2022 Volume 71 Issue 4 Pages 73-81
2022 Volume 71 Issue 4 Pages 73-81
Our understanding of the biology of the intestinal epithelium has advanced since the establishment of an organoid culture system. Although organoids have enabled investigation of the mechanism of self-renewal of human intestinal stem cells in vitro, it remains difficult to clarify the behavior of human normal and diseased intestinal epithelium in vivo. Recently, we developed a xenotransplantation system in which human intestinal organoids are engrafted onto epithelium-depleted mouse colons. This xenograft recapitulated the original tissue structures. Upon xenotransplantation, normal colon organoids developed normal colon crypt structures without tumorigenesis, whereas tumor-derived organoids formed colonic tumors resembling the original tumors. The non-tumorigenicity of human intestinal organoids highlights the safety of organoid-based regenerative medicine. As an example of regenerative medicine for short bowel syndrome, we devised a unique organ-repurposing approach to convert colons into small intestines by organoid transplantation. In this approach, the transplanted rat small intestinal organoids not only engrafted onto the rat colons but also remodeled the colon subepithelial structures into a small intestine-like conformation. Luminal flow accelerated the maturation of villi in the small intestine, which promoted the formation of a lymphovascular network mimicking lacteals. In this review, we provide an overview of recent advances in gastrointestinal organoid transplantation and share our understanding of human disease biology and regenerative medicine derived from these studies.
The intestinal epithelium is one of the most rapidly renewing tissues, and this homeostasis is strictly controlled by the intestinal stem cells located at the bottom of the crypts.1 Intestinal stem cells are defined by their capacity for multipotent differentiation and long-term self-renewal. Previously, long-term culture from primary adult tissue stem cells was not possible, which limited intestinal stem cell research to experiments in mice and cell lines. In 2007, in vivo genetic lineage tracing experiments using a genetically engineered mouse model demonstrated that the crypt base columnar cells, marked by the expression of LGR5, serve as intestinal stem cells.1 When Cre recombinase was activated by the administration of tamoxifen, LGR5-LacZ-positive cell-derived LacZ+ blue clones emanated from the crypt bottoms and later extended up the sides of the crypts. Owing to the discovery of LGR5, researchers have obtained a better understanding of the regulatory niche signals that cooperatively direct intestinal stem cells to perform their functions properly. By recapitulating the in vivo extracellular niche microenvironments of intestinal stem cells, Sato et al. established an in vitro three-dimensional (3D) culture system known as organoids, which allowed the long-term expansion of adult intestinal epithelium.2 After being established as a culture method for the mouse small intestine, this technique paved the way for establishing culture systems for human gastrointestinal tissues3,4,5,6 and various other organs.7,8,9 With the advent of organoids, the study of intestinal stem cells has made tremendous progress over the past 15 years.10 Organoids are useful not only as an in vitro research tool, but also as an in vivo research tool for studying human intestinal epithelium, and have potential as a cell source for transplantation. In this review, we highlight in vivo studies on intestinal organoids, including our own work.
