2021 Volume 70 Issue 1 Pages 19-23
2021 Volume 70 Issue 1 Pages 19-23
Proton pump inhibitors (PPIs) are widely used medicines worldwide. However, a rare etiology of syndrome of inappropriate secretion of antidiuretic hormone (SIADH) related to PPI was recently reported. Therefore, the putative role of PPIs in SIADH cannot be underestimated. A 78-year-old Japanese woman was admitted to our hospital for treatment of left Bell’s palsy. On admission, the patient was oriented with normal laboratory data, including a serum Na level of 135 mEq/L. Oral glucocorticoids and a proton pump inhibitor were initiated in combination with oral valaciclovir. Six days later, the patient’s consciousness became impaired. Laboratory data showed a serum Na level of 103 mEq/L, a urine Na level of 64.8 mEq/L, a urine K level of 43.6 mEq/L, and a urine osmolality of 450 mOsm/kg H2O. The patient met the criteria for SIADH. The initial treatment included water restriction and 3% hypertonic saline administration. The cessation of PPI significantly improved the urine diluting capacity and concomitantly increased serum Na, which indicated that the use of PPI had been responsible for the etiology of SIADH. The present case illustrates that physicians need to be aware of the uncommon adverse effects of PPI, such as SIADH.
Hyponatremia, defined as a serum Na concentration below 135 mEq/L, is a condition characterized by an excess of water without Na deficit caused by impaired excretion of free water.1 Renal free water excretion is tightly regulated by the serum antidiuretic hormone (ADH). In normal conditions, the serum osmolality plays an important role in regulating ADH release. An increase in serum osmolality of even 1% significantly increases serum ADH, with a proportional increase in urine concentration.2
In contrast to the normal response to serum osmolality, the syndrome of inappropriate secretion of ADH (SIADH) is a condition in which ADH is disproportionally secreted regardless of the serum osmolality. This inappropriate secretion results in water retention and subsequent hyponatremia. There are several etiologies of SIADH, including central nervous system disturbances, malignancy, recent surgery, and drugs.3
Proton pump inhibitors (PPIs) are one of the most widely administered drugs worldwide. However, there has been a growing concern that PPI use is associated with various adverse effects, including cardiovascular diseases,4Clostridium difficile infection,5 bone fractures,6 kidney damage, and electrolyte disturbances.7,8 Although electrolyte disturbances were considered to be a relatively rare side effect, hypomagnesemia, hypocalcemia, and hypokalemia have been reported.8 Hyponatremia related to PPI administration is less common, and its pathophysiology remains to be elucidated. Herein, we present the case of a female patient with hyponatremia due to SIADH, which might have been related to PPI use.
A 78-year-old Japanese woman with hyponatremia was referred to the Nephrology Department for evaluation. Six days earlier, the patient had been transferred to the Otorhinolaryngology Department because of the sudden onset of left facial paralysis. The patient’s medical history included hypothyroidism and she had been administered levothyroxine. On admission, the patient was alert and oriented. Cranial nerve examination demonstrated the inability to generate wrinkles on the left side of the forehead, loss of the left nasolabial fold, loss of taste sensation, and drooping of the left corner of the mouth. The patient did not drink alcohol, smoke tobacco, or use supplements. Laboratory tests were normal for kidney function, liver function, and electrolytes, including a serum Na level of 135 mEq/L (Table 1). Brain magnetic resonance imaging revealed no abnormalities in the internal auditory canal or the cerebellopontine angle. The patient was diagnosed with left Bell’s palsy. A dose of 50 mg/day of oral glucocorticoids was initiated. Proton pump inhibitor (esomeprazole) administration was concomitantly initiated to prevent glucocorticoid-induced gastric ulcer. Oral valaciclovir was also prescribed for 7 days.
| Day 1 | Day 6 | Day 11 | |
|---|---|---|---|
| Serum | |||
| Na mEq/L | 135 | 103 | 132 |
| K mEq/L | 3.7 | 3.5 | 3.2 |
| Cl mEq/L | 97 | 72 | 87 |
| BUN mg/dL | 10.6 | 7.6 | 7.6 |
| Creatinine mg/dL | 0.71 | 0.42 | 0.63 |
| Uric acid mg/dL | 4.4 | 1.9 | 3.1 |
| Alb | 4.0 | 3.9 | 3.0 |
| Osmolality mOsm/kg H2O | - | 218 | - |
| ADH pg/mL | - | 27.4 | - |
| Cortisol µg/dL | - | 30.8 | - |
| TSH µUI/mL | - | 3.43 | - |
| Free T4 ng/mL | - | 1.39 | - |
| Urine | |||
| Na mEq/L | - | 64.8 | 59.6 |
| K mEq/L | - | 43.6 | 10.2 |
| Cr mg/dL | - | 39.3 | 25.2 |
| Osmolality mOsm/kg H2O | - | 450 | 243 |
ADH, anti diuretic hormone; BUN, blood urea nitrogen; TSH, thyroid stimulating hormone; T4, Thyroxine 4.
