Abstract
The class 2 CRISPR-Cas endonuclease Cas12a (previously known as Cpf1) offers several
advantages over Cas9, including the ability to process its own array and the requirement
for just a single RNA guide. These attributes make Cas12a promising for many genome
engineering applications. To further expand the suite of Cas12a tools available, we tested
16 Cas12a orthologs for activity in eukaryotic cells. Four of these new enzymes
demonstrated targeted activity, one of which, from Moraxella bovoculi
AAX11_00205 (Mb3Cas12a), exhibited robust indel formation. We also showed that Mb3Cas12a
displays some tolerance for a shortened PAM (TTN versus the canonical Cas12a PAM TTTV).
The addition of these enzymes to the genome editing toolbox will further expand the
utility of this powerful technology.
Introduction
The ability to edit the genome of living cells enables a broad range of downstream genetic
analyses and has the potential for therapeutic use to resolve pathogenic mutations. Over the
past several years, enzymes from CRISPR-Cas systems, which provide bacteria and archaea with
adaptive immunity, have emerged as powerful tools for eukaryotic gene editing. In nature,
CRISPR-Cas systems acquire DNA snippets that match invading viruses or foreign nucleic
acids, creating a memory bank of infection. These snippets are then transcribed into short
RNA guides, which are used by Cas proteins to detect invading nucleic acids. Once a sequence
match is found, Cas nucleases destroy the foreign nucleic acid. In particular, Class 2
CRISPR-Cas systems are well-suited for development as molecular technologies because they
contain single effector enzymes. These effector enzymes, such as Cas9, are RNA-guided DNA
endonucleases that have been harnessed for a range of genome engineering
applications.1
Although Cas9 was the first such enzyme to be developed as a genome editing tool,2,3 three orthologs of Cas12a (a single RNA-guided class 2 effector
previously known as Cpf1) from Francisella novicida U112 (FnCas12a),
Acidaminococcus sp. BV3L6 (AsCas12a), and Lachnospiraceae
bacterium ND2006 (LbCas12a), have also been used for genome editing in eukaryotic
cells.4,5,6,7,8
Endonucleases of the Cas12a family differ from the Cas9 family in several ways: (i) Cas12a
utilizes T-rich protospacer adjacent motifs (PAMs) located 5′ of the targeted DNA sequence,
(ii) target cleavage occurs distally from the PAM and results in sticky-end overhangs, (iii)
Cas12a is guided by a single CRISPR RNA (crRNA) and does not require trans-activating CRISPR
RNA; and (iv) Cas12a possesses both RNase and DNase activity, which allows it to process its
own CRISPR array.7,9 These features make Cas12a particularly useful
in certain situations, such as targeting AT-rich genomic regions and multiplexed gene
targeting.8,10 Additionally, Cas12a has been shown to possess
non-specific single-stranded DNA cleavage activity after it has been activated by target
binding, which has been leveraged for nucleic acid detection.11,12,13,14 Finally,
Cas12a is more specific than Cas9 in certain contexts, making it well-suited to applications
in which high specificity is critical.15,16
Given previous work showing that different Cas enzyme orthologs exhibit a range of activity
in eukaryotic cells2,16,17 and indicating the potential advantages of Cas12a, we sought
to identify additional Cas12a orthologs with high activity in eukaryotic cells. Here we
examine 16 new Cas12a-family proteins for nuclease activity in human cells. We identify four
orthologs that can induce insertion/deletion (indel) events at targeted genomic loci. One
ortholog, from Moraxella bovoculi AAX11_00205 (Mb3Cas12a), exhibited
comparable activity to AsCas12a and LbCas12a when targeting sites containing TTTV (V=A, C,
or G) PAMs. We also show that Mb3Cas12a can recognize a TTN PAM, but with lower efficiency
than the conserved TTTV PAM. Together, these new orthologs expand the genome editing
toolbox, providing new enzymes that can be used for tailored applications.
