2025 Volume 50 Issue 5 Pages 223-233
2025 Volume 50 Issue 5 Pages 223-233
Perinatal perfluorooctane sulfonate (PFOS) exposure of the next generation through placenta and breast milk has been of high concern. Epidemiological and animal studies have reported that perinatal PFOS exposure is associated with neurodevelopmental disorders such as learning and autism spectrum disorders in children. However, the sensitive time window of perinatal PFOS exposure for neurodevelopment has yet to be elucidated. Here we examined differential effects of different time windows of postnatal PFOS exposure (postnatal day (PD) 1-7 or 8-14) on cognitive development and gene expression profiles in the hippocampus. Pups were exposed to PFOS from PD 1 to 7 (PD 1-7 group) or from PD 8-14 (PD 8-14 group) through breastfeeding by dams who received a daily gavage of 1 mg/kg body weight PFOS per day during each period. An object location test and an object recognition test revealed the impairment in spatial memory in PD 1-7 group at PD 70. Learning ability was also retarded in a visual discrimination test. According to RNA-seq analysis and real-time PCR, Serpina3g and Tmem91 were significantly downregulated in the hippocampus of PD 1-7 group at PD 21. These results suggest that the first 7 days after birth are critically vulnerable to PFOS exposure and consequent neurodevelopmental deficits rather than the late phase of postpartum. Our work puts a strong emphasis on the importance of monitoring PFOS concentration in pregnant women and potential impact on retardation of neurodevelopment in children.
For recent decades, per- and polyfluoroalkyl substances (PFAS) have been coming up in a worldwide debate about persistent organic pollutants (POPs). As the Stockholm Convention on POPs was issued in 2004 and has restricted production, industrial use, import and export, PFAS have been of great concern in regard to not only the environment, but also human exposure to PFAS through water, soil, air, and biota (Kannan et al., 2001).
Perfluorooctane sulfonate (PFOS), a member of the PFAS family, has been drawing significant attention due to the high level of its persistence in the life sphere, and in fact its adverse effects on human health have been widely reported (Espartero et al., 2022). According to past studies, it could be implied that the perinatal exposure to PFOS can cause neurodevelopmental toxicity in offspring. Most studies have focused on cognitive performance in children. Some epidemiological studies found that maternal PFOS exposure led to lower cognition (Luo et al., 2022; Goodman et al., 2023; Skogheim et al., 2020; Tanner et al., 2020). Rodent studies have also suggested that developmental exposure to PFOS reduces spatial learning and memory (Wang et al., 2015; Mshaty et al., 2020). Such neurobehavioral deficits are further corroborated by an electrophysiological study showing the inhibition of hippocampal long-term potentiation with reduction in membrane α-amino-3-hydroxy-5methyl-4-isoxazole propionic acid (AMPA) receptors (Zhang et al., 2019). Other studies have indicated that developmental exposure to PFOS altered the expression of genes related to synaptic plasticity and calcium signaling in the developing hippocampus (Liu et al., 2010; Wang et al., 2015).
There is a “sensitive” period in which the development of brain is prone to be affected by internal and external factors including environmental chemicals. During this period, the modification is so dynamic and but also steady that it can cause permanent outcomes in physiology, morphology, and behavior (Kroon et al., 2013; Marco et al., 2011; Meredith, 2015). Such sensitive time windows may vary according to the brain regions, structures, and causal factors and their mechanisms of action in the body system (Heyer and Meredith, 2017). In particular to the developing hippocampus, there are age-dependent increases in pathways associated with learning, memory, and cognition along with changes in neurotransmission, and synaptic function. Therefore, the substantial transcriptional maturation occurs during early postnatal development of the hippocampus, and expressions of genes related to neurodevelopmental disorders are also maximally modulated during these “sensitive time windows” (Olsen et al., 2023). Meanwhile, based on findings from both humans and animal studies after model validation of translating neurodevelopmental stages from animals to humans, some sensitive time windows are estimated to be prenatal for exposure to certain environmental toxicants such as polychlorinated biphenyls (PCBs), methylmercury, chiorpyrifos, organochlorines, and organophosphates, while others are estimated to be perinatal (lead) or postnatal (bisphenol A and arsenic) at a risk for neurodevelopmental disorders, including autism spectrum disorders (ASD), attention-deficit / hyperactivity disorder (ADHD), and schizophrenia (Heyer and Meredith, 2017). However, PFAS, including PFOS, have yet to be elucidated in terms of their sensitive time window for neurodevelopment. It is urgent to identify the time-specific effects of maternal PFOS exposure on neurodevelopmental toxicity as significant associations between maternal chemical levels and neurodevelopment in children have been reported in a recent large cohort in Japan (Kishi et al., 2021).