On the sixth day of hospitalization, the patient’s consciousness was impaired. Laboratory tests showed a serum Na level of 103 mEq/L, a urine Na level of 64.8 mEq/L, a urine K level of 43.6 mEq/L, and a urine osmolality of 450 mOsm/kg H2O (Table 1). The possible etiologies of hyponatremia were evaluated. During hospitalization, the patient ate about 50% of her meals (because of loss of taste sensation) and took about 500–700 mL of fluid in addition to meals. However, the patient denied any vomiting, diarrhea, or polydipsia. Medications associated with hyponatremia, such as antidepressants, anticonvulsants, antipsychotics, and vasopressin analogs, were not used. On examination, the vital signs were unremarkable, with a blood pressure of 118/60 mm Hg, a temperature of 36.5°C, and a heart rate of 68 beats/min. Physical examination showed no peripheral edema and no abnormal heart or respiratory sounds. The patient history and physical examination indicated that the patient was euvolemic. Additional laboratory examinations ruled out hypothyroidism or adrenal insufficiency. Inadequate oral intake might have contributed to the development of hyponatremia; however, it is not likely that this alone caused such a sudden decrease in serum Na levels from 135 to 103 mEq/L. As an underlying etiology of hyponatremia, SIADH has been suggested. Initial treatment was performed with fluid restriction combined with administration of 3% hypertonic saline at 0.6 mL/kg/h (Fig. 1). Hypertonic saline (3%) was used because the patient’s urine tonicity (urine Na + urine K) exceeded serum Na, which indicated that free water excretion was impaired and spontaneous improvement of serum Na seemed unlikely. After 2 days, serum Na improved to 113 mEq/L with a urine Na level of 33.1 mEq/L, a urine K level of 25.7 mEq/L, and a urine osmolality of 386 mOsm/kg H2O. At this point, the patient’s urine Na and urine osmolality were still elevated. Therefore, drug-induced SIADH was suspected. The medications used during hospitalization included glucocorticoids, levothyroxine, valaciclovir, and a proton pump inhibitor. The proton pump inhibitor was considered to be the likely causative factor for SIADH and was discontinued. Subsequently, serum Na gradually increased with a concomitant decrease in urine osmolarity. Administration of hypertonic saline was switched from 3% to 0.9% at 1 mL/kg/h until the patient could take enough food. She was discharged on the 15th day of hospitalization with a serum Na level of 135 mEq/L. Written informed consent was obtained from the patient for reporting this case.
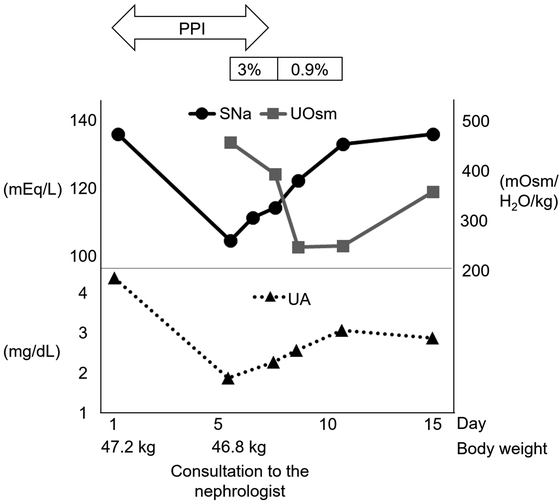
Clinical course. On admission, the laboratory data showed no abnormalities, with a serum Na level of 135 mEq/L. Six days after hospitalization, the serum Na level had dropped to 103 mEq/L. The patient showed signs of confusion. Therefore, 3% hypertonic saline was initiated. After discontinuation of PPI, urine osmolality decreased with accompanying improvement in serum Na. Hypouricemia occurred concomitantly with hyponatremia, and improved with increases in serum Na. Abbreviations: 3%, 3% hypertonic saline; 0.9%, 0.9% saline; PPI, proton pump inhibitor; SNa, serum sodium; UA, uric acid; and UOsm, urine osmolality.
