Article ID: 2016-0009-OA
Article ID: 2016-0009-OA
Anemia in chronic kidney disease (CKD) is a risk factor for cardiovascular diseases and is treated by long-acting erythropoiesis-stimulating agents (ESA). Although results of previous studies showed that the hemoglobin level could not be maintained at initiation of dialysis in CKD patients treated with recombinant human erythropoietin, it remains undetermined if long-acting ESA are effective to prevent the progression of anemia at initiation of dialysis. In the present study, hemoglobin levels in 40 CKD patients treated with darbepoetin alfa (DA) and 15 CKD patients treated with a continuous erythropoietin receptor activator (CERA) were retrospectively compared during the 6 months period prior to initiation of dialysis. Results showed that DA and CERA, respectively, maintained hemoglobin levels around 10 g/dL from 6 months to 1 month before dialysis. However, hemoglobin levels at initiation of dialysis were significantly decreased to 9.1 ± 1.2 g/dL in DA group and 9.0 ± 1.0 g/dL in CERA group, respectively. Although total doses of ESA used for 6 months were similar between two groups, DA-treated CKD patients received subcutaneous injections more frequently than patients treated with CERA. These results suggest that CKD patients are needed to receive more intense ESA therapy to prevent a decline in hemoglobin levels at initiation of dialysis, even under the use of long-acting ESA, and also raise a possibility that CERA is more useful than DA to reduce the number of injections during the pre-dialysis period.
Anemia is a common complication of chronic kidney disease (CKD) that occurs as a result of inadequate erythropoietin production by the damaged kidneys. It develops early in the course of CKD,1 and is associated with the reduced quality of life,2 the development of cardiovascular diseases such as left ventricular hypertrophy3 and congestive heart failure,4 as well as the increased mortality5 in pre-dialysis CKD patients. Anemia in CKD is treated by erythropoiesis-stimulating agents (ESA). Correction of anemia with ESA has been shown to improve the quality of life and left ventricular hypertrophy,6 and to prolong the kidney survival.7,8 Currently, the guidelines published by the Japanese Society for Dialysis Therapy recommend a target hemoglobin (Hb) concentration of 11.0 to 13.0 g/dL for pre-dialysis CKD patients.9
Two long-acting ESA, darbepoetin alfa (DA) and a continuous erythropoietin receptor activator (CERA, also known as methoxy polyethylene glycol-epoetin beta), have been approved for the treatment of anemia in patients with CKD. Whereas DA exhibits a half-life of 24–48 h in peritoneal dialysis patients,10 CERA has unique pharmacologic properties, acting differently from recombinant human erythropoietin (epoetin) and DA at the erythropoietin receptor level, with a long half-life of approximately 130 h.11 These half-life values are markedly longer than that of 8.5 h of epoetin. Both DA and CERA have been shown to successfully correct anemia and maintain stable Hb levels within the recommended target range in non-dialysis CKD patients at extended administration intervals.12,13,14,15 However, it has not been fully elucidated if they are also effective to prevent the progression of anemia at initiation of dialysis when Hb levels decrease most severely. Indeed, results of previous studies showed that the Hb level declined to 8.35 g/dL at initiation of dialysis in Japanese CKD patients treated with epoetin.16 In the present study, we therefore compared the efficacy of CERA and DA on anemia in pre-dialysis CKD patients during the 6 months prior to initiation of hemodialysis.
This study was a retrospective study conducted at a single center in Japan. The study protocol was approved by the Ethics Committee of Keio University. Outpatients who visited Keio University Hospital for treatment of CKD for more than 6 months prior to initiation of dialysis, and started hemodialysis therapy between January 1, 2014 and October 31, 2015 were recruited. They were divided into two groups, DA group and CERA group, based on the use of ESA during the pre-dialysis period. There were no restrictions for their primary physicians regarding the selection of ESA. Patients who had malignancies, hematological disorders, or liver cirrhosis, patients who received red blood cell transfusion, patients who were administrated both DA and CERA during the pre-dialysis period were excluded.
