2024 Volume 49 Issue 4 Pages 139-149
2024 Volume 49 Issue 4 Pages 139-149
Busulfan is an anticancer drug known to cause serious damage to seminiferous tubules in the testes and deplete germ cells in human and animal models. The testicular artery is anastomosed with deferential and cremasteric arteries and is divided into capsular arteries, which give rise to the centripetal arteries and then recurrent arteries. The arterial blood in the testicular tissue is supplied by such a consequent system of arterial vessels, in order from the peripheral to the central area. As anticancer drugs are generally distributed throughout the whole body via the bloodstream and the running and distribution of arteries differ among the testicular areas, we hypothesized that the efficacy of busulfan differs in different testicular areas, particularly between the central and peripheral areas. In this study, busulfan was intraperitoneally injected at 40 mg/kg body weight into C57BL/6J male mice. After 28 days, in busulfan-treated mice, the diameters of seminiferous tubules were significantly higher in the central than in the peripheral area of the testes. The seminiferous tubular areas also significantly decreased in the peripheral areas compared with the central areas. The number of germ cells per seminiferous tubule was significantly higher in the central than in the peripheral area. Sertoli cell nuclei were detached into the lumen in the peripheral area. The number of Leydig cells was significantly lower in the peripheral areas. These data suggest that the effects of busulfan differ between the central and peripheral areas of the testis at 4 weeks after busulfan administration.
Busulfan, a bifunctional alkylating agent, is one of the anticancer drugs (Lawson et al., 2021; Chiorcea-Paquim and Oliveira-Brett, 2023; Qu et al., 2019). Clinically, it is often used as a major component of chemotherapy-based conditioning for hematopoietic stem cell transplantation (Palmer et al., 2016; Huezo-Diaz et al., 2014). Allogeneic hematopoietic stem cell transplantation is a potentially curative treatment for hematological malignancies and nonmalignant diseases (Hassan and Andersson, 2013). Busulfan is cytotoxic to hematopoietic precursors and pluripotent stem cells. Additionally, lymphotoxic agents, i.e., cyclophosphamide or fludarabine, are administered with busulfan for myeloablative conditioning (Palmer et al., 2016; Ben-Barouch et al., 2016). However, anticancer drugs often affect the male reproductive system (Smith et al., 2015; Qu et al., 2019). The testicular toxicity of busulfan was first reported in the 1960s (Jackson et al., 1962). Busulfan yields serious damage to seminiferous tubules of the testes and depletes germ cells in human and animal models (Smith et al., 2015; Haghi-Aminjan et al., 2018). It targets the dividing mitotic spermatogonia, resulting in germ cell apoptosis and requiring several weeks to completely ablate germ cells from the testis as the residual postmitotic stages complete spermatogenesis and postmitotic germ cells are released into the seminiferous tubule lumen (Smith et al., 2015). Because alkylating chemotherapy, using busulfan, cyclophosphamide, and melphalan, has destructive effects on spermatogenesis and results in germ cell depletion, spermatogenesis recovery or fertility preservation is important for patients who desire to bear children (Huleihel and Lunenfeld, 2020). After chemotherapy, testicular sperm extraction (TESE) or microdissection testicular sperm extraction (micro-TESE) is one of the options for patients and often is applied to extract fresh sperm (Ashizawa and Kanda, 2020; Hsiao et al., 2011; Dabaja and Schlegel, 2013).
Busulfan is intravenously and intraperitoneally administered in human and experimental animal models, respectively. After administration, conventional anticancer drugs are generally distributed throughout the whole body via the bloodstream, and they affect both malignant and rapidly dividing normal cells of the bone marrow, gut, and lymphoid tissue; spermatogenic cells, and hair follicles (Links and Brown, 1999). In the testes, it is anatomically considered that busulfan and its metabolites are transported through testicular arteries and spread to the whole organ. The running and distribution in testicular arteries of humans, mice, and rats have already been reported. The testicular artery that branched from the abdominal aorta is anastomosed with deferential and cremasteric arteries and divides into capsular arteries, running beneath the tunica albuginea (Dudea et al., 2010; Mostafa et al., 2008). The capsular arteries give rise to centripetal arteries and then to recurrent arteries (Polguj et al., 2010; Dudea et al., 2010). They pass through the septations of the testicular parenchyma and supply blood in intratesticular areas. The arterial blood in the testicular tissue is supplied by such a consequent system of arterial vessels, in order from the peripheral to the central area. However, whether such running and distribution of arteries in the testes have effects on the testicular toxicity of busulfan remains unclear.
Busulfan is an anticancer drug known to exhibit high toxicity toward the male reproductive system; because anticancer drugs are generally distributed via the bloodstream (Links and Brown, 1999) and the running and distribution of arteries differ among the testicular areas, we hypothesized that the efficacy of busulfan differs in different testicular areas, particularly between the central and peripheral areas. If busulfan is indeed more absorbed in specific areas of the testes in a preferential manner, their testicular areas should be avoided in TESE and micro-TESE. To prove this hypothesis, this study evaluated testicular histology and compared the toxic effects of busulfan on the testes between the central and peripheral areas in busulfan-administered model mice.
Five-week-old C57BL/6J male mice were purchased from CLEA Japan Inc., Tokyo, Japan. and housed under a 12-hr light/dark cycle at a controlled temperature (23°C ± 2°C). Standard chow and water were provided ad libitum. One week later, busulfan was administered. All experiments were conducted as per the Committee for Proper Experimental Animal Use and Welfare, a peer-review panel established by The National Institute of Health Sciences (experimental approval no. 816, August 2, 2021).
Busulfan administrationTo generate the mouse model of infertility, a single intraperitoneal (i.p.) dose of busulfan of >30–40 mg/kg body weight has commonly been selected (Nakata et al., 2020; Gutierrez et al., 2016; Wang et al., 2010). In the present study, a two-i.p. dose schedule was designed to prevent the increase in the mortality rate of mice (Xie et al., 2020; Yokota et al., 2023). Then, the mice were randomly divided into two groups (6 per group). Busulfan (B-2635; Sigma–Aldrich, St. Louis, MO, USA) was dissolved in dimethyl sulfoxide (DMSO; 031-24051; CultureSure®; FUJIFILM Wako Pure Chemical Industries, Ltd., Osaka, Japan) at a 10 mg/mL concentration and was gradually added to nine times the saline volume (Otsuka Pharmaceutical Co., Tokyo, Japan) (final concentration: 1 mg/mL). Busulfan was intraperitoneally injected (twice daily at 20 mL/kg body weight) with a 3-hr interval between injections (01:00 pm and 04:00 pm, total dose of 40 mg/kg body weight). The control mice were injected with a vehicle containing 10% DMSO in saline. Several studies reported severe germ cell depletion in mice and rats 4–5 weeks after the administration of busulfan at 30–40 mg/kg body weight. In this study, after 28 days, the mice were deeply anesthetized with isoflurane and then euthanized. Testis samples were then collected (Fig. 1).