Materials and Methods
Computational search for Cas12a orthologs. Cas12a orthologs were selected
as previously described.7
Cell culture and transfection. HEK293T cells were maintained at 37°C with
5% CO2 in Dulbecco’s Modified Eagle Medium (Gibco) supplemented with 10% fetal
bovine serum (HyClone) and 2 mM GlutaMAX (Life Technology). For indel analysis, 22,000 cells
were seeded per well of a 96-well plate (Corning) 1 day before transfection. Each well was
transfected with 100 ng Cas12a-encoding plasmid (see the supplemental file) and 50 ng
guide-encoding plasmid or PCR fragment, or 150 ng Cas12a and guide-encoding plasmid, using
Lipofectamine 2000 (Thermo Fisher Scientific). Cells were harvested 3 days after
transfection using QuickExtract DNA extraction solution according to the manufacturer’s
protocol and analyzed by surveyor assay or deep sequencing. To generate Cas12a-containing
whole-cell lysate, 120,000 cells were seeded per well of a 24-well plate (Corning) 1 day
before transfection. Each well was transfected with 500 ng Cas12a-encoding plasmid, and cell
lysate was harvested 2 days after transfection.
In vitro PAM identification assay. The in vitro PAM identification assay
was performed as described previously.18
Briefly, whole-cell lysate from HEK293T cells overexpressing one of the Cas12a orthologs was
prepared with lysis buffer (20 mM HEPES, 100 mM KCl, 5 mM MgCl2, 1 mM DTT, 5%
glycerol, 0.1% Triton X-100) supplemented with EDTA-free Complete Protease Inhibitor
Cocktail (Roche). CrRNA with corresponding direct repeat sequences were transcribed in vitro
using custom oligonucleotides and a HiScribe T7 in vitro Transcription Kit (NEB) according
to the manufacturer’s recommended protocol for small RNA transcripts. The PAM library
consisted of a pUC19 plasmid carrying a degenerate 8-bp sequence 5′ of a 33-bp target
site.7 The library was pre-cleaved with
XmnI and column purified prior to use (Qiagen). Each in vitro cleavage reaction consisted of
1 μl 10× CutSmart buffer (NEB), 200 ng PAM library, 500 ng in vitro transcribed crRNA, 10 μl
cell lysate, and water for a total volume of 20 μl. Reactions were incubated at 37°C for 1 h
and stopped by adding 500 μl buffer PB (Qiagen) followed by column purification. Purified
DNA was amplified and sequenced using a MiSeq (Illumina) with a single-end 150-cycle kit.
Sequencing results were entered into the PAM discovery pipeline.7
Surveyor assay. The surveyor assay was performed as previous
described.19 Briefly, genomic regions
flanking a target site for each gene were amplified by PCR, and the products were purified
using a QiaQuick Spin Column (Qiagen). Total purified PCR products (400 ng) were mixed with
2 µL 10 Taq DNA Polymerase buffer (Enzymatics) and ultrapure water to a final volume of
20 µL. Re-annealing was achieved by heating to 95°C for 2 min followed by a slow cool down
to 10°C (∼2.5°C per min). Re-annealed products were treated with surveyor nuclease (IDT)
according to the manufacturer’s protocol. Cleavage products were then visualized on 10%
Novex TBE polyacrylamide gels (Life Technologies). Gels were stained with SYBR Gold DNA
stain (Life Technologies) for 10 min and imaged with a Gel Doc imaging system (Bio-Rad).
Deep Sequencing. Targeted regions were amplified using a previously
described two-step PCR protocol.19 Indels
were counted computationally as previously described.18 Briefly, each amplicon was searched for exact matches within a 70-bp
window around the cut site. For each sample, the indel rate was determined as (number of
reads with indel) / (number of total reads). Samples with fewer than 1000 total reads were
not included in subsequent analyses.
Results
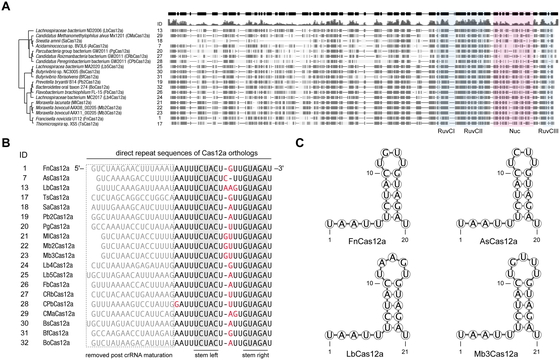
We selected 16 uncharacterized Cas12a-family proteins with varying degrees of homology to
three Cas12a orthologs (FnCas12a, AsCas12a, and LbCas12a)4,7 with
confirmed activity in eukaryotic cells (Fig. 1A).
