2017 Volume 67 Issue 3 Pages 45-53
2017 Volume 67 Issue 3 Pages 45-53
The aim of this study was to evaluate the effects of transcorneal electrical stimulation in subjects with primary open-angle glaucoma. Five eyes of four male subjects with primary open-angle glaucoma (average age: 52.25 ± 14.68 years) were enrolled. The subjects underwent transcorneal electrical stimulation every 3 months according to the following procedure. A Dawson-Trick-Litzkow electrode was placed on the cornea, and biphasic electric current pulses (10 ms, 20 Hz) were delivered using a stimulator (BPG-1,BAK Electronics) and a stimulus isolation unit (BSI-2). A current that evoked a phosphene that the subject perceived in the whole visual area was delivered continuously for 30 min. Humphrey visual field testing was performed after every third transcorneal electrical stimulation treatment. Changes in mean deviation (MD) values were evaluated with a linear regression model. Transcorneal electrical stimulation was performed 18.2 ± 9.4 times over a period of 49.8 ± 23.0 months. The average pretranscorneal electrical stimulation intraocular pressure, best corrected visual acuity, and MD values were 11.8 ± 1.79 mmHg, 0.14 ± 0.19 (logMAR) and −17.28 ± 6.24 dB, respectively. No significant differences were observed in intraocular pressure before and after transcorneal electrical stimulation. However, there was a significant positive linear relationship between changes in MD values and the number of transcorneal electrical stimulation treatments (R2 = 0.176, P = 0.005, Spearman correlation R =0.294, P = 0.008). Transcorneal electrical stimulation treatment may improve glaucomatous visual field defects in subjects with primary open-angle glaucoma. Large-scale studies are necessary to confirm these preliminary findings.
Glaucoma involves the progressive loss of retinal ganglion cells (RGCs) with concomitant visual field (VF) loss. Reducing intraocular pressure (IOP) is an effective treatment for open-angle glaucoma (OAG).1 However, glaucomatous VF loss can be seen in some patients with normal IOP, suggesting that keeping IOP low alone is not enough to inhibit glaucomatous optic neuropathy.
In a 1967 publication, Jarvik et al. reported the use of transcorneal electrical stimulation (TES), a noninvasive procedure involving electrical neuro-retinal stimulation, to treat retrograde amnesia in mice.2 Since then, many studies have described the use of TES to treat various diseases, including eye diseases. For example, Morimoto et al. reported that electrical stimulation has neuroprotective effects after optic nerve transection in adult rats,3 and Fujikado et al. described the neuroprotective effects of TES.4 TES has also been found to delay the degeneration of photoreceptors and the impairment of retinal function in Royal College of Surgeons rats used as an animal model of retinitis pigmentosa.5 In clinical studies, TES has been shown to improve visual acuity (VA) and/or VF in patients with various neuroretinal diseases, including retinal artery occlusion (RAO), nonarteritic ischemic optic neuropathy, Best vitelliform macular dystrophy, and traumatic optic neuropathy.6,7,8,9,10 TES also leads to improved electroretinography (ERG) findings and VF in patients with retinitis pigmentosa.11 To date, however, no information is available concerning the long-term therapeutic effects of TES on glaucoma.
The aim of this study was to evaluate the effects of TES on subjects with primary open angle glaucoma (POAG).
This was a prospective hospital-based case-series study carried out at Keio University Hospital, Tokyo, Japan. It was approved by the Ethics Committee of Keio University School of Medicine, and all procedures were in accordance with the Declaration of Helsinki. All subjects gave written informed consent prior to enrolment.