The organoid culture system reconstructs a niche; that is, a microenvironment essential for the maintenance of stem cell function, including growth factors and extracellular matrix that support the proliferation of tissue-derived stem cells ex vivo. For mouse small intestinal organoid culture, epidermal growth factor (EGF), a bone morphogenetic protein inhibitor (noggin), and a Wnt activator (R-spondin1) are added to the culture medium and embedded in Matrigel extracellular matrix.2 Although the composition of the stem cell niche varies by organ and species, the addition of Wnt-3A and a transforming growth factor-β inhibitor to the above conditions enabled the culture of mouse colon, and further addition of a p38 mitogen-activated protein kinase inhibitor enabled the culture of human intestinal organoids.3,11 However, these organoids have had difficulty maintaining a differentiated cell type because of their strong dependence on growth factors that inhibit differentiation. Although p38 inhibitor is required for long-term maintenance of human intestinal organoids, its removal is essential for the reproduction of secretory cells in vitro. Screening for biologically relevant niche factors produced by human intestinal subepithelial stromal cells revealed that the combination of insulin-like growth factor-1 and fibroblast growth factor-2 greatly promotes organoid growth and represents a niche factor for human intestinal stem cells that could replace EGF and a p38 inhibitor.6 Single-cell RNA sequencing revealed that intestinal epithelia cultured under these refined organoid culture conditions maintain a pattern of gene expression that is highly homologous to that of in vivo tissues. These conditions also promoted the growth of p38 inhibitor-sensitive colon tumor organoids, including patient-derived ulcerative colitis (UC)-associated dysplastic organoids, and promoted the efficiency of CRISPR–Cas9-mediated genome editing.6,12
The organoid culture method of normal intestinal stem cells is associated with a low risk of tumorigenesis because the cultivation of tissue stem cells occurs by simply taking intestinal tissue as the original source and adding factors. Unlike other organs that are difficult to access for tissue collection, the intestine has the unique advantage of being easily accessible using advanced endoscopic technology. Therefore, the application of patient-derived somatic epithelial stem cells in regenerative medicine, in which they are grown in vitro and used as cells for transplantation, has been eagerly anticipated. In 2012, Yui et al. reported the successful transplantation (via a transanal approach) and engraftment of colonic organoids (from enhanced green fluorescent protein transgenic mice) into ulcerated areas of the colon of Rag2-knockout mice with dextran sulfate sodium salt (DSS)-induced colitis.13 This success stimulated research in Japan towards a first-in-human trial of organoid transplantation for patients with UC. Subsequently, several groups successfully transplanted various mouse normal organoids into mice and successfully used collagen instead of Matrigel as extracellular matrix.14,15,16,17,18,19,20,21
Despite these studies in mice, no method had been developed to reconstruct human intestinal epithelium in vivo by transplanting normal human intestinal epithelium. Tumor cells grow subcutaneously and under the renal capsule in immunodeficient mice,4,22 but human normal intestinal cells cannot grow at such sites where niche factors that support epithelial growth are lacking. Therefore, the only way to study the human intestinal epithelium was to study it in humans themselves, but the administration of various drugs and gene editing for analysis in this context naturally involve ethical difficulties. Most study results were interpreted on the assumption that human intestinal epithelium would probably be similar to mouse epithelium. However, although the stem cell marker LGR5 was identified in mice,1 it has not been rigorously demonstrated whether LGR5 is a stem cell marker in humans. To address this issue, we sought to establish a method for xenotransplantation of human normal colonic organoids using immunodeficient mice. Because natural killer (NK) cell activity plays an important role in the rejection of xenotransplanted cells, NOD.Cg-PrkdcscidIl2rgtm1Sug/ShiJic (NOG) mice with multiple immune functional defects, including dendritic cells and the absence of T, B, and NK cells, were used as human colonic organoid recipients.23 After trying various epithelial abrasion methods, we decided to use ethylenediaminetetraacetic acid (EDTA), which has been proven to be optimal for epithelium removal.2,15 Importantly, the crypts of the colonic epithelium of the recipient mice must be completely removed, including the crypt base where the stem cells reside, or they will be repaired by the original epithelium, and the transplanted cells will fail to engraft. Using EDTA-based breaching with minimal damage to nonepithelial tissue, we were able to establish a technique for removing the crypts and replacing them with transplanted organoids.24,25 Organoid xenografts that have been fluorescently labeled by the piggyBac transposon system or lentivirus-based gene transfer can be observed endoscopically over time. When human colonic organoids were transplanted into the mouse colon using this technique, the structure reconstructed in the mouse colon was that of the human colonic crypt, which is clearly larger than the mouse crypt structure and has different mucus and other properties. This means that the stem cell intrinsic program of transplanted cells is preserved in different environments. Transplanted human colonic epithelial cells were found to grow tumor-free in the mouse colon for more than 10 months, providing the first significant data towards clinical application (Fig. 1), and showing that transplanted human intestinal organoids are not tumorigenic in vivo.24
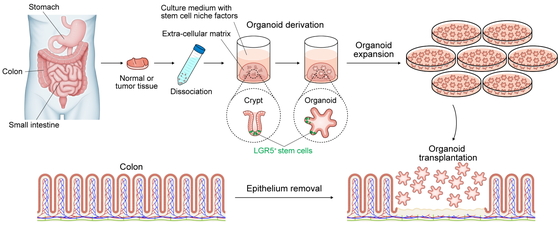
Derivation, expansion, and transplantation of intestinal organoids.