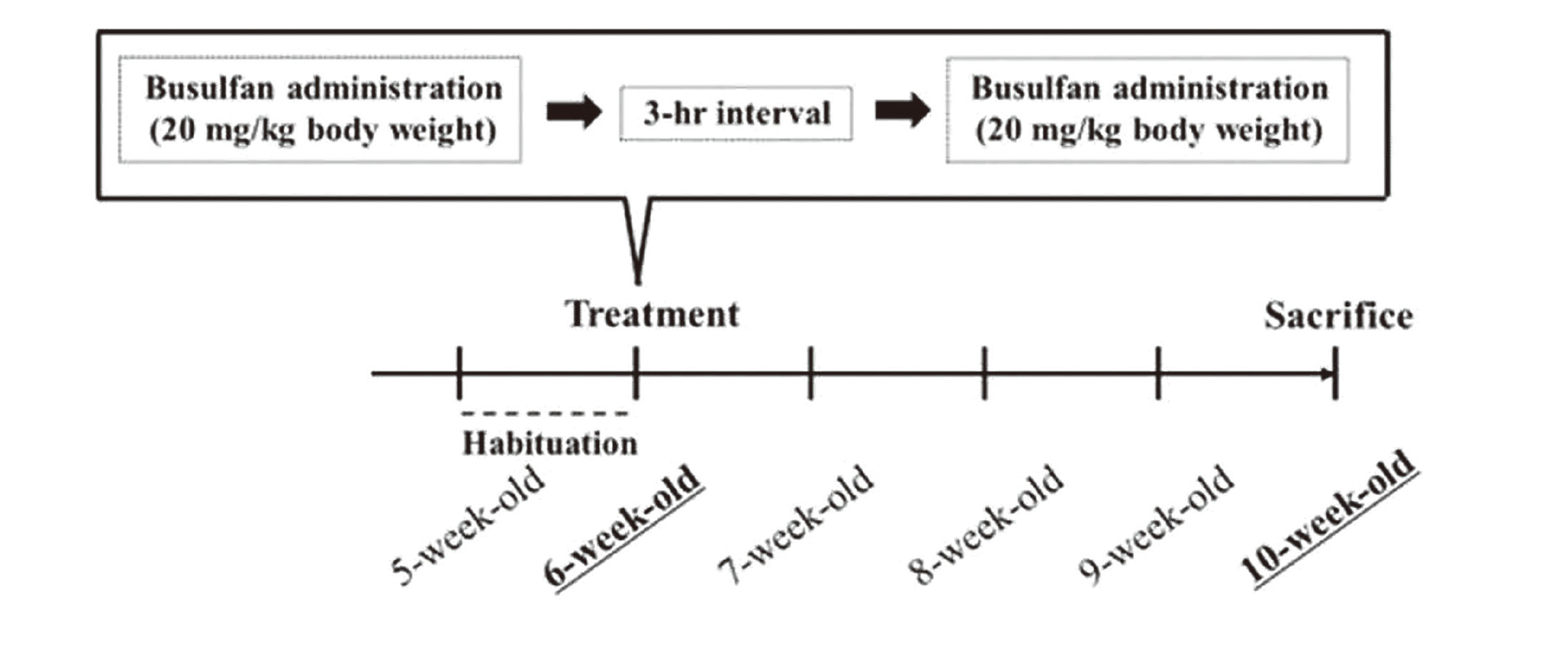
Schematic overview of busulfan administration. After habituation, busulfan was intraperitoneally injected (twice daily at 20 mL/kg body weight, a total dose of 40 mg/kg body weight) into 6-week-old C57BL/6J male mice.
After removal from control and busulfan-administered (BUS) mice, the right testis was immediately fixed with Bouin’s solution for 48 hr at 4°C, washed, dehydrated in a graded ethanol series, and embedded in paraffin (Paraffin wax II60; Sakura Finetek, Tokyo, Japan). Before the testis was fixed with Bouin’s solution, the tunica albuginea was punctured with a needle to allow rapid penetration of the fixative. Sections (4 μm) were obtained at 20–24-μm intervals and stained with Gill’s hematoxylin III and 2% eosin Y for examination under a light microscope (Keyence BZ-X800, Keyence Co. Ltd., Osaka, Japan). The major and minor axes of the testicular section were divided into three equal parts, and the central and peripheral areas are defined (Fig. 2). For each animal, 50-100 round seminiferous tubules were randomly selected from a specific area (central or peripheral area) of the testicular section. The diameters of seminiferous tubules, seminiferous tuble area, and seminiferous epithelial area were measured using the ImageJ 1.51J8 program (National Institutes of Health, USA). The Leydig cells were counted under 400X magnification in five distinct microscopic fields in a specific area (central or peripheral area) of the testicular section.