The direct repeat (DR) sequences of crRNAs associated with Cas12a orthologs show high levels
of homology (Fig. 1B) and are predicted to fold
into almost identical secondary structures (Fig.
1C). The homology is particularly strong at the stem structure and the AAUU motif
(Fig. 1C), which is required for efficient crRNA
maturation,9 suggesting that the
mechanism of crRNA maturation may be conserved within the Cas12a-family.
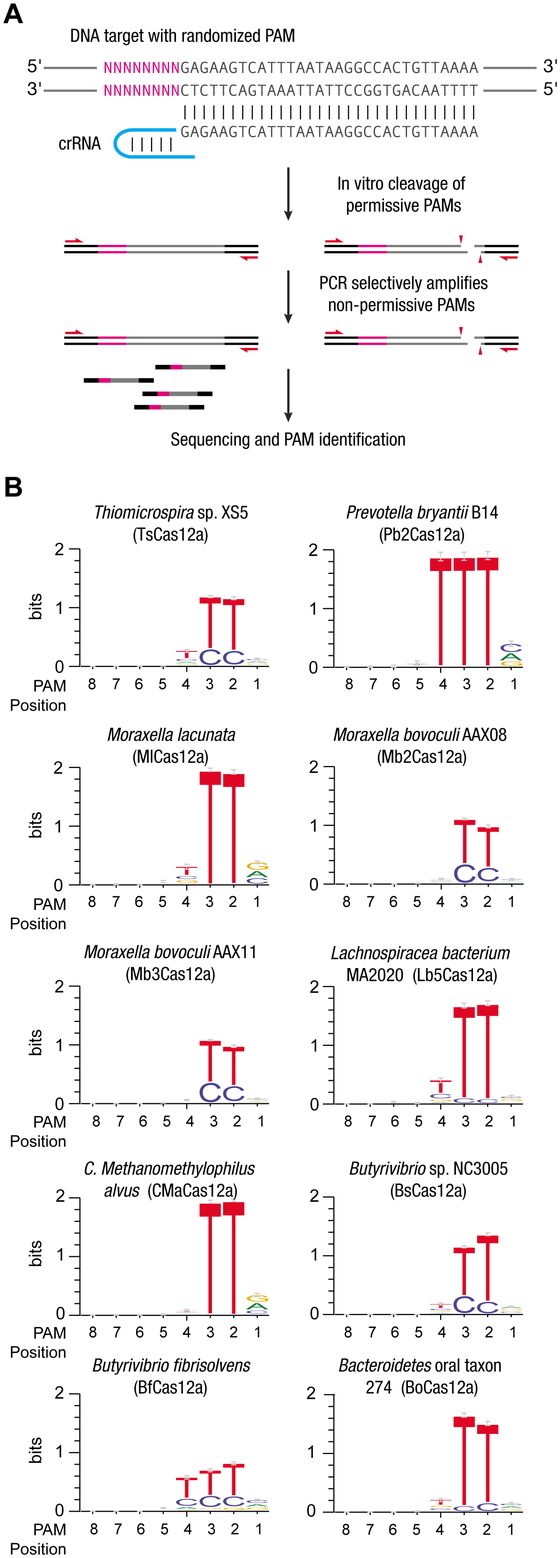
We performed a previously described in vitro assay18 to determine the sequence of the PAM for each Cas12a ortholog (Fig. 2A). Of the 16 new Cas12a proteins, ten were
active in vitro and recognized a T-rich PAM located 5′ of the targeted sequence (Fig. 2B), just as previously characterized Cas12a
proteins do.7
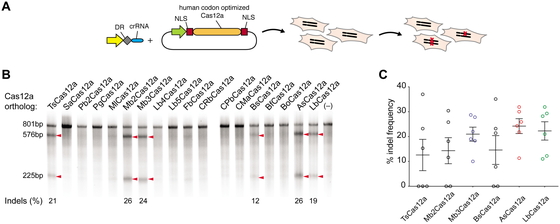
Next, we tested the 16 Cas12a orthologs for activity in human cells. We chose a previously
validated target within VEGFA, located next to a TTTG PAM that is
permissive to all Cas12a orthologs. HEK293T cells were transfected with plasmids encoding
humanized Cas12a orthologs together with PCR amplified fragments comprising a U6 promoter
fused to the corresponding crRNA sequence (Fig.