In a mouse model, we previously found that lactational PFOS exposure from postnatal day (PD) 1 to 14 through the breastmilk caused retardation in learning, memory, and motor performance in offspring (Mshaty et al., 2020; Ninomiya et al., 2022). In the present study, we further broke down the exposure period to some extent (PD 1-7 and PD 8-14) and aimed to specify the sensitive periods of PFOS exposure during brain development. The differential effects on cognitive function and gene expression profiles in the hippocampus were investigated using memory and learning tests and RNA-seq followed by real-time PCR, respectively.
The present study was approved by the council of the Animal Care and Experimentation Committee of Gunma University (protocol number: 19-075). Female C57BL/6J mice (Japan SLC, Hamamatsu, Japan) were mated with male C57BL/6J mice in a breeding room with a light cycle of 7:00-19:00, moderate temperature (22-24°C), and 30-60% humidity. Food and water were available ad libitum. The experimental design is described in Fig. 1A.

Change in body and brain development by different time periods of PFOS exposure. A Scheme describes the experiment schedule in the present study. B Changes in body weight at PD 21 according to the different time periods of exposure are shown. Body weights were significantly reduced in both the PD 1-7 and PD 8-14 groups compared to the control group. * p < 0.05, ** p < 0.01. C No change in brain weight was observed between groups. D Percentage of brain weight in body weight was higher in PFOS-exposed pups. *** p < 0.001
Lactational PFOS exposure was conducted as described in the previous studies (Mshaty et al., 2020; Ninomiya et al., 2022). In brief, linear-PFOS (L-PFOS, Purity ≥ 98.0%, Sigma Aldrich, St. Louis, MO, USA), the most dominant PFOS isomer among PFOS isomers (Benskin et al., 2010), was administered to dams by gavage at a concentration of 1 mg/kg body weight during lactation. This exposed the offspring exposed to PFOS through the breast milk. We preliminarily compared the PFOS effects on learning and memory among multiple doses (0.1 mg/kg, 0.25 mg/kg, and 1 mg/kg) (Mshaty et al., 2020). Only the dose of 1 mg/kg disrupted cognitive function in adult offspring (Mshaty et al., 2020). Therefore, the present experiments were conducted using a single dose (1 mg/kg), where we observed the endpoint of developmental neurotoxicity. In the present study, one group was given PFOS from postnatal day (PD) 1 to 7 (PD 1-7 group), and the other group was given PFOS from PD 8 to 14 (PD 8-14 group). Since PFOS was dissolved with 0.5% Tween 20 (Sigma Aldrich) in deionized water, control dams received the equivalent volume of 0.5% Tween 20 from PD 1 to 14 (sham group). There was no change in body weight of dams in each group (Supplemental Fig. 1). The average littermate sizes from control dams, PD 1-7 dams, PD 8-14 dams were 6, 6.2, 6.1, respectively. Also, no pups died during lactation. At PD 21, pups were isolated from their biological dams and sacrificed under an i.p. administration of a 100 mg/kg ketamine + 10 mg/kg xylazine anesthetic to sample hippocampal tissues for RNA sequencing and real-time PCR at PD 21, when the tissue structure appears distinct and is easy to isolate. The body weight and whole brain weight were measured when sampling. Only adult male offspring were used for the behavioral tests at PD 70. The use of female mice was avoided to rule out the hormonal effects on behavioral tests (Chari et al., 2020).