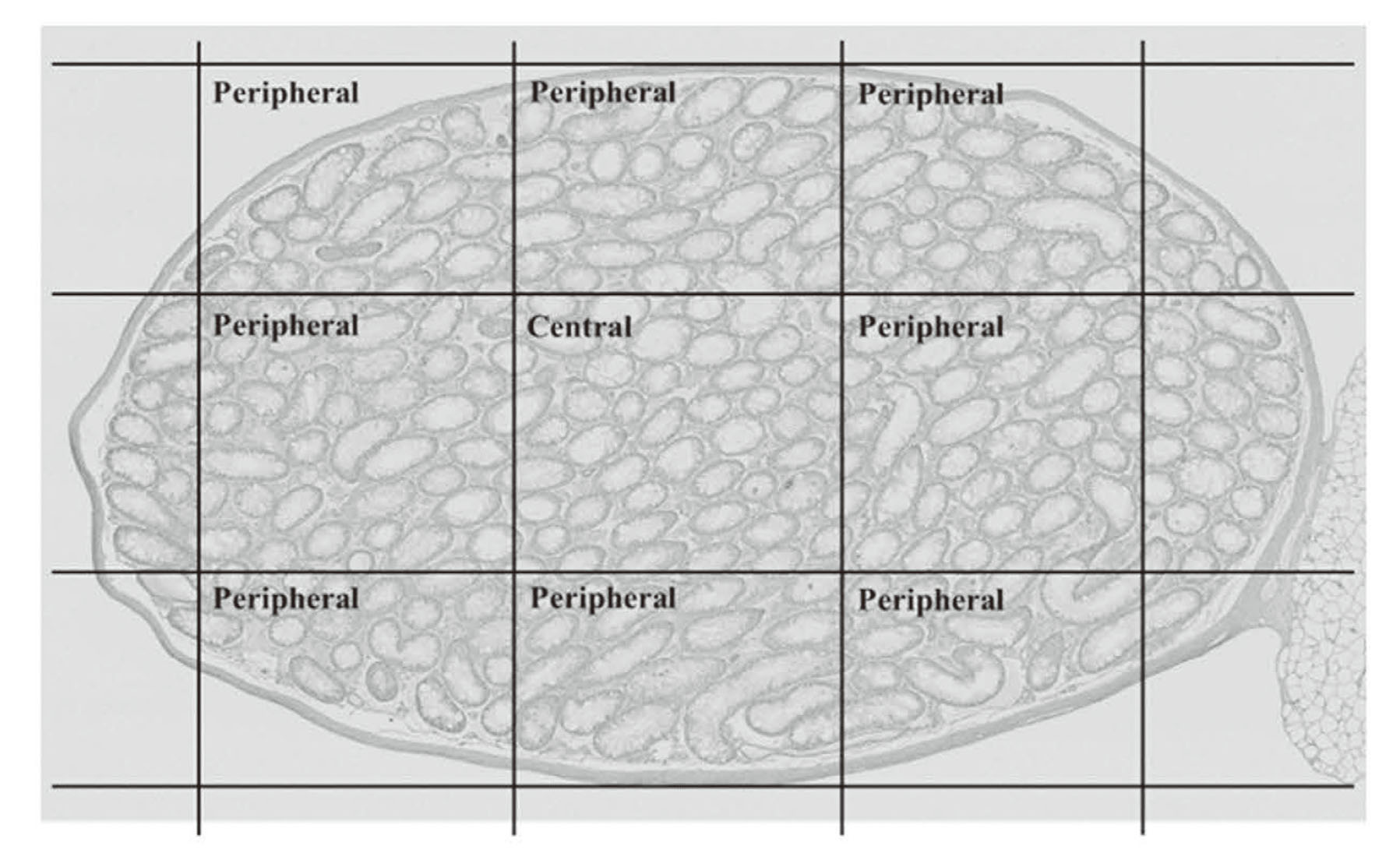
Histology of the testes from BUS mice. The major and minor axes of the testicular section were divided into three equal parts. The central and peripheral areas are defined as shown.
Another testis from control and BUS mice was embedded in Tissue-Tek OCT compound, frozen in liquid nitrogen, and stored at −80°C until examination. Embedded testes were dissected using a cryostat (CM3050S, Leica Microsystems, Tokyo, Japan) at 5 μm. The sections were fixed with acetone for 2 min at −20°C. For immunohistochemistry, separate sections were incubated with rabbit anti-SOX-9 polyclonal antibody (ab5535, Millipore; 1:2000 dilution), which recognizes a marker of Sertoli cells. Following an overnight reaction, sections were washed and incubated with a biotinylated anti-rabbit immunoglobulin IgG (1:200) for 30 min and labeled with avidin-biotinylated horseradish peroxidase (PK-6101, Vectastain ABC Elite Kit, Vector Laboratories, Burlingame, CA, USA) for 30 min at room temperature. After washing, the immunoreactivities were visualized with 3,3′-diaminobenzidine, and sections were counterstained with Gill’s hematoxylin III. Immunoreactive cells were observed using a light microscope.
Enzyme-linked immunosorbent assay (ELISA)Serum testosterone, luteinizing hormone (LH), and follicle-stimulating hormone (FSH) levels were measured using ELISA, as described by Miyaso et al. (Miyaso et al., 2021) and Endocrinetech ELISA kits (ERKR7016, ERKR7010, and ERKR7007, respectively) were used. Briefly, the whole blood (collected from retro-orbital plexus at euthanasia) was clotted and processed by standard techniques; the resulting serum samples were collected and stored at − 80°C until analysis. ELISAs were performed in accordance with the manufacturer’s protocols.
StatisticsStatistical analyses comparing the control and BUS mice or the central and peripheral areas in the testes were performed with Mann–Whitney U-test using the EZR version 1.36 (Saitama Medical Center, Jichi Medical University, Saitama, Japan) (Kanda, 2013). A p-value of < 0.05 was considered significant. Where appropriate, values are represented as the mean ± standard error of the mean (SEM).
The body weight of BUS mice was significantly lower than that of the control mice at 1, 2, 3, and 4 weeks after busulfan administration (Fig. 3). The testicular weight and relative testicular weight (organ weight/body weight × 100) were also significantly lower at 4 weeks after busulfan administration (Fig. 4).