The underlying mechanism of hyponatremia related to PPI use remains to be fully elucidated. However, as an underlying etiology of hyponatremia, administration of PPI was likely responsible for the development of SIADH in some cases.9,10,11 There are several test results that point to a diagnosis of SIADH. First, the presence of decreased urine diluting capacity is the essential feature of SIADH.12 For example, hyponatremia with high urine concentration (usually >300 mOsm/kg H2O) is a good indication that should raise the suspicion of SIADH. Second, SIADH is mostly associated with euvolemic hyponatremia.13,14 Urine Na levels combined with physical examinations are useful to evaluate volume status. A previous study illustrated the diagnostic value of urine Na levels in hyponatremic patients without edema. It showed that the mean urine Na was 72 mEq/L in patients with euvolemia (SIADH) compared to 18 mEq/L in patients with hypovolemic hyponatremia.13 Third, the coexistence of hypouricemia (<4 mg/dL) with increased fractional excretion of uric acid (FEUA) is often observed in patients with SIADH.15 Interestingly, in patients with SIADH, the improvement in hypouricemia and FEUA was concomitantly observed with the increase in hyponatremia.16 Lastly, before SIADH is diagnosed, other etiologies of euvolemic hyponatremia, including polydipsia, malnutrition, glucocorticoid deficiency, and hypothyroidism, should be excluded.
In our case, the clinical history and physical examination results suggested that the patient was euvolemic. The presence of high urine osmolality, high urine Na concentration, and the concomitant improvement in hyponatremia and hypouricemia were all consistent with the characteristics of SIADH. Finally, the detection of an elevated serum ADH level confirmed the diagnosis of SIADH. Before consultation, intravenous fluid was not administered; therefore, iatrogenic hypotonic fluid-induced hyponatremia was ruled out. Other causes of hyponatremia, including polydipsia, hypovolemic hyponatremia, heart failure, liver cirrhosis, adrenal insufficiency, thyroid dysfunction, and renal salt wasting, were all ruled out. The cessation of PPI significantly improved urine osmolarity and concomitantly increased serum Na. Although PPI-induced SIADH is considered to be rare, recent studies have reported this possibility.9,10,11 Concomitant with previous reports, we interpreted our clinical course as the putative involvement of PPI in SIADH. Therefore, we propose that physicians should consider PPI as a possible cause of SIADH. In general, some mechanisms of drug-induced SIADH are known.17 For example, carbamazepine and oxcarbazepine reportedly increase sensitivity to ADH. Chlorpropamide increases the number of vasopressin-2 (V2; antidiuretic) receptors in collecting tubules. Abuse of methylenedioxymethamphetamine is associated with the direct release of ADH and stimulation of thirst, which further worsens hyponatremia. However, PPI-induced SIADH is less common; therefore, the precise mechanism remains unclear and more research is needed.
PPIs are also known to cause acute interstitial nephritis (AIN). If PPI-induced AIN occurs concomitantly with SIADH, it will disturb free water excretion and exacerbate hyponatremia. It is important for physicians to evaluate whether AIN contributes to the development of hyponatremia. In our case, the patient did not show any allergic-type symptoms related to AIN and laboratory data did not indicate AIN, e.g., increased levels of eosinophilia or eosinophiluria. Therefore, we excluded the possibility of PPI-induced AIN. Furthermore, the possible contribution of valacyclovir to the development of hyponatremia should be addressed. A previous report described a patient who received valacyclovir and developed acute kidney injury concomitant with hyponatremia.18 Another report involved a patient who was administered acyclovir and developed hyponatremia.19 The latter report proposed a possible mechanism of hyponatremia due to SIADH, which might have been caused by herpes zoster virus infection and the administration of acyclovir. However, a causative relationship between valacyclovir and hyponatremia remains inconclusive. We speculate that the administration of valacyclovir in our case might have accelerated the development of PPI-induced SIADH.
Whether the type of PPI or the duration of PPI administration influences the development of hyponatremia remains unclear. One previous study showed that the hyponatremia effect was most pronounced for omeprazole, followed by lansoprazole and rabeprazole.8 In our case, esomeprazole was considered to be responsible for SIADH. Therefore, it should be noted that all types of PPI could be responsible for SIADH. Another study found that moderate hyponatremia was observed in 18.7–46.3% of elderly patients who took PPI for more than 1 year.20 In our case, both the onset and improvement of hyponatremia occurred quickly compared to previous cases.20,21 We speculate that the susceptibility to PPIs might vary between individuals.
In conclusion, we presented the case of a patient who rapidly developed hyponatremia that might have been related to PPI use. We emphasize that it is important to realize the uncommon adverse effect of PPIs, such as SIADH, regardless of the type of PPI or duration of use. This advice will help physicians to provide appropriate patient management in clinical practice.
The authors declare that no conflicts of interest exist.