Object location test and object recognition memory testAn object location test and an object recognition test were performed following the protocol in a previous study (Mshaty et al., 2020). Prior to the experiment, mice were habituated to the empty open field arena (30 × 30 × 39 cm, made of acryl) for 30 min. In the first session of the experiment, mice were introduced to two identical objects located diagonally at two corners of an open arena and allowed to explore for 5 min. In the second session after 24 hr, one of the objects was moved to the corner next to the other object. Mice were then allowed to explore the arena for 5 min during the object location test. Another 24 hr later, in the third session of the experiment, one of the objects was replaced by a novel object, but the location did not change for the object recognition test. Mice were again given 5 min to explore the arena. The exploring time for each object was measured in each session. Discrimination ratio was calculated as (timenovel – timefamiliar) / (timenovel + timefamiliar) to represent the preference for the novel object (Cavoy and Delacour, 1993).
Visual discrimination testLearning ability of the offspring was assessed by the pairwise visual discrimination test as previously described (Mshaty et al., 2020). Following overnight fasting, the 30-min habituation phase was conducted for five days to get mice used to the system, in which the food pellet was given to mice when they touched the paired screens in the chamber (O’Hara & Co., Ltd., Tokyo, Japan). In the habituation phase, the images appearing were the same between the paired screens. In the test phase, one screen displayed an image of horizontal strips and the other screen displayed an image of vertical strips, and they randomly switched in each trial (50 trials in total). Mice were rewarded with food pellets only when they touched the screen displaying horizontal strips (Correct). % Correct was calculated out of 50 trials for nine days.
RNA-sequencingThe RNA-seq was performed on the NovaSeq6000 platform by Macrogen Japan NGS service using an RNA sample extracted from hippocampal tissues at PD 21 from each group. Total RNA was extracted following the instructions for RNeasy Lipid Tissue Mini Kit (QIAGEN, Hilden, Germany). The quality of RNA was checked and all samples were confirmed as RNA integrity number (RIN) > 8. Library preparation was performed using the TruSeq stranded mRNA kit (Illumina), and paired-end sequencing of 101 bp was performed. BCL (base call) binaries were converted to FASTQ using the illumina package bcl2fastq. Removal of adapters and low-quality reads was performed using fastp (version 0.21.0). Reads after trimming were used for mapping to the mm10 reference genome and for read counting in the STAR (version 2.5.3a)-RSEM (version 1.3.3) pipeline. The median (minimum-maximum) number of reads uniquely mapped to the mm10 reference genome was 22.11 (19.82-27.15) million reads. The iDEGES-edgeR-edgeR pipeline of the TCC package (version 1.30.0) in R (version 4.2.1) was used to extract differential expressed genes (DEGs), and genes with FDR (adjusted p-value) < 0.05 were defined as the DEGs. Volcano plots were drawn using the EnhancedVolcano package (version 1.8.0) in R, and low-expressing genes with an average of less than 2 for all samples were removed.
Real-time PCRTotal RNA was prepared from hippocampal tissues using RNeasy Lipid Tissue Mini Kit (QIAGEN). 500 ng of total RNA was reverse-transcribed by ReverTra Ace® qPCR Master Mix with gDNA Remover (Toyobo Co., Ltd. Life Science Department, Osaka, Japan) to synthesize cDNA. Following the manufacture instruction, real-time PCR was performed using Thunderbird® Sybr qPCR Mix (Toyobo) and the Step One Real-Time PCR System (Thermo Fisher Scientific, Waltham, MA, USA). The following primers were used: Gdf1 (forward: AACTAGGGGTCGCCGGAAA, reverse: TCAAAGACGACTGTCCACTCG), Serpina3g (forward: CTTCCCAACGGCTGGAATCTA, reverse: ACTGTCCAATCAGGCATAGCG), Tmem91 (forward: GCAACCCTTACTGCCAAGCA, reverse: CTCTGGGGGACTCTGCAAAG), Dok3 (forward: ACGCTTGGCTGACTGTGTATC, reverse: GCTTCGCTCAGTGGTGGTAAT), Hbb-b1 (forward: GCACCTGACTGATGCTGAGAA, reverse: TTCATCGGCGTTCACCTTTCC), Tnfrsf25 (forward: CTGTGCCAGTGAGTCCCAG, reverse: CCGAGCAGTTCTCAAGGGTC), and Rpl22l1 (forward: CCTGGAGGTTTCATTTGGACC, reverse: ACGTAGCCAATCACGGAGATTA). 18S ribosomal RNA (Rn18s) and glyceraldehyde-3-phosphate dehydrogenase (Gapdh) were used as an internal control and geometric mean was calculated as previously done (Ninomiya et al., 2022).