Gastrointestinal epithelium can be cultured in vitro as organoids by isolating the crypts and adding growth factors. Organoids can be grown in expanded culture and transplanted to the site from which the original colonic epithelium was removed.
The establishment of this transplantation system allowed us to conduct experiments with normal intestinal epithelium, which could not be performed with human cells because of the lack of an adequate experimental system. First, we aimed to reveal the stem cell nature of LGR5-positive cells by demonstrating their multipotency and self-renewal capacities through genetic lineage tracing experiments. Gene editing cannot be performed in vivo on the human intestine but can be performed in vitro on human intestinal organoids. The system established in colorectal cancer organoids using CRISPR–Cas9 gene editing, in which LGR5-positive cell-derived cells are differentially fluorescently labeled by tamoxifen induction,22 was introduced into human normal colon organoids.24 The organoids were then xenotransplanted into the colon of NOG mice, resulting in mice with a human cell lineage, thereby enabling in vivo genetic lineage analysis in the human intestinal epithelium. Notably, we also found that human and mouse intestinal epithelial stem cells proliferate at different rates,24,26 suggesting the importance of conducting studies in human cells. Differences in cell composition and proliferation speed naturally suggest that drug reactivity and toxicity may also differ between humans and mice. Although much knowledge can be obtained from experiments with mice, studies using human cells are extremely important.27,28
Unexpectedly, removal of the epithelium also led to new insights into tissue regeneration. The injured area of the epithelium is repaired by the surrounding epithelial cells. Therefore, the injured area can be repaired by the transplanted organoids or adjacent epithelial cells, which can replace the epithelium even if the cells differ from the original cells. Colonic mucosal injuries created contiguously to the anus were repaired by adjacent anally derived squamous epithelium.29,30 This mechanism may be one explanation for the clonal field expansion of cells with inflammation-resistant somatic gene mutations and colitis-associated dysplasia in patients with UC characterized by chronic recurrent epithelial injury.12,31,32
Unlike normal intestinal epithelial cell transplantation, much experience has been accumulated with tumor cell transplantation using cell lines and organoids, including subcutaneous transplantation into nude mice and subrenal capsular transplantation in NOG mice.4,22,33 Although orthotopic transplantation of mouse or human tumor organoids by colonoscopy-based, surgery-based, or other local injection into the submucosal layer or intestinal wall has been actively performed,34,35,36,37,38 these techniques result in tumor growth from the serosal side rather than from the luminal side, which differs from the original growth and invasion of the tumor. Moreover , using this approach, low-grade tumors would be difficult to engraft. To resolve these problems, diseased cells need to be transplanted from the mucosal side into injured regions caused by the DSS-induced colitis39 or EDTA-based breaching models.40,41,42 Because normal human cells can be transplanted, the addition of genetic alterations in multiple steps can prospectively provide new insights into tumorigenesis. Genotype–phenotype studies can be performed to observe the phenotypic changes that occur in vivo as a result of acquired genetic changes. In other words, diseased cells can be created from normal cells and reproduced in vivo. For instance, we have successfully transplanted human colonic organoids that expressed BRAFV600E and R-spondin rearrangements (EIF3E-RSPO2 fusion) into the mouse colon. These xenografts showed serrated changes, but further introduction of GREM1 overexpression promoted polypoid tumor formation, reproducing the unique endoscopic and histologic features of traditional serrated adenoma (TSA), including slit-like serrations and penicillate nuclei. These findings indicate that the ectopic expression of GREM1 is important in pathologic changes, consistent with GREM1 upregulation in clinical TSA compared with the level in adjacent superficially serrated adenoma.40 Prospective genetic analysis has advantages over marker-based speculation for understanding the effects of specific genetic alterations in diseased human tissues. In vivo studies with organoid transplantation provide pathological insights to bridge the gap between genetic analysis and the histological phenotypes of tumors (Fig. 2A).