VariablesBaseline characteristics (age, gender, height, body weight, primary cause of CKD, past history of cardiovascular disorders), clinical data (systolic and diastolic blood pressure, use of angiotensin converting enzyme inhibitors or angiotensin receptor blockers, use of iron supplementation, and dates and doses of injection of ESA), and laboratory data (Hb, mean corpuscular volume, total protein, albumin, urea nitrogen, creatinine, estimated glomerular filtration rate (eGFR), calcium, inorganic phosphate, iron, total iron binding capacity, ferritin, transferrin saturation, C-reactive protein, and cardio-thoracic ratio) were obtained from medical records.
Statistical analysisData are reported as percentages or mean ±standard deviation. Categorical data were compared by means of the chi-square test or Fisher's exact test. Continuous variables were tested by unpaired t test or one-way factorial ANOVA with a post hoc Fisher protected least significant difference test. When the data failed to pass the normality test, Wilcoxon rank sum test or Kruskal-Wallis test with Dunn's post hoc test was performed. Association between Hb levels and creatinine levels, as well as association between changes in Hb levels during 1 month before the initiation of dialysis and creatinine levels, were assessed by Pearson's regression analysis. P values <0.05 were considered significant.
A total of 90 CKD patients started hemodialysis therapy between January 1, 2014 and October 31, 2015 in our hospital. Out of the 90 patients included in the study, 36 were excluded from the analysis for one of the following reasons: the patients who visited our hospital for less than 6 months before starting dialysis, the patients who had malignancies, hematological disorders or liver cirrhosis, the patients who received red blood cell transfusion, and the patients who were treated by the combinatorial use of DA and CERA. Eventually, 40 CKD patients treated with DA and 15 CKD patients treated with CERA over 6 months prior to initiation of hemodialysis were analyzed in the present study.
The characteristics of the patients showed no significant differences in gender, height, weight, systolic and diastolic blood pressure, primary cause of CKD, past history of cardiovascular diseases, the use of angiotensin converting enzyme inhibitors or angiotensin receptor blockers, and iron supplement use between DA and CERA groups, although DA group was significantly older than CERA group (Table 1). Laboratory data at initiation of dialysis also showed no significant differences in serum levels of urea nitrogen, creatinine, total protein, albumin, calcium, inorganic phosphate, iron, total iron binding capacity, transferrin saturation, ferritin, and C-reactive protein between these groups (Table 2). Although the parameters for iron deficiency, such as iron, total iron binding capacity, transferrin saturation and ferritin, were not different between DA and CERA groups, mean corpuscular volume was smaller in CERA group than DA group. Reticulocyte levels were unavailable in most patients. Cardio-thoracic ratio and eGFR did not differ between the two groups.
During the 6-months pre-dialysis period, serum levels of creatinine gradually increased in both groups (Figs. 1A–1C). Indeed, serum levels of creatinine 6 months before dialysis were 6.0 ± 1.6 mg/dL in DA group and 5.0 ± 1.7 mg/dL in CERA group, respectively (Table 3), and they were not significantly different between the two groups (P = 0.056). At initiation of hemodialysis, they were 9.9 ± 2.9 mg/dL in DA group and 10.0 ± 3.1 mg/dL in CERA group, respectively, and also did not differ between the two groups (P = 0.828). Hb levels were kept constant from 6 months before dialysis to 1 month before dialysis, although they significantly decreased at initiation of dialysis in both groups (Figs. 1D–1F). Hb levels at initiation of dialysis were similar between DA group (9.1 ± 1.2 g/dL) versus CERA group (9.0 ± 1.0 g/dL). The monthly doses of ESA did not change significantly from 6 months before dialysis to initiation of dialysis in each group (Figs. 1G and 1H). In addition, total amounts of ESA, as well as total costs of ESA, used for 6 months were not different between the two groups (Figs. 2A and 2B). However, as shown in Fig. 2C, DA group received subcutaneous injections of ESA more frequently (6.6 ± 2.5 times/6 months) than CERA group (4.3 ± 2.3 times/6 months).