Statistical analysisData were analyzed using GraphPad Prism version 10 for Mac OS (San Diego, CA, USA, www.graphpad.com). Ordinary one-way ANOVA followed by Dunnet’s multiple comparison test was performed for body weight, brain weight, object location test, and object recognition test. Two-way ANOVA followed by Dunnett’s multiple comparison test was performed for visual discrimination test. Unpaired t-test was conducted for real-time PCR. The significant value was considered p < 0.05. All data were expressed as mean ± standard error of the mean (SEM).
To examine the effects of lactational PFOS exposure on body growth, body weight and brain weight of the offspring were measured at PD 21. Body weight of PFOS-exposed groups was significantly decreased compared to the control group (Fig. 1B). The PD 1-7 group especially showed a greater reduction (22.03% reduction, control (n = 8): 7.838 ± 0.4276 g vs. PD 1-7 (n = 9): 6.111 ± 0.1033 g, p < 0.01) than PD 8-14 did (15.31% reduction, control (n = 8): 7.838 ± 0.4276 g vs. PD 8-14 (n = 8): 6.638 ± 0.3615 g, p = 0.03). There was no change in brain weight between groups (control (n = 8): 0.4082 ± 0.0075 g vs. PD 1-7 (n = 9): 0.3987 ± 0.0079 g, p = 0.52, control (n = 8): 0.4082 ± 0.0075 g vs. PD 1-7 (n = 9): 0.3986 ± 0.0043 g, p = 0.54, Fig. 1C); however, the percentage of brain weight to body weight was higher in the PFOS-exposed group (F = 9.006, p < 0.01, control (n = 8): 5.292 ± 0.2303% vs. PD 1-7 (n = 9): 6.528 ± 0.1036%, p < 0.001, control (n = 8): 5.292 ± 0.2303% vs. PD 1-7 (n = 9): 6.111 ± 0.2782%, p = 0.02, Fig. 1D), indicating that developmental exposure to PFOS may affect body growth but not brain growth.
PFOS exposure during PD 1-7 impaired memoryAn object location test and an object recognition test were conducted to assess memory in each group (Fig. 2A). In the object location test, there was a significant difference in discrimination index between groups (F = 7.98, p < 0.01, Fig. 2B). Post hoc analysis revealed a significant lower discrimination index in the PD 1-7 group compared to the control group (control (n = 20): 0.2272 ± 0.04773 vs. PD 1-7 (n = 18): 0.002711 ± 0.04191, 95% CI = 0.09004 to 0.359, p < 0.001), but not in the PD 8-14 group (control (n = 20): 0.2272 ± 0.04773 vs. PD 8-14 (n = 16): 0.1866 ± 0.03408, 95% CI = -0.09821 to 0.1795, p = 0.73). Similarly, the discrimination index differed between groups in the object recognition test (F = 5.604, p < 0.01, Fig. 2C). Post hoc analysis revealed a significant lower discrimination index in the PD 1-7 group compared to the control group (control (n = 20): 0.243 ± 0.04916 vs. PD 1-7 (n = 18): 0.03722 ± 0.05175, 95% CI = 0.05129 to 0.3603, p < 0.01), but not in the PD 8-14 group (control (n = 20): 0.243 ± 0.04916 vs. PD 8-14 (n = 16): 0.2331 ± 0.04476, 95% CI = -0.1496 to 0.1694, p = 0.99). Taken together, these results indicate that PFOS exposure during the first 7 days after birth may be the most vulnerable to memory deficit.

Effect of PFOS exposure on memory at different time window. A Scheme of object location test (OLT) and object recognition test (ORT) B Object location test. The PD 1-7 group showed significantly lower discrimination index compared to the control group. C Object recognition test. The PD 1-7 group had a showed significantly lower discrimination index compared to the control group. ** p < 0.01, *** p < 0.001.