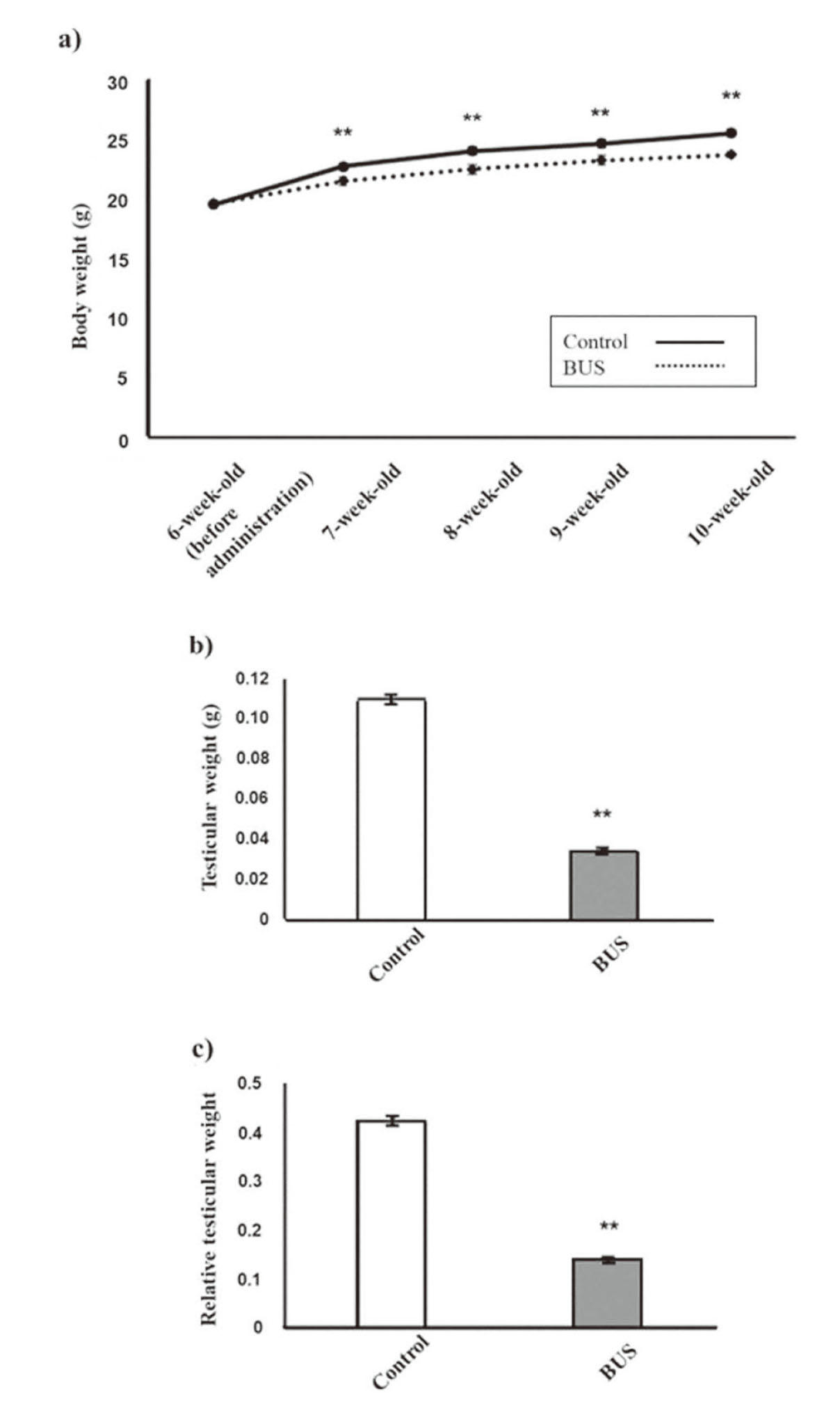
Body and testicular weights of BUS mice. Body weight (a), testicular weight (b), and relative testicular weight (c) of the control and BUS mice. **p < 0.01 compared to the control.

Gross morphology of the testes from BUS mice. Representative morphology of the testes from control (a) and BUS (b) mice at 4 weeks after busulfan administration. The scale bar is 5 mm.
The diameters of seminiferous tubules were significantly lower in the BUS mice than those in the control mice both in the central and peripheral areas (Fig. 5 and Table 1). In control mice, no significant differences in seminiferous tubule diameters were found between the central and peripheral areas, whereas in BUS mice, seminiferous tubule diameters were significantly lower in the peripheral area than in the central area (Table 1).
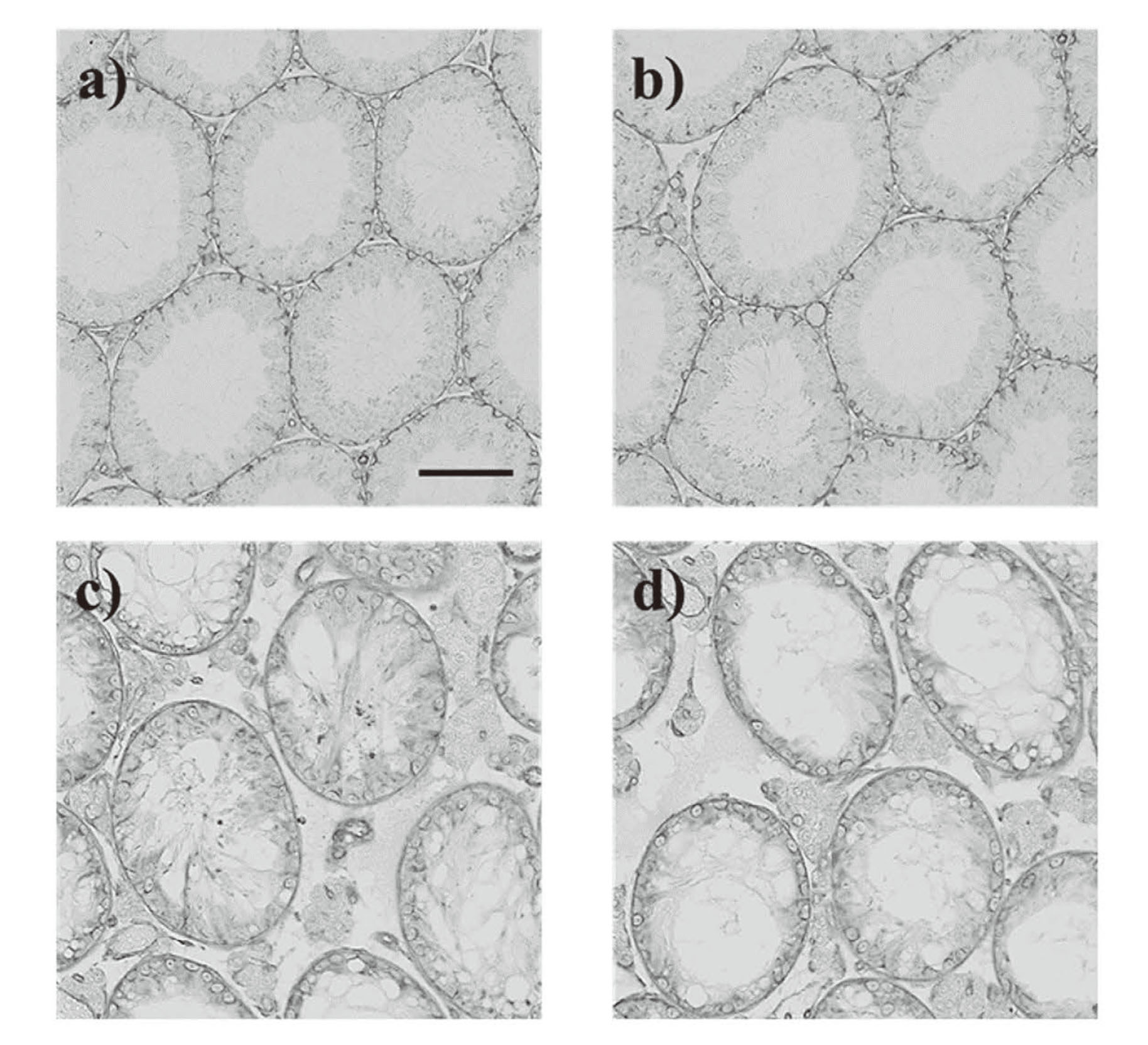
Testicular morphology of BUS mice. Representative micrographs of seminiferous tubules in the central (a) and peripheral areas (b) of the testes from control mice and central (c) and peripheral areas (d) of the testes from BUS mice. Representative H&E-stained micrographs are provided. The scale bar is 100 µm.