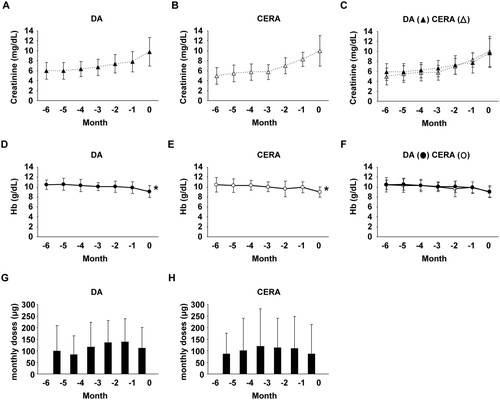
Monthly changes in serum creatinine levels, Hb levels, and ESA doses in pre-dialysis CKD patients treated with DA and CERA. A-C: Serum levels of creatinine increased in CKD patients treated with DA (A) and CERA (B) from 6 months before initiation of dialysis (−6) to initiation of dialysis (0). Two graphs in Figures 1A and 1B are combined in Figure 1C. D-F: Hb levels were kept constant from 6 months before initiation of dialysis (−6) to 1 month before dialysis (−1), but they decreased at initiation of dialysis (0) in both CKD patients treated with DA (D) and CERA (E). Two graphs in Figures 1D and 1E are combined in Figure 1F. G, H: Monthly ESA doses were not significantly altered in CKD patients treated with DA (G) and CERA (H). DA: n=40; CERA: n=15. *P<0.05 compared with the levels at 6-months before initiation of dialysis.
| DA (n=40) | CERA (n=15) | P-value | |
|---|---|---|---|
| age (years) | 69.4 ± 12.4 | 59.7 ± 14.2 | 0.016 |
| male (%) | 70.0 | 66.6 | 1.000 |
| height (cm) | 161.0 ± 8.6 | 160.4 ± 7.3 | 0.794 |
| body weight (kg) | 58.9 ± 15.4 | 63.4 ± 9.7 | 0.302 |
| systolic blood pressure (mmHg) | 136 ± 22 | 145 ± 21 | 0.172 |
| diastolic blood pressure (mmHg) | 74 ± 15 | 73 ± 14 | 0.738 |
| CKD etiology | |||
| diabetes (%) | 32.5 | 40.0 | 0.888 |
| hypertension (%) | 27.5 | 26.7 | 1.000 |
| primary glomerular diseases (%) | 25.0 | 20.0 | 1.000 |
| polycystic kidney disease (%) | 5.0 | 0.0 | 1.000 |
| others (%) | 10.0 | 13.3 | 0.660 |
| history of CVD (%) | 17.5 | 26.7 | 0.468 |
| ACEI or ARB use (%) | 30.0 | 46.7 | 0.401 |
| iron supplement use (%) | 22.5 | 26.7 | 0.734 |
CVD: cardiovascular disease; ACEI: angiotensin converting enzyme inhibitors; ARB: angiotensin receptor blockers
| DA (n=40) | CERA (n=15) | P-value | |
|---|---|---|---|
| urea nitrogen (mg/dL) | 99 ± 29 | 83 ± 24 | 0.056 |
| creatinine (mg/dL) | 9.9 ± 2.9 | 10.0 ± 3.1 | 0.828 |
| eGFR (mL/min/1.73 m2) | 4.8 ± 1.8 | 4.9 ± 1.6 | 0.712 |
| total protein (g/dL) | 6.0 ± 0.8 | 5.9 ± 0.7 | 0.615 |
| albumin (g/dL) | 3.2 ± 0.7 | 3.2 ± 0.6 | 0.872 |
| calcium (mg/dL) | 7.9 ± 1.0 | 7.5 ± 1.0 | 0.279 |
| inorganic phosphate (mg/dL) | 6.8 ± 1.8 | 6.3 ± 1.6 | 0.512 |
| iron (μg/dL) | 63.6 ± 42.1 | 60.9 ± 27.5 | 0.736 |
| total iron binding capacity (μg/dL) | 242.1 ± 58.5 | 238.2 ± 48.9 | 0.832 |