Learning ability was tested using a visual discrimination test (Fig. 3A). Over 9 days of trials, there was a significant difference in % correct between groups. In particular, PD 1-7 group displayed lower scores compared to the control group on day 3 (control (n = 20): 69.5 ± 0.1.782% vs. PD 1-7 (n = 18): 59.917 ± 2.016%, 95% CI = 3.366 to 15.8, p < 0.01), day 4 (control (n = 20): 74.3 ± 2.332% vs. PD 1-7 (n = 18): 62.714 ± 3.032%, 95% CI = 2.726 to 20.45, p < 0.01), day 6 (control (n = 20): 85.4 ± 1.459% vs. PD 1-7 (n = 18): 72.667 ± 4.058%, 95% CI = 2.499 to 22.97, p = 0.01), day 7 (control (n = 20): 87.2 ± 1.67% vs. PD 1-7 (n = 18): 78.415 ± 2.893%, 95% CI = 0.9801 to 16.59, p = 0.03), and day 8 (control (n = 20): 88.7 ± 1.46% vs. PD 1-7 (n = 18): 80.111 ± 2.975%, 95% CI = 0.8025 to 16.38, p = 0.03). These results indicate that PFOS exposure during postnatal 7 days may cause learning retardation.

Effect of PFOS exposure on learning at different time window. A Scheme of visual discrimination test B Dunnett’s multiple comparisons test revealed that the PD 1-7 group had a significantly lower discrimination index compared to the control group, particularly on days 3, 4, 6, 7, and 8. * p < 0.05, ** p < 0.01, *** p < 0.001.
Given the effects of developmental exposure to PFOS on memory and learning, we assumed gene expression profiles may be modified in PFOS-exposed pups. To identify the causal genes, RNA-seq was performed for each group. In comparison between the control group and the PD 1-7 group, there was significant suppression of the expressions of Gdf1, Serpina3g, Tmem91, Dok3 in the PD 1-7 group (Fig. 4A). In comparison between the control group and the PD 8-14 group, there was significant suppression of the expressions of Hbb-b1, Tmem254c, Tnfrsf25, Rpl22l1 in the PD 7-14 group (Fig. 4B). To confirm the differential expressed genes from RNA-seq, real-time PCR was performed for four candidate genes whose expressions were significantly suppressed in the PD 1-7 group (Gdf1, Serpina3g, Tmem91, Dok3). mRNA levels of Serpina3g (Fig. 4C) and Tmem91 (Fig. 4D) were significantly decreased in the PD 1-7 group ([Serpina3g] control (n = 5): 1.00 ± 0.1193 vs. PD 1-7 (n = 5): 0.4792 ± 0.04392, t = 4.098, df = 8, p < 0.01, [Tmem91] control (n = 5): 1.00 ± 0.2205 vs. PD 1-7 (n = 5): 0.334 ± 0.04657, t = 2.955, df = 8, p = 0.02). There was no change in mRNA levels of Gdf1 (Fig. 4E) and Dok3 (Fig. 4F) between groups ([Gdf1] control (n = 5): 1.00 ± 0.339 vs. PD 1-7 (n = 5): 1.094 ± 0.1735, t = 0.2475, df = 8, p = 0.81, [Dok3] control (n = 5): 1.00 ± 0.1287 vs. PD 1-7 (n = 5): 0.9177 ± 0.1787, t = 0.3737, df = 8, p = 0.72). mRNA levels of differentially expressed genes in the PD 8-14 group were also quantified; however, none of them were changed in real-time PCR (Supplemental Fig. 2). These results ensured the downregulation of Serpina3g and Tmem91 in the hippocampus following the PD 1-7 PFOS exposure.
