2008, p. 53).
Resin monomers activate the intrinsic apoptosis pathwayThe intrinsic apoptotic pathway, also known as mitochondrial apoptosis, is mediated mainly by the complex reciprocal effects of pro- and antiapoptotic proteins in the Bcl-2 family (Singh et al., 2019). Bcl-2 family proteins are classified into three different functional subfamilies. The first group consists of apoptosis-inhibiting proteins, including Bcl-2, Bcl-xl, and Mcl-1, which restrain cell death by binding to proapoptotic Bcl-2 proteins. The second group comprises proapoptotic proteins, including Bax and Bak, which directly contribute to mitochondrial outer membrane permeabilization (MOMP). The third group, known as the apoptosis initiator group, also includes proapoptotic proteins consisting of proteins containing only the highly conserved BH3 structural domain, including Bad, Bid, Bim, Puma, and Noxa. When they interact directly and activate Bax and Bak, they are called 'direct activators', and when they bind antiapoptotic proteins and replace direct activators, they are called 'sensitizers' (Chipuk et al., 2010). In the absence of apoptosis-inducing stress, the antiapoptotic proteins Bcl-2, Bcl-xl, etc., are located mainly on the outer mitochondrial membrane (OMM), whereas the majority of the proapoptotic proteins, e.g., Bax, Bad, and Bak, are found mainly in the cytoplasm and are shuttled between the cytoplasm and the OMM at different rates (Todt et al., 2015). The prosurvival proteins Bcl-2 or Bcl-xl form a heterodimer located on the OMM with the proapoptotic proteins Bax or Bak to maintain normal mitochondrial morphology and block apoptosis. After a cell receives an apoptotic signal, activated cytoplasmic p53 competes with Bax or Bak to bind the antiapoptotic proteins Bcl-2 and Bcl-xl in the heterodimer, thereby releasing Bax and Bak. Free Bax and Bak travel from the cytoplasm to the OMM and form oligomers, known as BAX/BAK dynamic lipid pores, which alter the MOMP and drive apoptosis (Dai et al., 2023; Edlich, 2018; Peña-Blanco and García-Sáez, 2018).
In HEMA-treated cells, an increase in ROS levels is closely associated with a significant effect on exacerbating the decreased MMP in mitochondria (Schweikl et al., 2014). Cellular ATP production cannot be separated from the oxidative phosphorylation of the ETC (OXPHOS) driven by the MMP. In TEGDMA-treated preodontoblasts, MOMP alterations lead to significant changes in the proton gradient across the inner mitochondrial membrane (IMM). During this time, the MMP disrupts ETC activity, leading to a decrease in ATP production, which triggers an increase in mtROS production that exacerbates oxidative cellular damage and contributes to apoptosis (Sb et al., 2019).
Alterations in the MMP result in the activation of the mitochondrial calcium uniporter (MCU), which can rapidly transport Ca2+ from the cytoplasm and endoplasmic reticulum into the mitochondrial matrix (Alevriadou et al., 2021). The influx of Ca2+ into mitochondria activates the machines that induce the posttranslational modification (PTM) of tricarboxylic acid cycle (TCA) dehydrogenases, which in turn promotes respiration and ROS production and exacerbates changes in the MMP (Wescott et al., 2019). Recently, IMM penetration due to a disruption of intracellular Ca2+ homeostasis and mitochondrial Ca2+ overload has also been shown to induce cell death through MOMP, which is regulated independently of Bcl-2 family proteins (Quarato et al., 2022).
The mitochondrial permeability transition pore (mPTP) is located between the IMM and OMM and consists of various proteins, such as Cyt C, OMI (also called HTRA2), and SMAC (also called DIABLO), whose molecular composition has not yet been fully characterized. Under normal physiological conditions, ATP synthase is driven by oxidative phosphorylation to maintain the MMP and intracellular and extracellular ionic homeostasis. However, when resin monomers induce a series of events, such as a decrease in the MMP, Ca2+ overload, an increase in ROS production, and glutathione oxidation, which disrupts MOMP, the permeability transition pore (PT) completely opens (Zorov et al., 2014), resulting in two outcomes. First, ionic homeostasis is disrupted, leading to an increase in the number of protons within the cytoplasm, a decrease in pH, calcium overload, oxidative phosphorylation uncoupling, a rapid decrease in ATP levels, and a series of apoptotic events. Second, the membrane is depolarized, reducing the MMP and causing the swelling of the mitochondrial matrix and further release of proteins from the IMS to result in cell death (Xu et al., 2020). Cyt C escapes from cristae into the IMS through BID-mediated cristae remodeling, followed by release into the cytoplasm through an OMM channel (Yamaguchi et al., 2008). These released Cyt C molecules bind to apoptotic peptidase-activating factor 1 (APAF1) in the cytoplasm to form apoptotic bodies and promote caspase-9 activation. SMAC and OMI inhibit antiapoptotic X-linked inhibitor of apoptosis protein (XIAP) (Duchen, 2004). As a key executor of apoptosis, the downstream protein caspase-3 is activated and translocated from the cytoplasm to the nucleus, where it undergoes substrate cleavage, triggering apoptosis (Alevriadou et al., 2021; Baldion et al., 2021; Quarato et al., 2022; Singh et al., 2019). The general principles by which resin monomers activate the intrinsic apoptosis pathway are illustrated in Fig. 1.