3A). Four of the new Cas12a orthologs [Thiomicrospira sp. Xs5
(TsCas12a), Moraxella bovoculi AAX08_00205 (Mb2Cas12a), Moraxella
bovoculi AAX11_00205 (Mb3Cas12a), and Butyrivibrio sp. NC3005
(BsCas12a)] were able to induce detectable indel events, as measured by surveyor nuclease
assay (Fig. 3B). We tested these orthologs with
six additional guides targeting either DNMT1 or EMX1 next
to TTTV PAMs (Fig. 3C) and compared them to the
activity of AsCas12a and LbCas12a. For all four new Cas12a enzymes, indel frequencies of
>20% could be detected for at least two guides, but only Mb3Cas12a was able to induce
robust indel levels with all six guides comparable to those of AsCas12a and LbCas12a. The
apparent difference in activities between Mb2Cas12a and Mb3Cas12a was somewhat surprising
given that these orthologs share a predicted homology of 94.7%.
Because Mb3Cas12a was predicted to recognize a less restrictive PAM than the TTTV consensus
PAM of AsCas12a and LbCas12a (Fig. 2B), we tested
the ability of Mb3Cas12a to cleave endogenous DNA at TTN PAMs. To this end, we designed 64
guides: 16 guides for DNMT1, EMX1,
GRIN2b, or VEGFA, targeting next to any combination of
NTTN PAMs. To compare the activity of Mb3Cas12a, AsCas12a, and LbCas12a at NTTN PAMs, we
transfected HEK293T cells with two plasmids, one expressing Cas12a and one expressing the
crRNA, and assessed indel frequencies at each target site by deep sequencing. The average
activity at TTTV PAMs was approximately 18% for Mb3Cas12a, 28% for AsCas12a, and 13% for
LbCas12a (Fig. 4A). A few guides targeting next to
NTTN PAMs (three for MbCas12a and one for AsCas12a) resulted in activity between 25–45%
indels. However, whereas Mb3Cas12a performed better than AsCas12a and LbCas12a at NTTN PAMs,
the average activity was relatively low with approximately 5.3% for Mb3Cas12a, approximately
2.7% for AsCas12a, and approximately 1.4% for LbCas12a. Comparing activity across all VTTN
PAMs revealed statistically significant differences in indel activity between Mb3Cpf1 and
LbCpf1 (mean 5.26% vs 1.38%, P = 0.0117), but not between Mb3Cpf1 and
AsCpf1 (mean 5.26% vs 2.69%, P = 0.1343)
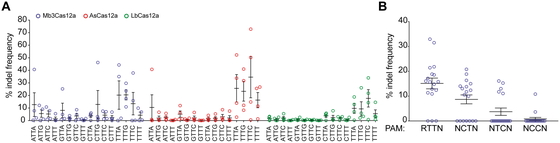
Based on the in vitro PAM screen, Mb3Cas12a tolerates Cs or Ts within its PAM. To assess
the tolerance for Cs at position 2 and 3 of the Mb3Cas12a PAM, we used 18 guides targeting
DNMT1 or EMX1 next to RTTN and NYYN PAMs (R=A or G, Y=C
or T). HEK293T cells were transfected with a single plasmid expressing Mb3Cas12a and crRNA.
The activity of each guide was determined using the surveyor nuclease assay. Guides
targeting next to RTTN, RCTN, and RTCN PAMs had an average activity of approximately 15%,
approximately 9%, and approximately 4%, respectively; however, guides targeting next to RCCN
PAMs were mostly inactive (Fig. 4B). Taken
together, our data show that Mb3Cas12a is active in human cells and shows robust activity at
TTTV PAMs at levels comparable to those of AsCas12a and LbCas12a. Furthermore, Mb3Cas12a can
reliably target sites with RTTV PAMs, albeit with lower overall activity.
Discussion
Reaching the full potential of CRISPR-based genome editing will require a suite of tools to
ensure that there are optimal enzymes for a range of genomic contexts. This will be
particularly important for the therapeutic deployment of CRISPR, where the target site will
be constrained by the genetic variations found in individual patients. Moreover, to tackle
the full landscape of pathogenic mutations, a number of different gene editing strategies
will be needed beyond simple gene knockout. Consequently, having an array of Cas enzymes
that can be used in human cells is essential for the continued development of this
technology.