SubjectsFive eyes of four male subjects with POAG were enrolled. The average age of the subjects was 52.25 ± 14.68 years (mean ± standard deviation) (Table 1). Glaucoma specialists (NO, IK) carried out the following tests on all subjects: best-corrected visual acuity (BCVA) measurement, IOP measurement (by Goldmann applanation tonometry), slit lamp microscopy, gonioscopy, fundus examination, and visual field measurement (with a Humphrey visual field analyzer [HFA]). All subjects were familiar with HFA testing, having been subjected to it at least twice in the 9 months before they entered the study. All subjects had typical glaucomatous optic disc cupping and concomitant glaucomatous VF defects (mean deviation; MD <–12 dB). Average pre-TES IOP, BCVA, and MD values were 11.8 ± 1.79 mmHg, 0.14 ± 0.19 (logMAR), and −17.28 ± 6.24 dB, respectively.
| Case | Eye | Type of glaucoma | Age (years) | VA pre-TES (LogMar) | IOP pre-TES (mmHg) | Baseline MD pre-TES (dB) |
|---|---|---|---|---|---|---|
| 1 | R | POAG | 34 | 0 | 9 | −13.44 |
| 2 | L | NTG | 69 | 0.3 | 12 | −28.14 |
| 3 | R | POAG | 57 | 0.4 | 12 | −16.59 |
| 3 | L | POAG | 57 | 0 | 14 | −12.95 |
| 4 | L | NTG | 49 | 0 | 12 | −15.28 |
R, right eye; NTG, normal-tension glaucoma.
The baseline MD pre-TES values are the average of those obtained on the last two examinations before TES.
The sensitivity of the visual field to the presented stimulus is recorded for each test location. The threshold calculated for that point is compared to a database of normal individuals of similar age. The average of these deviations over all test points is called the mean deviation (MD).
TES procedureThe cornea and conjunctiva were anesthetized with 0.4% oxybuprocaine hydrochloride and covered with 3% hyaluronic acid and 4% chondroitin sulfate (Viscoat, Alcon Japan, Tokyo, Japan). A Dawson-Trick-Litzkow electrode (Tomey, Nagoya, Japan) was placed on the cornea (Fig. 1a). Biphasic electric current pulses were delivered using a stimulator (BPG-1, BAK Electronics, Mount Airy, MD, USA) through a stimulus isolation unit (BSI-2, BAK Electronics) (Fig. 1b). The current of the pulses (duration: 10 ms, frequency: 20 Hz) was increased to determine the threshold current necessary to elicit a phosphene. The current that evoked a phosphene that the patient perceived over almost the whole visual area was determined (409.38 ± 390.28 μA, [range 100–1800]), and this level of current was delivered continuously for 30 min via biphasic pulses (Fig. 2). We performed stimulation with 300 μA or less when subjects felt brightness throughout the visual field with 300 μA or less. When the brightness did not change even with stimulation of more than 500 μA, we performed TES with an amplitude (300–500 μA) at which patients did not feel pain on the skin. TES was performed in all cases by NO or IK.

Equipment used for transcorneal electrical stimulation.
(a) Photograph of an eye with a Dawson-Trick-Litzkow electrode in place. The cornea and conjunctiva were anesthetized with 0.4% oxybuprocaine hydrochloride and covered with 3% hyaluronic acid and 4% chondroitin sulfate. The electrode was placed on the conjunctiva and the lower part of the cornea. (b) Stimulator and stimulus isolation units. Biphasic electric current pulses were delivered using a stimulator (large box, BPG-1) through a stimulus isolation unit (small box, BSI-2). The images in Figure 1a is of one of the article’s authors.

Current intensity evoking a phosphene covering more than half of the visual field. The current of the pulses (duration: 10 ms, frequency: 20 Hz) was increased to determine the threshold current necessary to elicit in each subject a phosphene perceived to cover almost the whole visual area. The mean threshold current was 409.38 ± 390.28 μA (range 100–1800) and, once determined, was delivered continuously for 30 min via biphasic pulses.
TES was performed once every 3 months. The examiners who performed VF testing were blinded to TES treatment. Humphrey VF testing and multifocal ERG (mfERG) were performed after every third TES treatment. During the course of the study, topical medication to decrease IOP was administered as necessary.