Resin monomers induce apoptosis by enhancing the positive feedback loop between mitochondrial ROS and abnormal mitochondrial dynamics, resulting in exacerbated mitochondrial dysfunction and cell apoptosis. RM: resin monomer; DSB: double-strand break; ATM: ataxia-telangiectasia mutated; ROS: reactive oxygen species; ETC: electron transport chain; pmf: proton motive force; MOMP: mitochondrial outer membrane permeabilization; MCU: mitochondrial calcium uniporter; TCA: tricarboxylic acid cycle; APAF1: apoptotic peptidase-activating factor 1.
Mitochondria maintain the dynamic balance of the mitochondrial network through continuous fission and fusion (Krifka et al., 2013, 2011; Sb et al., 2019; Wang et al., 2021b, 2021a). TEGDMA-induced apoptosis is associated with oxidative stress in mitochondria, mitochondrial dysfunction, and morphological abnormalities of mitochondria. These three processes promote each other, forming a vicious cycle and accelerating the process of apoptosis (Huang et al., 2018; Ježek et al., 2018; Sb et al., 2019; Zhang et al., 2016).
Dynamin-related protein 1 (Drp1) and fission 1 (Fis1) are proteins critical for mitochondrial fission. An increase in mitochondrial fission significantly affects the number and shape of mitochondria, respiratory enzyme activity and ATP production (Huang et al., 2015). In TEGDMA-stimulated preodontoblasts, mitochondrial OS activates Fis1, which recruits Drp1 from the cytoplasm to mitochondria, where Drp1 oligomers form and induce mitochondrial fission (Sb et al., 2019). In this process, Bax expression is increased, the MMP is decreased, and MOMP is increased (Xian and Liou, 2019). Drp1 and mtROS enhance each other, exacerbating mitochondrial dysfunction and promoting apoptosis (Sb et al., 2019).
Fusion can lead to the elimination of irreversibly damaged mitochondria to maintain mitochondrial homeostasis (Gao and Hu, 2021). It is controlled by optic atrophy 1 (Opa1), which mediates IMM fusion, and mitofusin-1 (Mfn1) and mitofusin-2 (Mfn2), which mediate OMM fusion (Malka et al., 2005; Pernas and Scorrano, 2016). Opa1 oligomers maintain tight cristae structures, and their two forms, L-Opa1 and S-Opa1, are essential for mitochondrial homeostasis. TEGDMA induces the rapid cleavage and accumulation of L-Opa1 to yield s-Opa1 in preodontoblasts, leading to mitochondrial rupture and the release of IMS proteins such as Cyt C, which causes apoptosis (Quintana-Cabrera et al., 2018; Sb et al., 2019). In addition, the hydrolytic cleavage of the Opa1 protein is correlated with OS, while the inhibition of Opa1 cleavage is essential for preventing ATP depletion-induced apoptosis (Sb et al., 2019). Thus, Opa1 may be a key protein in mitochondria during resin monomer-induced cellular OS, which leads to apoptosis. To date, the expression of Mfn1 and Mfn2 protein levels in resin monomer-treated cells has not been found to be significantly altered, and their importance needs to be further explored (Sb et al., 2019). The general principles of OS causing morphological changes in mitochondria to promote apoptosis are illustrated in Fig. 2.