Here we examined 16 new Cas12a family proteins for potential use in genome editing. Four of
these, Thiomicrospira sp. Xs5 (TsCas12a), Moraxella
bovoculi AAX08_00205 (Mb2Cas12a), Moraxella bovoculi AAX11_00205
(Mb3Cas12a), and Butyrivibrio sp. NC3005 (BsCas12a), exhibited activity in
human cells. We chose HEK293 cells as a model for exploring these new Cas12a orthologs
because there is a wealth of published data available on the efficiencies of other Cas
enzymes in these cells. Previously, we observed only weak activity of FnCas12a in mammalian
cells.7 However, a recent study found
that FnCas12a exhibits robust activity in plant cells,4 indicating that Cas12a orthologs might have different activities
depending on the organism. Therefore, it may be informative in future studies to test the
activities of these new Cas12a orthologs in different cell types and organisms.
Further analysis of the PAM requirements of the most active new ortholog, Mb3Cas12a, showed
that it has a less restricted PAM (TTV) than AsCas12a and LbCas12a, which are active only at
the canonical TTTV PAM. Alignment of Mb3Cas12a to other Cas12a orthologs did not suggest any
immediate reason for the more relaxed PAM (data not shown), and further work will be
required to investigate the structural basis for this altered PAM requirement.
Given the advantageous properties of Cas12a, such as its inherent high specificity and
distinct PAM preference, this family of enzymes represents a powerful addition to the gene
editing toolbox. Here, we further expanded the utility of Cas12a by identifying new
orthologs that are active in human cells.
Author Contributions
B.Z., J.S., and F.Z. conceived this study. B.Z. and J.S. performed the experiments with
help from all authors. O.A. and J.G. analyzed PAM detection data. D.S. contributed to
computational analysis of Cas12 orthologs. F.Z. supervised the research. B.Z. and F.Z. wrote
the manuscript with input from all authors.
Acknowledgments
We thank R. Macrae, R. Belliveau, G. Faure, and L. Gao for discussions and support. J.S. is
supported by the Human Frontier Science Program. F.Z. is a New York Stem Cell
Foundation–Robertson Investigator. F.Z. is supported by National Institutes of Health grants
(1R01-HG009761, 1R01-MH110049, and 1DP1-HL141201); the Howard Hughes Medical Institute; the
New York Stem Cell, Edward Mallinckrodt, Jr., and G. Harold and Leila Mathers Foundations;
the Poitras Center for Psychiatric Disorders Research at MIT; the Hock E. Tan and K. Lisa
Yang Center for Autism Research at MIT; J. and P. Poitras; and the Phillips Family. F.Z. is
a co-founder and advisor of Beam Therapeutics, Editas Medicine, Arbor Biotechnologies,
Sherlock Biosciences, and Pairwise Plants. The authors plan to make the reagents widely
available to the academic community through Addgene and to provide software tools via the
Zhang lab website (zlab.bio). A patent has been filed relating to the presented data.
Conflicts of Interest
The authors have declared that no conflict of interest exists.
References
- 1.Zhang F. Development of CRISPR-Cas systems for
genome editing and beyond. 52, 653 (2019).DOI:10.1017/S0033583519000052
- 2. Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib
N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F: Multiplex genome engineering using
CRISPR/Cas systems. Science 2013; 339: 819–823. PMID:23287718,
DOI:10.1126/science.1231143
- 3. Mali P, Yang L, Esvelt KM, Aach J, Guell M,
DiCarlo JE, Norville JE, Church GM: RNA-guided human genome engineering via Cas9. Science
2013; 339: 823–826. PMID:23287722, DOI:10.1126/science.1232033
- 4. Endo A, Masafumi M, Kaya H, Toki S: Efficient
targeted mutagenesis of rice and tobacco genomes using Cpf1 from Francisella novicida. Sci
Rep 2016; 6: 38169. PMID:27905529, DOI:10.1038/srep38169
- 5. Kim Y, Cheong SA, Lee JG, Lee SW, Lee MS, Baek
IJ, Sung YH: Generation of knockout mice by Cpf1-mediated gene targeting. Nat Biotechnol
2016; 34: 808–810. PMID:27272387, DOI:10.1038/nbt.3614
- 6. Ma S, Liu Y, Liu Y, Chang J, Zhang T, Wang X, Shi
R, Lu W, Xia X, Zhao P, Xia Q: An integrated CRISPR Bombyx mori genome editing system with
improved efficiency and expanded target sites. Insect Biochem Mol Biol 2017; 83: 13–20.