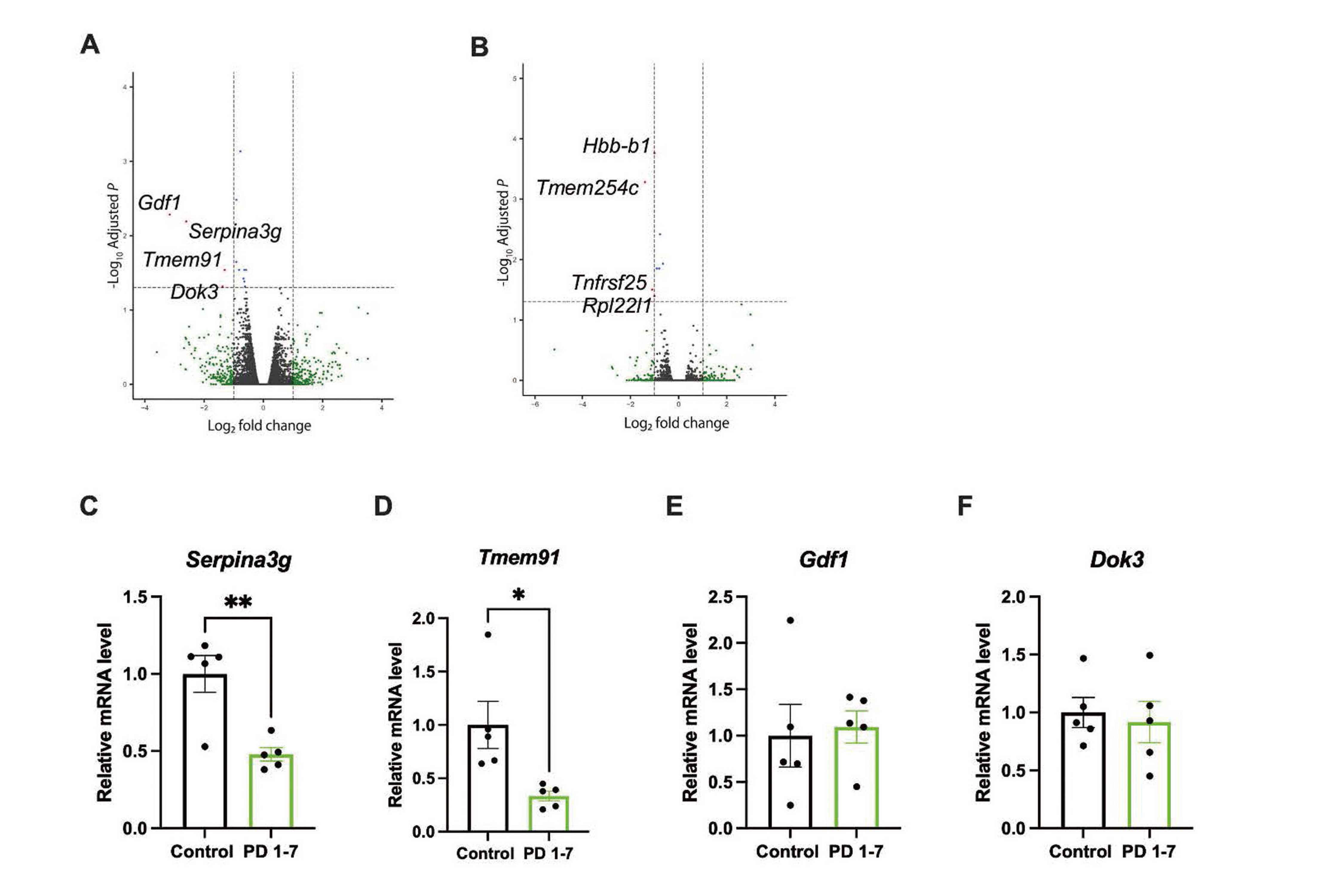
Differential expressed genes in PD 1-7 group and mRNA levels. A RNA-seq was performed using P21 hippocampus. Volcano plot for differential expressed genes between the control group and the PD 1-7 group. Four genes (Gdf1, Serpina3g, Tmem91, Dok3) were significantly downregulated in the PD 1-7 group. B Volcano plot for differential expressed genes between the control group and the PD 8-14 group. Four genes (Hbb-b1, Tmem254c, Tnfrsf25, Rpl22l1) were significantly downregulated in the PD 8-14 group. C, D, E and F Real-time qPCR for Serpina3g, Tmem91, Gdf1 and Dok3 mRNA, respectively to confirm the RNA-seq results using the same samples. Serpina3g and Tmem91 were significantly downregulated in the PD 1-7 group. No differences were found in Gdf1 and Dok3 mRNA levels between groups.
The present study explored the sensitive time window for exposure to PFOS especially in terms of brain development. The pups were exposed to PFOS through breast milk of dams given PFOS by gavage during PD 1-7 or PD 8-14. Body weight of pups exposed to PFOS was significantly reduced compared to the control group; however, there was no change in brain weight. According to the object location test, object recognition test, and visual discrimination test, memory and learning were most impaired in the PD 1-7 group. RNA-seq from PD 21 hippocampus revealed slight changes between groups but the mRNA levels of Gdf1, Serpina3g, Dok3, and Tmem91 were significantly reduced in the hippocampus of the PD 1-7 group. This mRNA profile does not overlap with and is different from that of the PD 8-14 group.