| Central | Peripheral | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | BUS | Control | BUS | ||||||||||
| Diameter of seminiferous tubule (µm) | 233.65 | ± | 5.26 | 141.54 | ± | 2.62*** | 238.22 | ± | 3.59 | 130.14 | ± | 1.87***, +++ | |
| Seminiferous tubule area (×10-3/mm2) | 46.67 | ± | 1.78 | 15.36 | ± | 0.502*** | 45.522 | ± | 1.91 | 14.15 | ± | 0.322***, + | |
| Seminiferous epithelial area (×10-3/mm2) | 33.47 | ± | 1.21 | 7.29 | ± | 0.209*** | 34.31 | ± | 1.38 | 7.13 | ± | 0.179*** | |
| Relative seminiferous epithelial area (Seminiferous epithelial area to seminiferous tubular area) | 0.744 | ± | 0.020 | 0.488 | ± | 0.009*** | 0.790 | ± | 0.021 | 0.518 | ± | 0.010***, + | |
Values are expressed as the mean ± SEM of data from three animals per group (for central or peripheral areas, 50-100 seminiferous tubules per animal; total 200-300 seminiferous tubules per group).
***p < 0.001 versus the control group in the central area and peripheral area
+p < 0.05 versus the central area in BUS mice
BUS, busulfan.
Additionally, areas of seminiferous tubules were significantly lower in the BUS mice than in the control mice both in the central and peripheral areas. The control mice showed no significant change in seminiferous tubular areas between the peripheral and central areas; meanwhile, in the BUS mice, significant decreases were observed in seminiferous tubular areas in the peripheral areas compared with the central areas. For seminiferous epithelium, many vacuoles were observed in BUS mice (Fig. 5). The seminiferous epithelial areas were significantly lower in the BUS mice than in the control mice both in the central and peripheral areas. No difference in the area of the seminiferous epithelium of the tubules was observed between the central and peripheral areas both in the control and BUS mice. The relative seminiferous epithelial areas (seminiferous epithelial area to seminiferous tubular area) significantly decreased in the BUS mice as compared with the control mice in both the central and peripheral areas. In the control mice, the relative seminiferous epithelial areas did not significantly change between the central and peripheral areas, whereas in the BUS mice, significant increases were observed in the relative seminiferous epithelial areas in the peripheral areas compared with the central areas.
The detachment of Sertoli cell nuclei in the BUS miceIn the peripheral areas of the testes from BUS mice, some seminiferous tubules showed detachment of Sertoli cell nuclei into the lumen (Fig. 6). The detached Sertoli cell nuclei were rarely observed in the central areas. In the control mice, detached Sertoli cell nuclei were not detected both in the central and peripheral areas (Data not shown).
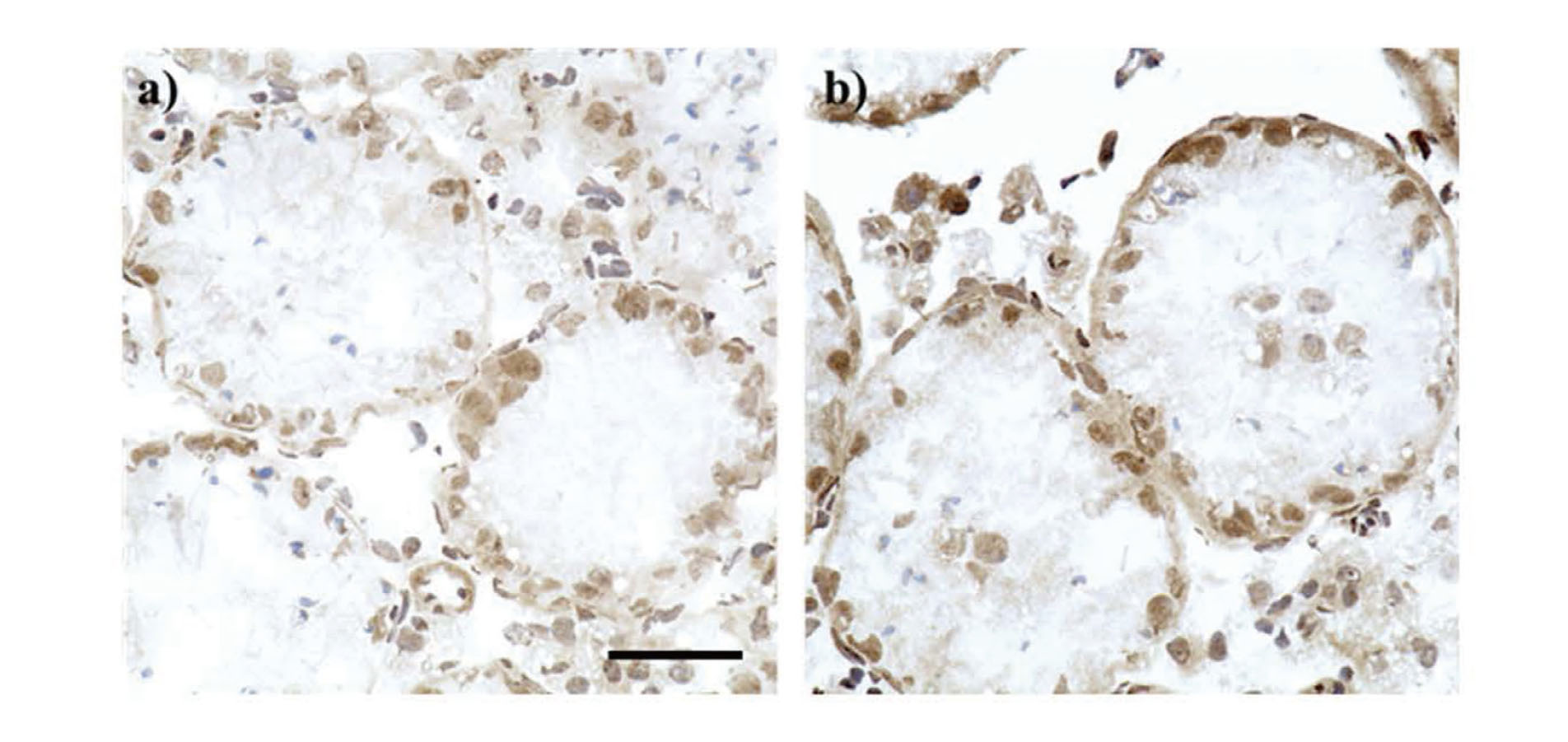
Detachment of Sertoli cell nuclei in BUS mice. Representative micrographs of Sertoli cells immunostained with anti-SOX-9 antibody in the central (a) and peripheral areas (b) in the testes from BUS mice. The scale bar is 50 µm.