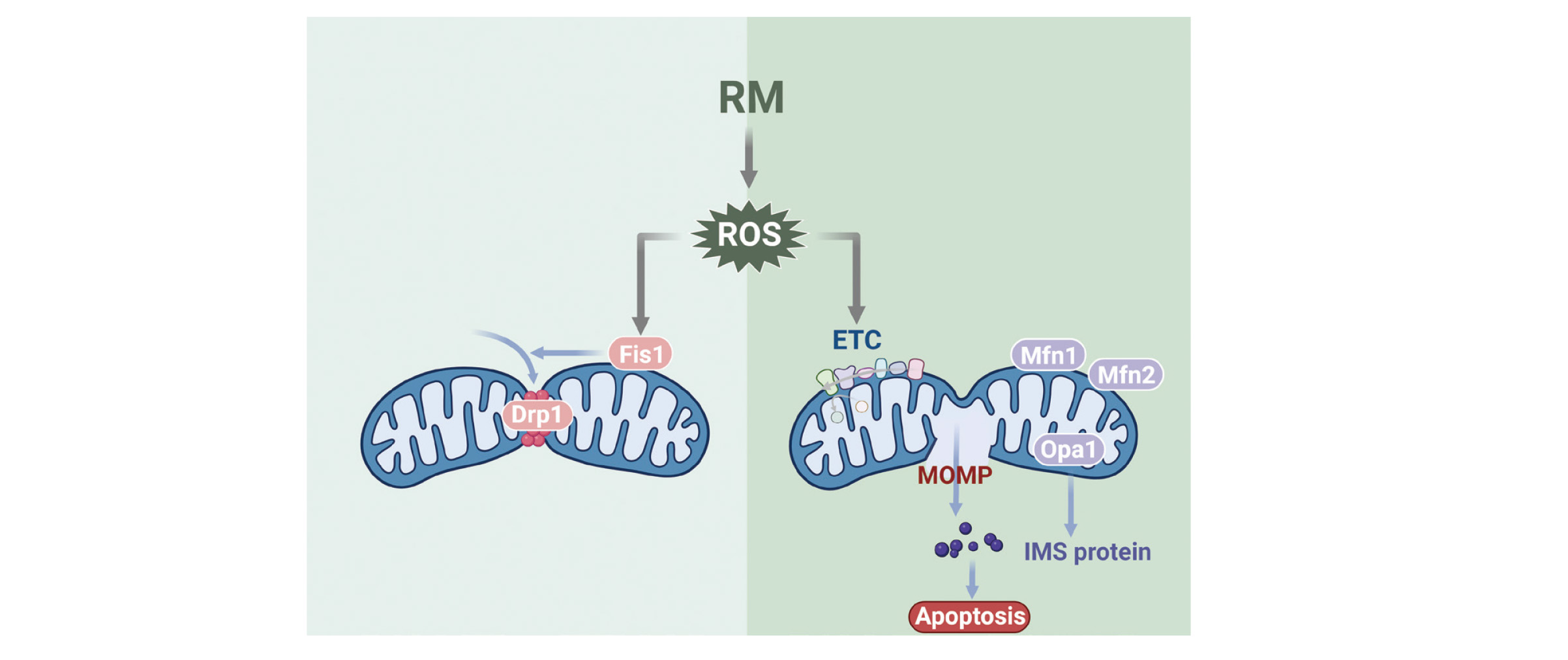
Resin monomers induce ROS production and cause morphological changes in mitochondria to promote apoptosis. Drp1: dynamin-related protein 1; Fis1: fission 1; Mfn1: mitofusin-1; Mfn2: mitofusin-2; Opa1: optic atrophy 1; IMS: intermembrane space.
The pulp‒dentin complex is a collective term for dentin and pulp tissue. It contains cells such as odontoblasts, fibroblasts, immune cells, vascular cells, nerve cells and mesenchymal stem cells (MSCs) or dental pulp stem cells (DPSCs), among others, and includes other components such as the dentin matrix and pulp matrix. Together, these proteins maintain the internal structure and function of the tooth and are involved in tooth repair and defense mechanisms (Gronthos et al., 2000; About, 2011; Diogenes, 2020). Apoptosis is the main manifestation of resin monomer-induced pulp damage, usually as a result of cytotoxicity (Gallorini et al., 2014). It leads directly or indirectly to cell loss, weakened tissue repair, increased oxidative stress and the activation of inflammatory responses.