Developmental PFOS exposure has been a serious concern as it has been reported to be associated with neurodevelopmental disorders such as learning disorder (Luo et al., 2022; Goodman et al., 2023; Skogheim et al., 2020; Tanner et al., 2020) and ASD (Shin et al., 2020) in children. In addition to our previous reports on the effects of lactational PFOS exposure on learning, memory, and motor function (Mshaty et al., 2020; Ninomiya et al., 2022), some animal studies found that PFOS exposure during development, either gestation or lactation, deranged neurobehavioral development (Fuentes et al., 2007; Lau et al., 2003; Wang et al., 2015; Yu et al., 2009; Zhang et al., 2019; Johansson et al., 2008). These findings led to an embargo on PFOS use worldwide; however, PFOS has widely accumulated in the environment to date due to its chemical stability. High concentrations of PFOS have been detected in the water environment on a global scale, thus exposure through drinking water and seafood has been of high concern (Soleman et al., 2023; Muir and Miaz, 2021; Fujii et al., 2024). Considering the neurotoxicity of PFOS, it is important to monitor effects on offspring next generations of PFOS-exposed mothers and to assess the sensitive period of PFOS exposure for neurological development.
The major routes of PFOS exposure during development can be through either placenta and breast milk. Both epidemiological and animal experimental studies have reported no distinct difference in PFOS accumulation in serum of offspring between gestational and breastfeeding exposure, showing prenatal and postnatal exposure to PFOS equally contribute to infant PFOS serum concentrations (Yu et al., 2009; Gyllenhammar et al., 2018). In terms of neurodevelopment, it has been reported that spatial learning and memory abilities were impaired to a greater extent in prenatal exposure group than in postnatal exposure group (Wang et al., 2015), whereas another rodent study revealed that gestational exposure to PFOS caused no alteration in development of learning in offspring (Lau et al., 2003). Meanwhile, we previously found that lactational PFOS exposure (PD 1-14) reduced learning and memory in male offspring during adulthood (Mshaty et al., 2020). These discrepancies between findings suggest that the specific time window for neurodevelopmental vulnerability to PFOS exposure has remained controversial. For further analysis, we broke down the exposure period into PD 1-7 and PD 8-14 in the present study. Behavioral analyses indicated a consistent tendency in which the PD 1-7 group was more affected in terms of memory and learning compared to the PD 8-14 group. These findings strongly support previous studies considering postnatal period as a sensitive period for susceptibility to PFOS exposure and also underscore that early postpartum phase is especially sensitive to PFOS-induced neurodevelopmental deficits rather than the late phase. This may contribute to the setting of the exposure time period in further studies exploring the neurodevelopmental effects.