Spermatogenesis disorder was observed, but germ cells were found in both the central and peripheral areas in BUS mice. The germ cell number per seminiferous tubule was significantly higher in the central area than in the peripheral area in BUS mice (Fig. 7).
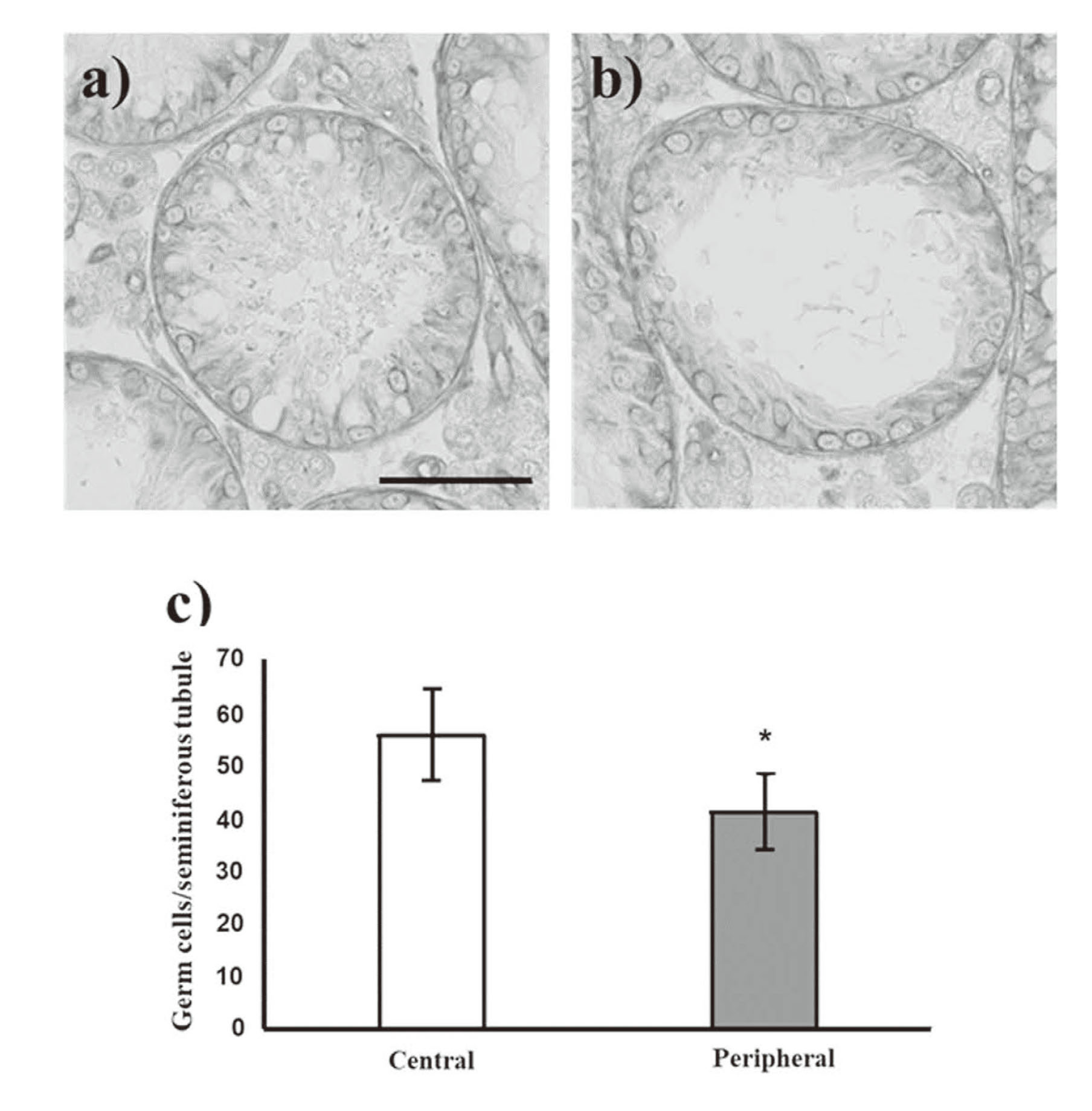
Number of germ cells in seminiferous tubules of BUS mice. Representative micrographs of germ cells per seminiferous tubule in the central (a) and peripheral areas (b) in the testes from BUS mice. The scale bar is 100 µm. The germ cell number per seminiferous tubule between the central and peripheral areas in BUS mice (c). Values are expressed as the mean ± SEM of data from three animals per group (for central or peripheral areas, 50-100 seminiferous tubules per animal; total 200-300 seminiferous tubules per area). *p < 0.05 compared to the control.
At 4 weeks after administration, serum testosterone and LH levels decreased in BUS mice compared with those in control mice (Fig. 8a and 8b), but not significantly. In contrast, the serum FSH levels increased in BUS mice (Fig. 8c). However, these differences were not significant.