This approach allows the transplantation of not only colorectal cancer but also gastric cancer (GC) organoids.41 Diffuse GC exhibits a unique histology and is subdivided into signet-ring cell carcinoma (SRCC) and non-SRCC; the latter is also referred to as poorly cohesive carcinoma not otherwise specified (PCC-NOS). In the stomach, transplantation from the mucosal surface is also useful for research aimed at elucidating the invasion mechanism. However, there are limitations in transplantation experiments of human GC in the mouse model, in which it is difficult to perform gastroscopy. Taking advantage of the preserved properties of transplanted cells, we attempted to transplant diffuse GC organoids into the rectum of mice, where colonoscopy can easily be performed. Organoid engraftment of diffuse GC was successfully performed, and endoscopic observation confirmed that the xenografts increased in size diffusely over time. Interestingly, the xenotransplanted GC tissue showed heterogeneity between the luminal side and the area near the fibroblasts where the crypt base was originally located. Because fibroblasts provide growth factors such as Wnt and R-spondin, the histological appearance suggested that the transplanted GC cells were differentiating in a growth factor-dependent hierarchical manner as they moved away from the fibroblasts. In other words, PCC-NOS and SRCC are not separate, but rather PCC-NOS differentiates into SRCC. Similar changes in morphology related to fibroblast positioning were observed in a model in which these organoids were transplanted into the stomach wall. SRCC is sometimes referred to as undifferentiated-type GC, but it actually has a “differentiated” GC histology. In vitro experiments also confirmed that PCC-NOS differentiated into SRCC when Wnt and R-spondin were removed from the medium.41 Evidently, cells from different organs are able to remodel the colon while retaining their unique identity.
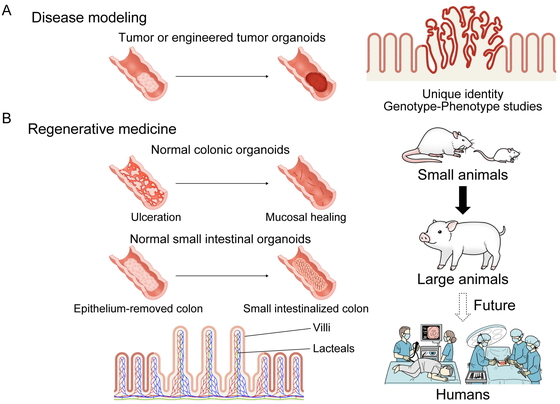
Tissue reconstruction and future prospects of transplanted organoids.
(A) Diverse organoids, including human genetically engineered artificial colorectal tumors and diffuse gastric cancer, can be transplanted into the mouse colon, and the unique properties of the transplanted cells are preserved in vivo. (B) Normal colonic organoid transplantation repairs ulcerations in the colon. Transplantation of normal small intestinal organoids into the epithelium-removed colon results in the formation of small intestinal villi including lacteals. In regenerative medicine applications, we are advancing from validation at the small-animal level to that at the large-animal level. Future work is expected to lead to organoid-based treatment of patients with intractable diseases using endoscopy or surgery.
The small intestine plays an important role in sustaining life, including the digestion of food and absorption of nutrients. For patients with UC, which is one of the most common diseases of the large intestine, many biologics, small molecule inhibitors, and other treatments are being actively developed, and new therapeutic agents are being launched one after another. In contrast, there are diseases of the small intestine, once referred to as the “dark continent” because of the difficulty of examining it, for which sufficient treatment methods have not yet been established. Most notably, short bowel syndrome (SBS), which occurs when a large amount of the small intestine has been surgically removed, results in the inadequate absorption of water and nutrients, commonly leading to diarrhea, dehydration, nutritional deficiencies, and weight loss. Because nutrient absorption from the intestine is impaired in SBS, nutritional support is the central focus of treatment, and in severe cases, lifelong central venous nutrition is required. There are many associated complications of SBS, such as catheter-related infections, thrombosis, and liver dysfunction, which can sometimes be fatal.43 Glucagon-like peptide (GLP)-2 analogs, which are promising drugs that improve villus resorptive function,44,45 have been approved and used worldwide, but their effectiveness is obviously limited for patients with only a small residual length of small intestine. Intestinal lengthening procedures such as serial transverse enteroplasty may also be effective in cases with some residual autologous intestine,46 but are ineffective if the remaining small intestine is too short.