It is expected to clarify the mechanism underlying PFOS-induced neurobehavioral changes; nevertheless, little is known about how PFOS perturbs the development of central nervous system at the molecular level. PFOS actions in the other parts than the brain have been gradually demonstrated such as alteration of membrane fluidity (Hu et al., 2003), interference with gap junctional intercellular communication (Hu et al., 2002), disorganization of fatty acid and lipid metabolism (Hu et al., 2005), and disruption of internal hormone regulation (Chang et al., 2008; Austin et al., 2003) by utilizing transcriptional analysis in the liver (Bjork et al., 2008; Hu et al., 2005; Yeung et al., 2007; Krøvel et al., 2008) and the lung (Grasty et al., 2005). These previous studies have indicated tissue-specific variation in gene expression profiles following PFOS exposure and expectation for the unique pattern of transcriptome in the brain. However, in the present study, we found only 4 genes differentially expressed in the hippocampus of the PD 1-7 and PD 8-14 groups at PD 21. In the PD 1-7 group, Serpina3g and Tmem91 were significantly downregulated in both RNA-seq and real-time PCR (Fig. 4). In the hippocampus, Serpina3g has been reported to have pro-survival activity in reactive astrocytes, microglia and neurons (Sathyanarayana et al., 2008; Xiao et al., 2023). A previous study found that viral delivery of Serpina3g gene to the hippocampus protected neurons from oxidative stress in vitro and prevents progression of Alzheimer’s disease in vivo (Xiao et al., 2023), indicating the protective role of Serpina3g in cognitive function. In addition, a human study revealed a negative correlation between SERPINA3, an orthologue to murine Serpina3g, in cerebrospinal fluid and cognitive impairment based on Mini-mental state examination score (DeKosky et al., 2003). Although the age of PD 21 is too young to be related to such age-related neurodegenerative diseases, downregulation of Serpina3g in earlier life could affect later life considering our recent finding that early-life lactational PFOS exposure caused Alzheimer’s disease-like behaviors in middle-aged mice (Ninomiya et al., 2024). Therefore, it is likely that postnatal PFOS exposure may mediate hippocampal expression of Serpina3g and cognitive function though life from development to neurodegeneration. Tmem91, another differentially expressed gene following PFOS exposure during PD1-7, encodes a transmembrane protein that mediates the regulation of cell migration and invasion, constructs ion channels, and participates in the immune response (Chang et al., 2017; Ishikawa and Barber, 2008; Wrzesiński et al., 2015). Whereas most studies focus on its role in cancer rather than in cognitive function (Oktay and Jones, 2015), a relationship between the copy number variation of Tmem91 and thyroid disease has been suggested (Jin et al., 2018). Given that PFOS perturbs thyroid hormone metabolism in vitro (Fujiwara et al., 2023) and thyroid hormone status of both mothers and infants (Boesen et al., 2020) and that maternal hypothyroidism causes cognitive impairment in offspring (Min et al., 2016; Amano et al., 2018), there is a potential involvement of Tmem91 in cognitive functional development through thyroid hormone action.
There are a few limitations in the present study. First, the combined effect of PFOS isomers was not investigated. Electro-chemical fluorination (ECF) is applied in the manufacturing process of PFOS. This process results in a final mixture of PFOS that consists not only of L-PFOS (approximately 70%) but also branched isomers such as mono-methyl-substituted PFOS or di-methyl-substituted PFOS, collectively referred to as “branched PFOS” (Benskin et al., 2010). Consequently, PFOS is detected in human serum as a mixture of these isomers (Kang et al., 2023). Ideally, it would be preferable to examine the toxicity of the PFOS isomer mixture. However, since the neurotoxicity of PFOS is not well understood, the present study focused on examining the toxicity of L-PFOS, the predominant isomer in the mixture. Second, PFOS transfer from dams to pups was not directly demonstrated as we lack the measurement of concentration levels in breast milk and pups’ sera. Also, PFOS accumulations in target organs such as the brain should be examined. Future studies are expected to measure PFOS concentrations in serum, breast milk, and several tissues, which the present study lacks due to the technical unavailability. Finally, although the present study specifically focused on neurological development following lactational PFOS exposure, the indirect effect caused by general toxicity cannot be ignored, because the suppression of body weight gain was observed (Fig. 1B). This change is consistent with findings from epidemiological surveys summarized in the Agency for Toxic Substances and Disease Registry (ATSDR) report (Rogers et al., 2021). Failure to thrive may negatively impact a child's overall well-being, including neuronal development. Thus, it would be toxicologically meaningful to investigate whether there are effects on cognitive function at doses that do not cause fluctuations in toxicological indicators, such as body weight reduction.
Our work has demonstrated that the first 7 days after the birth is critically vulnerable to PFOS exposure and consequent neurodevelopmental deficits rather than the later phase of postpartum. On the other side, the expressions of genes related to neurobehavioral development or cognitive function were not much affected in PFOS-exposed hippocampus at PD 21. Taken together with the previous findings, this finding may implicate that PFOS immediately perturbs gene expression profiles in the brain following the exposure and brings about adverse effects on neurobehavioral development in the long run. Thus, the present study underlines that maternal PFOS concentration should be carefully monitored and sets off an alarm over the potential retardation of neurodevelopment.
This work was supported by the JSPS KAKENHI Grant Numbers 22J11280 (to A.N.) and JP24710068 (to A.H.).
Conflict of interestThe authors declare that there is no conflict of interest.