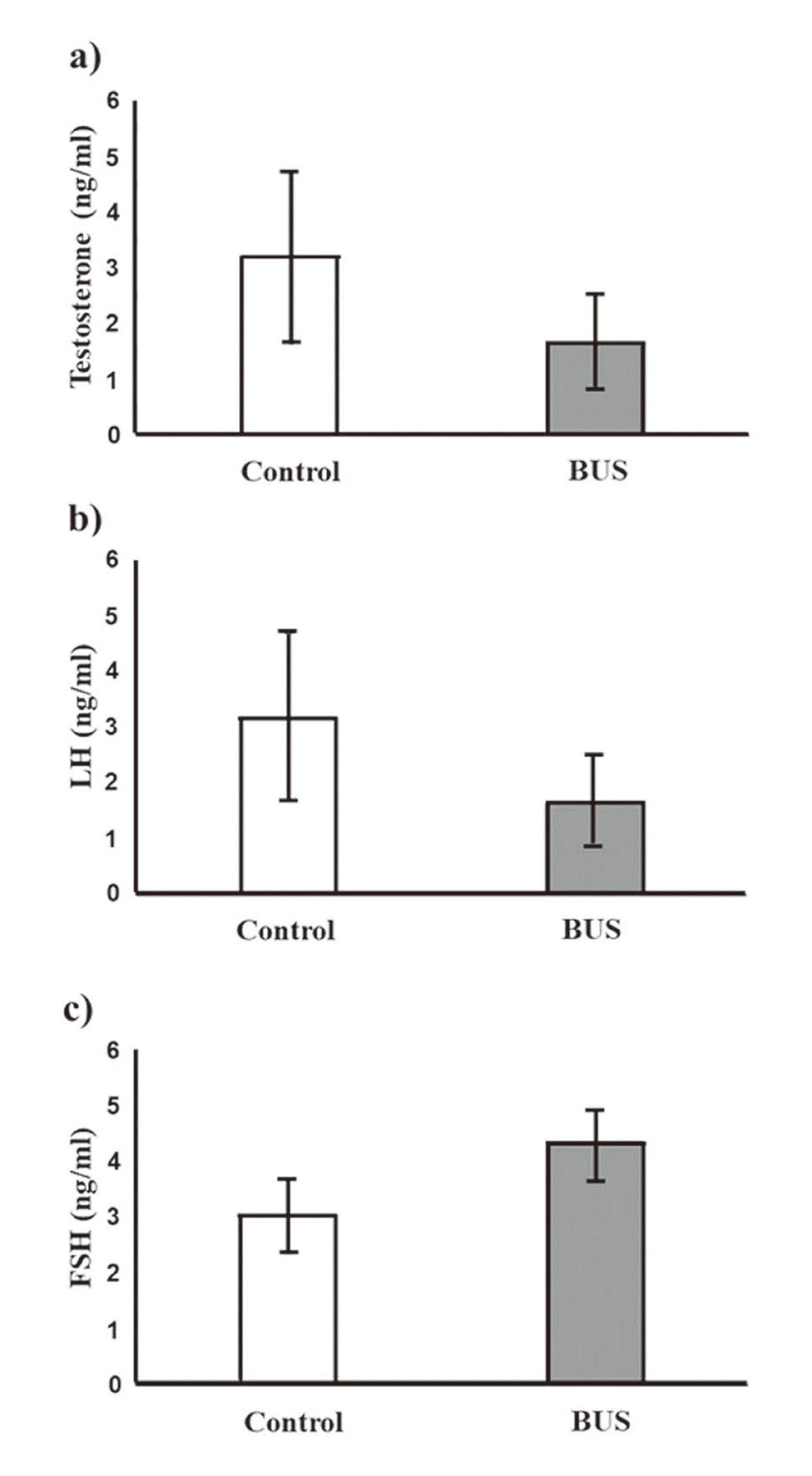
Serum hormone levels in BUS mice. Serum testosterone (a), LH (b), and FSH (c) levels at 4 weeks after BUS administration were assessed using ELISA. Values are expressed as the mean ± SEM of 6 samples per group.
No differences in the number of Leydig cells were observed between control and BUS mice (Table 2). In control mice, no significant differences in the number of Leydig cells were found between the central and peripheral areas, whereas in BUS mice, the number of Leydig cells was significantly lower in the peripheral area than in the central area.
| Control | BUS | |||
|---|---|---|---|---|
| The Leydig cell number per total (central + peripheral) interstitial area (×103/mm2) | 1.73 ± 0.130 | 1.80 ± 0.0923 | ||
| Central | Peripheral | |||
| Control | BUS | Control | BUS | |
| The Leydig cell number per central or peripheral interstitial area (×103/mm2) | 1.74 ± 0.187 | 1.93 ± 0.132 | 1.73 ± 0.186 | 1.67 ± 0.126+ |
Values are expressed as the mean ± SEM of data from three animals per group (for central or peripheral areas, 15 distinct microscopic fields per animal).
+p < 0.05 versus the central area in BUS mice
BUS, busulfan.
Busulfan is a well-known anticancer drug that causes germ cell depletion in the testes. It has been intraperitoneally administered to mice at 30–40 mg/kg body weight, resulting in severe ablation of germ cells from the testis (Nakata et al., 2020; Gutierrez et al., 2016; Wang et al., 2010). In this study, the diameters of seminiferous tubules were found to be significantly bigger in the central area than in the peripheral area in testes of BUS mice, whereas germ cell ablation was observed in the whole testis. The seminiferous tubular areas also significantly decreased in the peripheral areas compared with the central areas. The germ cell number was significantly higher in the central area in BUS mice. Moreover, Sertoli cell nuclei were detached into the lumen in the peripheral area. The serum testosterone levels decreased in BUS mice compared with those in control mice; however, not significant. The number of Leydig cells per unit area was significantly lower in the peripheral areas as compared with central areas in BUS mice. To the best of our knowledge, for the first time, it was clarified that the toxic effects of busulfan on testicular histology at 4 weeks after administration differ between the peripheral and central areas of the testes.