| transferrin saturation (%) | 27.2 ± 18.3 | 26.2 ± 12.3 | 0.705 |
| ferritin (ng/mL) | 146.0 ± 103.7 | 190.0 ± 126.1 | 0.305 |
| mean corpuscular volume (fL) | 92.6 ± 3.7 | 87.8 ± 4.4 | 0.003 |
| C-reactive protein (mg/dL) | 1.78 ± 2.57 | 1.21 ± 1.72 | 0.642 |
| cardio-thoracic ratio (%) | 56.4 ± 7.7 | 56.2 ± 7.4 | 0.705 |
| DA (n=40) | CERA (n=15) | P-value | |
|---|---|---|---|
| urea nitrogen (mg/dL) | 62 ± 18 | 51 ± 20 | 0.060 |
| creatinine (mg/dL) | 6.0 ± 1.6 | 5.0 ± 1.7 | 0.056 |
| albumin (g/dL) | 3.6 ± 0.8 | 3.6 ± 0.7 | 0.873 |
| calcium (mg/dL) | 8.4 ± 0.7 | 8.3 ± 0.7 | 0.621 |
| inorganic phosphate (mg/dL) | 5.2 ± 0.9 | 4.8 ± 0.7 | 0.963 |
| C-reactive protein (mg/dL) | 0.45 ± 0.84 | 0.14 ± 0.22 | 0.191 |
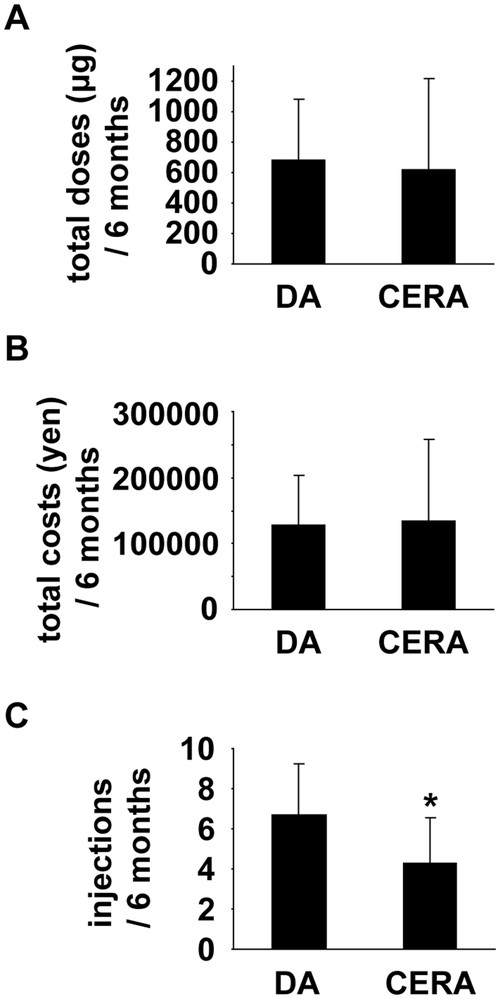
The doses, costs, and frequency of ESA injections in pre-dialysis CKD patients treated with DA and CERA. The total amounts of ESA (A), the total costs of ESA (B), and the frequency of subcutaneous injections of ESA (C) during 6 months before initiation of hemodialysis were compared among CKD patients treated with DA (n=40) versus CERA (n=15). *P<0.05 compared with DA-treated CKD patients.
The guidelines published by the Japanese Society for Dialysis Therapy9 recommend the dose reduction or interruption of ESA therapy if the Hb level exceeds 13 g/dL in pre-dialysis CKD patients (or exceeds 12 g/dL in pre-dialysis CKD patients with a history of serious cardiovascular disease). We examined the number of pre-dialysis CKD patients whose Hb level was elevated more than these levels. One in 40 CKD patients treated with DA and 1 in 15 CKD patients treated with CERA, respectively, had such an episode of Hb overshoot. The incidence of Hb overshoot was not significantly different between DA and CERA groups.
Finally, we examined the relationship between Hb levels and serum levels of creatinine at initiation of dialysis in both groups together. No significant correlation was observed between these parameters (r =0.074, P = 0.590, n =55). In addition, no significant correlation was observed between changes in Hb levels during the 1 month before initiation of dialysis and serum levels of creatinine at initiation of dialysis (r =0.201, P = 0.297, n =55), suggesting that the decline in Hb levels at initiation of dialysis is not simply caused by the decline in kidney function.