Values analyzedBCVA, IOP, and changes in MD values as determined with the HFA central 30–2 program (Swedish Interactive Threshold algorithm 30–2) were analyzed. Changes in MD were calculated as the MD value after TES treatment minus the value at baseline.
The mfERG findings were processed with Visual Evoked Response Imaging System software (VERIS science 4.1.1, Mayo, Inazawa, Japan). The stimulus was composed of 61 hexagonal elements, but hexagons lying on the horizontal midline were not used in the analysis. The initial negative wave (N1) and the first positive peak (P1) were examined. We also analyzed the latencies and amplitudes of both N1 and P1.
Statistical analysisThe Spearman correlation coefficient, a linear regression model, and a mixed effect model were used to evaluate BCVA, IOP, changes in MD values, and N1 and P1 amplitudes and latencies. SPSS software (version 23, IBM, NY, USA) was used for the analyses. A P value less than 0.05 was considered statistically significant.
During the study period, TES was performed 18.2 ± 9.4 times (mean ± standard deviation, range 6–27) over a period of 49.8 ± 23.0 months (range 11–68 months).
There was no significant change in MD value in any patient by linear regression analysis (P > 0.05, Fig. 3 and 4). However, there was a significant positive linear relationship between changes in MD values and the number of TES treatments (R2 = 0.176, P = 0.005; Spearman correlation R = 0.294, P = 0.008) (Fig. 5).
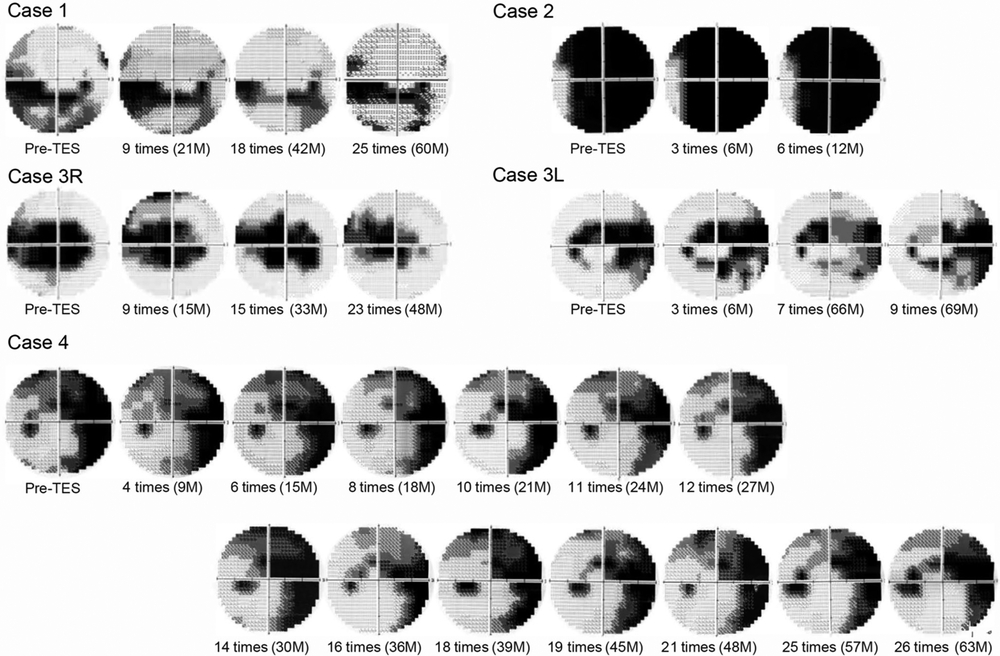
Changes in the visual field of each patient after transcorneal electrical stimulation. The visual fields at least show no worsening during the treatment period. The visual fields were measured by the HFA central 30-2 program (Swedish Interactive Threshold algorithm 30-2).

Changes in MD values of each patient. There were no significant associations between MD values and the number of TES treatments in any patient (P > 0.05).