The only curative treatment for SBS is intestine transplantation, in which a healthy small intestine is transplanted into the recipient. However, in these cases, lifelong use of immunosuppressive drugs is required, and because the small intestine is highly susceptible to rejection compared with other organs, patient management after transplantation is often difficult, and the 5-year graft survival rate is below 60%.47 Despite an estimated potential intestinal transplant waiting list of fewer than 200 patients in Japan, only 35 intestinal transplants have been performed in 31 patients from 1996 to 2020.48 In Japan, intestinal transplant has been covered by the national health insurance system since 2018. Although the number of intestinal transplants is expected to increase in the future, this procedure has not become a definitive treatment option.
Treatment options for SBS are significantly more limited than for diseases of other organs, but there are high expectations for the use of regenerative medicine in this field. However, the small intestine has a complex architecture, including crypt-villous structures composed of diverse epithelial cells, lymphatic and vascular channels, nerves, and muscular layers; moreover, it must also perform a diverse range of functions such as digestion, absorption, peristalsis, and secretion. The concept of using regenerative medicine for the small intestine has been unrealistic until now because it is extremely difficult to create the small intestine itself outside the body and on a human scale. The same difficulties apply to organoids because, as cells, organoids alone cannot regenerate the small intestine. However, upon noting the similarities between the colon and small intestine below the submucosa, we wondered whether the colon could be reconstituted as the small intestine by replacing only the epithelium of colonic structures with the small intestine through organoid transplantation (Fig. 2B).
We first simply adopted the method established for human colonic organoid transplantation directly in human small intestinal organoid transplantation.49 We considered that this approach would be successful given the previous success in transplantation of mouse small intestinal organoids into the mouse colon.15 As expected, the transplanted human small intestinal epithelium formed villous structures not found in the large intestine and expressed proteins involved in digestion and absorption that are unique to the small intestine (e.g., sucrase-isomaltase, ileal bile acid transporter). To our surprise, we noticed that the transplantation of small intestinal organoids led to the formation of lymphatic vascular structures unique to the small intestine located in the center of the villi (lacteals), which are essential for lipid absorption. Furthermore, these lacteal-like structures had the ability to absorb administered fluorescent cholesterol, indicating that the epithelium reconstructed by organoid transplantation had an absorptive function. However, the reconstructed villus structures were immature compared with the original mature human small intestinal villi. Small intestinal organoid transplantation in mice15 or humans49 with only a very small epithelial replacement of the colon near the anus with insufficient villus formation is not expected to be a treatment option to expand small intestinal function, and therefore has no clinical significance. We considered the need to promote the maturation of transplanted small intestinal villi and pondered how to achieve this goal. We then focused on flow as a factor that promotes villus formation. Luminal flow is faster in the small intestine, but slower in the large intestine. In clinical practice, the loss of luminal flow because of dietary restrictions or stoma construction causes villus atrophy. Together with confirmation of the effect of flow on villus maturation in vitro as described below, we realized the importance of reconstructing the small intestinal epithelium at the original position of the small intestine where flow is present.