Our data showed that busulfan results in higher toxic effects in peripheral areas as compared with central areas. Such differences in the toxic effects of busulfan could be related to differences in the running and distribution of arteries between the peripheral and central areas of the testes. Therefore, the toxic effects of busulfan observed herein might reflect differences in blood supply between the peripheral and central areas of the testes. As an alternative possibility, busulfan might adversely affect the peripheral areas of the testes earlier than the central areas because, anatomically, the arterial blood in the testicular tissue is supplied in order from the peripheral area to the central area (Dudea et al., 2010; Mostafa et al., 2008; Polguj et al., 2010). However, the detailed mechanism remains unclear. To evaluate the effects of busulfan between peripheral and central areas in detail, further research is required. The evaluation of the toxic effects of busulfan on the testes at an earlier stage than that in this study, for example, 1 or 2 weeks after administration, may present useful information.
Herein, busulfan was intraperitoneally injected at 40 mg/kg body weight. Reportedly, this dose causes severe germ cell depletion (Nakata et al., 2020; Gutierrez et al., 2016; Wang et al., 2010). However, the effects of higher or lower doses of busulfan on the central and peripheral areas of the testes remain unclear. Our results suggest the importance of evaluating the toxic effects of busulfan at other doses and examining whether such differences in busulfan-induced toxicity between the central and peripheral areas in testes are dose-dependent. For example, busulfan administration at lower doses might reveal the differences in busulfan-induced effects between the central and peripheral areas more clearly than the dose used in this study. Moreover, it is possible that lower-dose busulfan administration will clearly reveal the specific testicular area in which even hypotesticular toxicity exerts toxic effects, and the identification of this area is extremely important for testicular toxicity assessments. Furthermore, it is possible that busulfan treatment at higher doses than those used herein or excessive doses will mask the differences in toxic effects between the central and peripheral areas of the testes. Notably, Nakata et al. reported that the branching points in the seminiferous tubules may be more vulnerable to busulfan-induced damage based on the three-dimensional analysis (Nakata et al., 2020). In future, combinational evaluation using both two- and three-dimensional analyses might clarify testicular areas vulnerable to busulfan-induced damage.
Chemotherapy using busulfan, cisplatin, and other alkylating agents can have a profound negative effect on spermatogenesis by causing germ cell depletion, chromosomal aberrations, maturation arrest, mutagenesis, and impaired spermatozoa motility (Polland and Berookhim, 2016; Gutierrez et al., 2016). In particular, cytotoxic chemotherapy may result in irreversible spermatogenic dysfunction. Studies reported that approximately two-thirds of patients have azoospermia after chemotherapies (Schmidt et al., 2004; Hsiao et al., 2011). Although cryopreservation of mature sperm offers an excellent opportunity to preserve the fertility potential before initiating chemotherapy, the penetration rate of sperm banking is low due to various reasons (Hsiao et al., 2011; Dabaja and Schlegel, 2013). In these cases, treatment with TESE or micro-TESE and intracytoplasmic sperm injection (ICSI) are useful techniques for men with azoospermia after chemotherapy to father a child. TESE and micro-TESE are surgical techniques for sperm retrieval. In TESE or micro-TESE, surgeons must select seminiferous tubules to include more germ cells for ICSI or in vitro fertilization. Although busulfan has adverse effects on spermatogenesis and fully results in germ cell depletion, this study identified that the central area in the testes from busulfan-administered mice has less adverse effects of busulfan than the peripheral area on the testes from mice at 4 weeks after administration. This result suggests the possibility of improved TESE and micro-TESE in the testes after chemotherapy.
Several studies reported that germ cell depletion and spermatogenesis disorder following busulfan administration recover after several months in experimental mice (Wang et al., 2010; Nakata et al., 2020). This study showed that the diameters of seminiferous tubules and germ cell count were significantly higher in central areas at 4 weeks after busulfan administration; however, histological examination was not performed after several months and during the recovery period in this experimental model. Testicular areas where spermatogenesis preferentially recovers remain unknown, and therefore, the mechanisms of spermatogenesis recovery should be investigated. Several studies showed that the possibility of fertility restoration following busulfan treatment heavily depends on the drug dose and the number of surviving spermatogonial stem cells (SSCs) (La and Hobbs, 2019). Hence, our future study aims to identify testicular areas preferentially including the seminiferous tubules with recovered spermatogenesis and evaluate the number of SSCs in seminiferous tubules at ≥ 10 weeks after busulfan administration.
Busulfan has been clinically used as a chemotherapeutic agent for patients with hematological malignancies and nonmalignant diseases. However, it has been known to affect the testes and spermatogenesis. Jackson et al. reported testicular toxicity of busulfan in 1962 (Jackson et al., 1962). Thereafter, several studies reported the occurrence of spermatogenesis disorders and germ cell depletion following busulfan administration. For the first time, we clarified that the toxic effects of busulfan on testicular histology at 4 weeks after administration differ between the peripheral and central areas of the testes. Note that there is a possibility that the degree of penetration of fixative solution differs between central and peripheral areas in testes, because fixative solution generally infiltrates testicular tissue in order from the peripheral to the central area. In this study, we confirmed that there was no difference in testicular morphology, for example the diameters of seminiferous tubules, between central and peripheral areas in control mice, suggesting that the difference in penetration degree of fixative solution between central and peripheral areas in testes hardly affected its morphology in this study. The difference in penetration degree of fixative solution between central and peripheral areas in testes should be considered; meanwhile our data suggest the usefulness of comparing the peripheral and central areas in evaluating the effects of testicular toxicants. Moreover, note that differences in fixative solution also generally influence histological morphology in testes. In this point, we have already confirmed that testes fixed with 4% paraformaldehyde solution also show a result analogous to that of the present study for testicular morphology (Supplemental data). On the other hand, the detailed mechanism by which busulfan causes different toxic effects between the peripheral and central areas of the testes and differences of toxic effects by busulfan between peripheral areas remain unclear. Evaluation of the vascular system in testes may give a clue to this mechanism. Additionally the toxic effects were not compared between peripheral and central areas of the testes for other alkylating drugs, such as cisplatin, cyclophosphamide, and nitrogen mustard. Further studies are required to evaluate the effects of other chemotherapeutic drugs and testicular toxicants in each testicular area.
We would like to thank Enago (https://www.enago.com/) for their English language editing service. This study was supported by the Japan Agency for Medical Research and Development (grant numbers 21mk0101210h0201 and 22mk0101210h0202).
Conflict of interestThe authors declare that there is no conflict of interest.