Changes in MD values (differences between post-TES values and baseline MD). There was a significant positive linear relationship between changes in MD values and the number of TES treatments (R2 = 0.176, P = 0.005; Spearman correlation R = 0.294, P = 0.008).
The average IOP before TES treatment was 11.8 mmHg, and at 6 months, 12 months, and 18 months after the series of TES interventions, it was 11.3 mmHg, 12.0 mmHg, and 15.7 mmHg, respectively. Thus, there was no significant difference in IOP before and after TES treatment (P > 0.05, mixed model). Nor was any significant difference observed in N1 latency or amplitude in either the superior area or the inferior area before and after TES treatment (P > 0.05, a mixed model, Fig. 6), or in P1 latency or amplitude in either area (P > 0.05, mixed model, Fig. 7). BCVA at baseline was 0.14 ± 0.19 (mean ± standard deviation, logMAR), and after the last TES it was 0.68 ± 0.90. All subjects had superficial punctate keratopathy after TES treatment. One patient developed a cataract during the study period. Decreased VA in association with the progression of glaucomatous VF loss was noted in only one eye during TES treatment.

Mean N1 amplitude and latency on multifocal ERG of the five eyes. No significant differences were observed in N1 latency or amplitude in either the superior or inferior area before and after TES treatment (P > 0.05, mixed effect model). (a) N1 amplitude in the superior field, (b) N1 amplitude in the inferior field, (c) N1 latency in the superior field, (d) N1 latency in the inferior field.

Mean P1 amplitude and latency on multifocal ERG of the five eyes. No significant differences were observed in P1 latency or amplitude in either the superior or inferior area before and after TES treatment (P > 0.05, mixed effect model). (a) P1 amplitude in the superior field, (b) P1 amplitude in the inferior field, (c) P1 latency in the superior field, (d) P1 latency in the inferior field.
This is the first study describing the effects of long-term TES treatment on patients with glaucoma. It showed that the number of TES treatments is positively associated with changes in MD values, suggesting that TES may improve RGC function.
An important issue is whether it is possible to improve VF in patients with glaucoma. It is widely believed that genuine improvements in glaucomatous VF do not occur. Nevertheless, our findings that TES improved glaucomatous VF is supported by several reports describing improvements in glaucomatous VF defects in humans.12,13,14,15 Caprioli et al. investigated the proportions of VF locations decaying or improving before and after surgery in POAG patients treated with trabeculectomy: 30% of locations improved preoperatively, and 44% improved postoperatively.16 In their control group, 34% of locations improved during the first half of follow-up, and 35% improved during the second half of follow-up; a chi-squared comparison of the rates of improvement in these two groups was statistically significant (P < 0.0001), suggesting that trabeculectomy can improve glaucomatous VF defects. Musch et al. also found that some patients participating in the Collaborative Initial Glaucoma Treatment Study showed VF improvement over a long period after starting initial treatment; they also showed, importantly, that measures of better IOP control during treatment were significantly predictive of VF improvement, thereby indicating that improvement was not just a learning effect or chance.17 Caprioli et al. attributed the improvements they observed in glaucomatous VF loss to the revitalization of RGCs. We believe that TES may revitalize RGC function by mimicking the IOP lowering effect of trabeculectomy, thereby improving glaucomatous VF defects. However, the exact mechanism by which TES improves these defects remains to be elucidated.
Morimoto et al. investigated the effects of TES after optic nerve (ON) transection in rats.3,18 Seven days after transection, the number and survival rate of RGCs were measured, and the RGC survival rate in the TES group was found to be significantly higher than that in the controls. The levels of insulin-like growth factor 1 (IGF-1), a neurotrophic factor, in the retinas of rats not undergoing ON transection also increased significantly after TES, as determined by RT-PCR and western blot analysis. In the intact retina, IGF-1 is located in the basal endfeet of Muller cells,3 and TES promotes the level of IGF-1 secretion from Muller cells. A high dose of JB-3, an IGF-1 receptor antagonist, reduced the survival of RGCs after TES in rats undergoing ON transection.3 These results indicate that IGF-1 plays a role in the survival of injured RGCs.