PMID:28189747, DOI:10.1016/j.ibmb.2017.02.003
- 7. Zetsche B, Gootenberg JS, Abudayyeh OO, Slaymaker
IM, Makarova KS, Essletzbichler P, Volz SE, Joung J, van der Oost J, Regev A, Koonin EV,
Zhang F: Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell
2015; 163: 759–771. PMID:26422227, DOI:10.1016/j.cell.2015.09.038
- 8. Zetsche B,et al: Multiplex gene editing by
CRISPR–Cpf1 using a single crRNA array. Nat Biotechnol 2016.
PMID:27918548
- 9. Fonfara I, Richter H, Bratovič M, Le Rhun A,
Charpentier E: The CRISPR-associated DNA-cleaving enzyme Cpf1 also processes precursor
CRISPR RNA. Nature 2016; 532: 517–521. PMID:27096362,
DOI:10.1038/nature17945
- 10. Wang M, Mao Y, Lu Y, Tao X, Zhu J: Multiplex gene
editing in rice using the CRISPR-Cpf1 system. Mol Plant 2017; 10: 1011–1013.
PMID:28315752, DOI:10.1016/j.molp.2017.03.001
- 11. Chen JS, Ma E, Harrington LB, Da Costa M, Tian X,
Palefsky JM, Doudna JA: CRISPR-Cas12a target binding unleashes indiscriminate
single-stranded DNase activity. Science 2018; 360: 436–439. PMID:29449511,
DOI:10.1126/science.aar6245
- 12. Li SY, Cheng QX, Liu JK, Nie XQ, Zhao GP, Wang J:
CRISPR-Cas12a has both cis- and trans-cleavage activities on single-stranded DNA. Cell Res
2018; 28: 491–493. PMID:29531313, DOI:10.1038/s41422-018-0022-x
- 13. Gootenberg JS, Abudayyeh OO, Kellner MJ, Joung J,
Collins JJ, Zhang F: Multiplexed and portable nucleic acid detection platform with Cas13,
Cas12a, and Csm6. Science 2018; 360: 439–444. PMID:29449508,
DOI:10.1126/science.aaq0179
- 14. Li SY, Cheng QX, Wang JM, Li XY, Zhang ZL, Gao S,
Cao RB, Zhao GP, Wang J: CRISPR-Cas12a-assisted nucleic acid detection. Cell Discov 2018;
4: 20. PMID:29707234, DOI:10.1038/s41421-018-0028-z
- 15. Kleinstiver BP, Tsai SQ, Prew MS, Nguyen NT,
Welch MM, Lopez JM, McCaw ZR, Aryee MJ, Joung JK: Genome-wide specificities of CRISPR-Cas
Cpf1 nucleases in human cells. Nat Biotechnol 2016; 34: 869–874. PMID:27347757,
DOI:10.1038/nbt.3620
- 16. Strecker J, Jones S, Koopal B, Schmid-Burgk J,
Zetsche B, Gao L, Makarova KS, Koonin EV, Zhang F: Engineering of CRISPR-Cas12b for human
genome editing. Nat Commun 2019; 10: 212. PMID:30670702,
DOI:10.1038/s41467-018-08224-4
- 17. Ran FA, Cong L, Yan WX, Scott DA, Gootenberg JS,
Kriz AJ, Zetsche B, Shalem O, Wu X, Makarova KS, Koonin EV, Sharp PA, Zhang F: In vivo
genome editing using Staphylococcus aureus Cas9. Nature 2015; 520: 186–191. PMID:25830891,
DOI:10.1038/nature14299
- 18. Gao L, Cox DB, Yan WX, Manteiga JC, Schneider MW,
Yamano T, Nishimasu H, Nureki O, Crosetto N, Zhang F: Engineered Cpf1 variants with
altered PAM specificities. Nat Biotechnol 2017; 35: 789–792. PMID:28581492,
DOI:10.1038/nbt.3900
- 19. Hsu PD, Scott DA, Weinstein JA, Ran FA, Konermann
S, Agarwala V, Li Y, Fine EJ, Wu X, Shalem O, Cradick TJ, Marraffini LA, Bao G, Zhang F:
DNA targeting specificity of RNA-guided Cas9 nucleases. Nat Biotechnol 2013; 31: 827–832.
PMID:23873081, DOI:10.1038/nbt.2647