In the previous section, we mentioned several times that resin monomers cause the apoptosis of multiple cells in the pulp‒dentin complex through mitochondria-mediated oxidative stress. On this basis, we would like to further elaborate on the multiple types of apoptosis-induced damage to the pulp‒dentin complex. First, we discuss the adverse consequences of apoptosis in terms of cell differentiation and mineralization capacity. The actions of HEMA and TEGDMA on human pulp stem cells result in a surge of MtROS, which lead to decreased abilities of human pulp stem cells to proliferate, migrate, and differentiate into odontoblasts and significantly reduced expression of mineralization-related genes (Bakopoulou et al., 2011; Tao et al., 2024). TEGDMA acts on pulp cells and downregulates their specific odontogenic cellular functions, including alkaline phosphatase activity, matrix mineralization capacity, and the expression of relevant mineralization genes (Galler et al., 2011). TEGDMA, HEMA, UDMA and BisGMA inhibit the differentiation of pulp fibroblasts to odontoblasts and suppress the expression of mineralization-related genes and matrix mineralization (About et al., 2002). Apoptosis of odontoblasts directly reduce the capacity of dentin to produce, mineralize, and repair dentin, and apoptosis may lead to damage to the supporting cells surrounding the nerve fibers of the pulp. All of the above processes have the potential to increase tooth sensitivity to external thermal and other stimuli and weaken tooth defenses. In addition, BPA, for example, disrupts the number and function of innate and adaptive immune cells, including decreasing the levels of Treg cells, anti-inflammatory cytokines, and chemokines, while increasing the levels of proinflammatory cytokines (Xu et al., 2013). The apoptosis of other cells in the pulp, such as fibroblasts and immune cells, also disrupts the structural integrity of the pulp tissue and the immune defense system, increasing the risk of pulp infection or lesions. Apoptosis is often accompanied by the activation of the inflammatory response, and prolonged pulp inflammation may trigger pulp necrosis, resulting in a failure to provide normal nutritional and supportive functions. In severe cases, this inflammation even spreads to the apical region and causes destruction, which may ultimately lead to a poor outcome in the form of root canal treatment or tooth extraction.
In conclusion, long-term apoptosis may lead to structural and holistic dysfunction of the pulp‒dentin complex, jeopardizing dental health. Therefore, exploring the mechanism of apoptosis induced by resin monomers is essential for developing potential preventive and therapeutic strategies, such as optimizing the original monomer composition, increasing the antioxidant content, and improving resin restorative materials (Zhu et al., 2015; Liu et al., 2017; Yu et al., 2024).
In summary, resin monomers usually contain lipophilic functional groups such as aromatic rings, ester groups, and acrylate double bonds, which make them susceptible to penetrating cell membranes and further affecting the redox balance of cells in the pulp‒dentin complex. Methacrylate resin monomers generate the vast majority of ROS in a cell through OXPHOS, which is mediated by the ETC. Resin monomers cause oxidative damage to DNA by binding directly to the nucleophilic center of DNA or indirectly through excessive ROS production. The DSB-ATM-p53 pathway is subsequently triggered, leading to the activation of two key proteins in the Bcl-2 family, BAX and BAK, in mitochondria. The BAX/BAK pore induces MOMP, which causes a decrease in the MMP, and proteins such as Cyt C are then released from the mitochondrial membrane into the cytoplasm. Cyt C activates caspase-3, which leads to apoptotic cell death. During this process, the ETC and pmf promote each other, leading to increased ROS generation. A change in membrane potential leads, on the one hand, to a disruption of the mitochondrial electron respiratory chain, a decrease in cellular ATP levels, and, ultimately, mitochondrial dysfunction. On the other hand, it also leads to Ca2+ influx, causing tricarboxylic acid cycle (TCA) dysfunction and leading to mPTP opening. In mitochondria, a vicious cycle among oxidative stress, mitochondrial dysfunction, and abnormal mitochondrial dynamics is thus generated, accelerating apoptosis.
Due to the incomplete polymerization of resin monomers, free-state peripulpal monomers may cause irreversible damage to the pulp–dentin complex, leading to apoptosis, resulting in pulp sensitivity and other adverse effects. Although recent toxicological studies on resin monomers are still limited, other important apoptotic proteins may be involved in the mitochondria-mediated apoptotic process and are awaiting discovery. Moreover, the extent to which crosstalk among different organelles affects the apoptotic process needs to be confirmed by further studies; in any case, our goal is to understand the precise mechanisms underlying cell death induced by resin monomers and to find strategies to reduce or eliminate their toxicity in the future through advances in material technology.
FundingThe study was supported by a grant from the Science and Technology Project of Guangzhou City (grant number: 201903010038).
Conflict of interestThe authors declare that there is no conflict of interest.