Tagami et al. reported that axonal regeneration after ON transection was promoted by daily TES treatment in rats.19 This result was also related to IGF-1 levels. TES may promote RGC function by promoting axonal regeneration in human POAG patients.
Another possible explanation for improved VF is increased ocular blood flow. Inomata et al. showed that the light reflectance changes in the posterior retina induced by TES were slow in two healthy rhesus monkeys, and also that there was a strong correlation between the slow components of the intrinsic signal and blood flow changes.20 This finding suggests that TES increases blood flow. Kurimoto et al. reported that chorioretinal blood flow midway between the optic disc and the macula increased after TES in healthy human subjects.21 The Leuven Eye Study showed that patients with OAG had lower blood velocities in the central retinal vessels than the controls did.22 From these studies, we can hypothesize that TES has an effect on the retinal vessels and improves chorioretinal blood flow, which may improve RGC function.
Our study revealed no significant differences in latencies and amplitudes on ERG before and after TES, suggesting that the side effects of TES on retinal function in POAG subjects is minimal. Shatz et al. demonstrated that latencies and amplitudes on ERG in patients with retinitis pigmentosa during TES treatment did not change significantly,11 and Naycheva et al. also reported similar results in RAO subjects before and after TES.8 However, some researchers have reported improved mfERG responses. Oono et al. reported significant reductions in N1 and P1 latency after TES in subjects with RAO, although the N1 and P1 amplitudes did not change significantly.9 Inomata et al. demonstrated that N1 and P1 amplitudes improved significantly after TES in some patients with longstanding RAO.10 Although differing mfERG responses have been reported in previous TES studies, at least no deterioration in responses after TES treatment has been reported, which indicates that TES has no harmful effects on the retina. In 2015, Machida et al. reported that a focal photopic negative response on the ERG indicates RGC function in OAG subjects.23 In future studies, it would be helpful to use focal macular ERG or full-field photopic ERG to objectively evaluate RGC function.
Many studies have shown improved BCVA after TES in subjects with nonarteritic ischemic optic neuropathy, RAO, traumatic optic neuropathy, or Best vitelliform macular dystrophy.6,7,10 However, we observed no improvements in BCVA in our study. This may be partially explained by the specific conditions of two of our subjects. In case 2, slight changes were difficult to detect because central VF had already been lost before TES. This patient experienced central vision loss, probably as a result of the progression of glaucomatous VF loss. In case 4, we could not evaluate the actual effect of TES on BCVA because the patient developed a cataract. After the study, this patient had cataract surgery, following which BCVA improved. Further study is needed to evaluate the effects of TES on BCVA in subjects with POAG.
As far as we know, no studies examining the effects of TES on IOP have been reported, but our study revealed no significant differences in IOP before and after TES treatment. Reducing IOP is the standard treatment for glaucoma, so we gave our subjects topical IOP-lowering drugs, and IOP was maintained at a low level. Therefore, we could not confirm whether the effects on glaucoma we saw were the result of IOP control or TES treatment, although we can say that VF loss may have progressed without TES. For confirmation, we need to carry out further studies with a control group.
This study is subject to several limitations. First, we did not have a control group. It is possible that improvements in VF might have been observed in a control group, although, in general, glaucomatous VF defects do not improve. Second, our sample of POAG patients undergoing TES treatment was small. Third, we changed the topical anti-glaucoma treatment during the study period, which may have affected our results.
In conclusion, patients with POAG are able to tolerate TES treatment for long periods, and TES may have beneficial therapeutic effects in some cases of progressive glaucomatous optic neuropathy. Large-scale studies are necessary to confirm our preliminary findings.
The authors received no financial support for this study.
We have no conflict of interest to declare.