We elected to use the rat SBS model, which is larger than the mouse and therefore better suited for establishing complex surgical techniques. Rat organoids could not be cultured under the same culture conditions as for mice,2,3 but refined culture conditions made it possible to establish luciferase-transgenic rat organoids for syngeneic transplantation and bioluminescence monitoring.6,49 We then created a “small intestinalized colon” (SIC), in which the colonic epithelium was removed, and the small intestinal organoids were transplanted to replace the colonic epithelium. The same rats were used as an SBS model to confirm the efficacy of the treatment. As a surgical technique, to prevent the passage of stool to allow the transplanted organoids to grow robustly, the 4-cm-long epithelialized intestine was temporarily attached to the abdominal wall after organoid transplantation to allow a wide area of organoid engraftment without bowel obstruction. However, as expected, there was no luminal flow in the transplanted area and there was insufficient villi formation under those conditions. We then interposed the SIC to the original site of the small intestine where flow was present, and, as we had intended, we were able to confirm the formation of villi in the SIC. Total removal of the jejunoileum caused weight loss and severe diarrhea, and the condition of the rats deteriorated drastically within 2 weeks. In contrast, weight gain was observed in rats in which the SIC was constructed, and it was remarkable to consider that the generation of only a few centimeters of SIC could contribute to survival. However, clinical studies have confirmed that the presence or absence of as little as 10 cm of small intestine can affect the prognosis of human SBS patients,50,51 whose original small intestine length was approximately 5–7 m. In fact, weight gain was observed in SBS rats with only a few centimeters of healthy terminal ileum remaining, supporting the finding that the generation of the SIC ensured the rats’ survival.49 Nevertheless, we considered that other parties may cast doubt over these results, and as long as surgical experiments are performed, it may be possible that the surgeon’s manipulation affected the results, even if unintentionally. Therefore, to minimize experimental bias, we conducted the experiments in a blinded fashion using organoids in which either ileum or colon organoids were transplanted into rats without informing the surgeon and care staff of their cell types. These experiments demonstrated prolonged survival in the ileal organoid-transplanted SBS rat group compared with the colon organoid-transplanted group and extensive engraftment of transplanted cells in rats with increased body weight. The SIC also formed lacteals and blood vessel networks, confirming that it would be a functional small intestinalized graft with intestinal peristalsis and absorption function.49
Inspired by the findings obtained from organoid transplantation, we investigated whether mechanical forces on the luminal surface could be an inductive cue for villus formation.49 To demonstrate the involvement of flow, we aimed to evaluate villus maturation in vitro. However, conventional 3D organoids and induced pluripotent stem-derived organoids are not suitable as an evaluation system because they do not exhibit villus formation or mature enterocyte marker expression. Therefore, we decided to add flow to 2D-cultured intestinal organoids. Normally, when cultured in 2D, organoids show a monolayer structure, but when flow was artificially added with a shaker, a villus-like structure formed. This change is not seen in colonic organoids, indicating that the small intestinal epithelium forms villus-like structures in a flow-dependent manner. RNA-sequencing also confirmed the expression of mature absorptive epithelial markers, leading to the establishment of a new in vitro culture evaluation system for small intestine function.49
We believe that this transplantation method, for which the therapeutic concept has been confirmed in small animals, is technically feasible and has therapeutic potential in large animals and humans (Fig. 2B). This organoid transplantation is attracting substantial attention as a potential game changer in the treatment of SBS.52 Alternative approaches, such as combining organoids with human decellularized intestinal scaffolds to create intestinal tracts, are also possible.53,54 If the first-in-human trial of endoscopic colonic organoid transplantation demonstrates the safety of organoid transplantation, it will provide significant supportive data for small intestinal organoid transplantation. However, there remains a need for further verification of the safety of small intestinal organoid transplantation, which will require further surgical work. We are currently conducting such research at Keio University School of Medicine using pigs as a preclinical model. We are currently developing methods based on surgery, but we believe that the possibilities for organoid transplantation treatment will continue to expand in the future. These are expected to include minimally invasive treatments combined with endoscopy and the treatment of diseases through the development of medical devices and technologies.
We have developed a novel platform for epithelial cell transplantation using various types of organoids. Organoid transplantation has great potential to promote investigation of the biology of human diseases and to advance the development of regenerative medicine.
This work was supported by the Japan Agency for Medical Research and Development (AMED) (grant numbers JP21ek0109523 and JP21bm0704069), AMED-CREST (grant number JP18gm1210001), Japan Society for the Promotion of Science (grant numbers JP21K19540 and JP20H03746), the Mochida Memorial Foundation for Medical and Pharmaceutical Research, the Takeda Science Foundation, and Keio Gijuku Fukuzawa Memorial Fund for the Advancement of Education and Research.
T.S. is the holder of several patents related to organoid culture. The remaining authors declare that they have no conflicts of interest related to this article.