In the present study, we examined the effect of DA and CERA on anemia in pre-dialysis CKD patients during the 6 months period before initiation of hemodialysis. The results showed that DA and CERA, respectively, maintained Hb levels around 10 g/dL from 6 months before dialysis to 1 month before dialysis, but they both failed to prevent a decline in Hb levels at initiation of dialysis. Hb levels at initiation of dialysis decreased to 9.1 ± 1.2 g/dL in DA group and 9.0 ± 1.0 g/dL in CERA group, respectively. Results also showed that CKD patients treated with DA received more subcutaneous injections than those treated with CERA, although total doses and total costs of ESA used during the 6-months pre-dialysis period were similar between the two groups. The reduction in the number of subcutaneous injection is of importance, because administration of ESA is painful and some CKD patients receive injections with fear. As such, results of the present study suggest that: 1) CKD patients are required to get more attention to prevent the progression of anemia immediately before dialysis; and 2) CERA is, possibly, more useful than DA to reduce the number of subcutaneous injections during the pre-dialysis period.
Results of previous studies showed that hemodialysis patients had a high mortality rate within 120 days after initiation of hemodialysis,17 and that the incidence of cardiovascular events in the first week after initiation of hemodialysis was much higher than the subsequent period.18 Because Hb levels decrease most severely at initiation of dialysis16 and because anemia is a risk factor for the development of cardiovascular diseases and the increased mortality,3,4,5 it is highly possible that the prevention of a decline in Hb levels at initiation of dialysis improves the prognosis in CKD patients. In support of this, results of recent studies showed that low Hb level at hemodialysis initiation was a significant risk factor for coronary artery disease and cerebrovascular disease.19 Based on the results described above and those of the present study, it is suggested that CKD patients need larger doses and/or more frequent injections of ESA immediately before dialysis, even under the use of long-acting ESA.
The target Hb level for renal anemia is 11–13 g/dL in patients with CKD stage 3–5 according to the guidelines of the Japanese Society of Dialysis Therapy.9 However, mean Hb levels in both DA and CERA groups did not achieve the target in the present studies. In spite of the guideline recommendation, monthly ESA doses were not increased during the observation period. There are several explanations about this phenomenon. First, ESA are expensive, and patients sometimes refuse to increase the doses. Second, some patients are unable to visit the hospital frequently for personal reasons. Third, some nephrologists might consider the negative effect of ESA on the risk of cardiovascular diseases. Indeed, there are several studies reporting that higher Hb targets by ESA therapy did not improve the outcome in pre-dialysis CKD patients.20,21,22 These reasons may affect the fact that, even under the treatment with ESA, mean Hb levels were below 11.0 g/dL during the observation period.
Recently, two groups have independently reported retrospective studies similar to our present studies.23,24 Results of both studies showed the decline in Hb levels at initiation of dialysis in CKD patients treated with epoetin, DA, or CERA. Any one of these ESA failed to maintain target Hb levels at initiation of dialysis, as we have shown. As compared to our present study, the advantage of these studies is that they analyzed larger number of CKD patients. On the other hand, the disadvantage of these studies is that they enrolled CKD patients who started dialysis from 2007 to 2014 in one study23 and from 2009 to 2015 in another study.24 During these long periods, the guideline has been revised,9 and many papers which may affect the decision of the nephrologists regarding the ESA therapy have been reported. Nonetheless, results of the previous studies and our present study all suggest that more intense ESA therapy is needed for pre-dialysis CKD patients to avoid the progression of anemia immediately before initiation of dialysis.
In the present study, the efficacy of DA and CERA was analyzed as equivalent ESA dose. Indeed, some previous studies used a dose conversion ratio of DA:CERA =1:0.93 for non-dialysis CKD patients,13 whereas others used a ratio of 1:1.23,24 Even if the conversion ratio of 1:0.93 was adapted, the conclusions of the present study were unaffected.
There are several limitations in the present study. First, the number of CKD patients treated with DA versus CERA was quite different. Second, the age was also different between the two groups. These differences may contribute to the efficiency of ESA on anemia treatment. Moreover, although alcohol consumption and smoking might influence renal anemia, the history of these habits could not be determined accurately because of the nature of retrospective studies. Prospective randomized studies are needed in future.
In summary, results of the present study suggest that CKD patients need to be treated with more intense ESA therapy to prevent the progression of anemia immediately before dialysis, and that CERA is, possibly, more useful than DA to reduce the number of subcutaneous injections during the pre-dialysis period. It is of interest to determine the relationship between Hb level and the prognosis of CKD patients enrolled in the present study in future.
M.H. received grants from Kyowa Hakko Kirin Co., Ltd.. and Chugai Pharmaceutical Co. However, these corporations were not involved in designing or conducting this study. T.Y. has no conflicts of interest to declare.