Article ID: 2023-0018-OA
Article ID: 2023-0018-OA
The aim of this study was to identify 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG-PET/CT) parameters that could predict the prognosis of patients with esophageal cancer before and after undergoing chemoradiation therapy. We retrospectively reconstructed images under the same conditions for patients who underwent pre- and post-treatment 18F-FDG-PET for chemoradiation therapy for esophageal cancer. Correlations between 2-year survival rates and pre-treatment values, differences between pre- and post-treatment quotients, and their ratios were examined for various standardized uptake values (SUV), metabolic tumor volumes (MTV), and each SUVmean (Mean SUV)*MTV (Vol.mean). We enrolled 29 patients who underwent pre-and post-treatment 18F-FDG-PET. The median overall survival was 21.4 months (range, 3.6–100.9 months). Pre-treatment MTV had the most favorable hazard ratio (HR) for survival. However, the MTV product (Vol.meanQ), SUV corrected for basal metabolic rate using Mifflin-St Jeor estimation (BMR.ms), Vol.mean (SUVmeanQ) using the qPET method, SUVmean, and HR using Vol.meanQ corrected for body weight were nearly equivalent. No significant results were obtained for the pre- and post-treatment quotients. The pre- and post-treatment Vol.meanQ is a useful prognostic parameter that considers the effect of age-related loss of lean body mass. The use of parameters, including metabolism, will facilitate more appropriate use of 18F-FDG-PET before and after chemoradiation therapy.
Most esophageal cancers are regional or distant metastatic cases (locally advanced disease) at the time of initial diagnosis, and multimodal therapy including surgery is the standard treatment.1,2 We have previously reported favorable long-term outcomes for patients with esophageal cancer.3 Definitive chemoradiotherapy has the merit of esophageal preservation and is one of the standard treatments.1,2 However, there are known early and late adverse events caused by chemoradiation4; therefore, it is useful to predict the therapeutic effect to determine the treatment strategy.
18F-Fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG-PET/CT) has been established as a useful diagnostic modality in oncology because of its excellent tumor detection abilities. It is widely used for detecting distant metastases, unknown primary tumors, and tumor recurrence.5 With regard to esophageal cancer, Marzola et al. reported that 18F-FDG-PET was not suitable for the detection of tumors with very small volumes, although it was superior to CT/endoscopic ultrasound, especially for the detection of distant metastases.6
18F-FDG-PET/CT is widely used for not only tumor detection but also for determination of therapeutic effects because it provides semi-quantitative numerical values.7,8 In recent years, its utility for determining the prognosis after concurrent chemoradiotherapy (CCRT) in lymph node-positive patients has also been investigated.9 Although this modality is widely used to determine therapeutic effects via comparisons before and after treatment,10,11 the treatment efficacy is based on visual assessment of uptake, and the details of reliable parameters for predicting the patient’s prognosis remain unclear.
Studies on prognosis prediction based on the judgment of therapeutic effects are being conducted, with several studies reporting that the standardized uptake value (SUV) was a useful predictor in oral cancer,12,13 small cell lung cancer,14 and lymphoma.15 However, another study reported that the metabolic tumor volume (MTV) was a more reliable predictor than SUV.16 Therefore, it is unclear which parameter is more reliable. Although both pre- and post-treatment values appear to be powerful parameters for prognosis prediction, the disadvantage lies in the fact that post-treatment parameters are required and cannot be predicted before treatment.
The purpose of this study was to examine 18F-FDG-PET/CT parameters before and after treatment and determine the useful parameters for predicting the prognosis before and after chemoradiation for esophageal cancer. In addition, we examined the robustness of SUV and MTV values through detailed examination of changes in their usefulness depending on metabolic correction.
All procedures were in accordance with the ethical standards of the responsible committee at which the studies were conducted (Ethics Committee of Keio University School of Medicine; 31 July 2015; No. 2015/20150137) and were in accordance with the 1964 Declaration of Helsinki and later versions. Informed consent was obtained from all patients.
PatientsWe retrospectively reviewed our institutional database of electronic medical records and all available 18F-FDG-PET/CT imaging studies between 1 August 2012 and 31 December 2019. During this period, 112 patients with esophageal squamous carcinoma received CCRT treatment. We included patients who received CCRT as the first-line treatment and underwent 18F-FDG-PET/CT before and after CCRT. The first 18F-FDG-PET/CT (pre-test) examination was performed within 50 days before CCRT initiation, and the second examination was performed 14–150 days after CCRT completion (post-test). Patients with a history of chemotherapy or radiotherapy for other cancers and those with metastatic recurrence were excluded.
18F-FDG-PET/CT image acquisitionFollowing confirmation of a fasting blood glucose level below 6 mmol/L (≥6 h of fasting), we intravenously administered 18F-FDG. Approximately 60–75 min (until 31 August 2016: 60 min; from 1 September 2016: 75 min according to changes in facility policies) after 18F-FDG uptake, a topogram was obtained in the craniocaudal direction to plan for the scan. This was followed by CT (without contrast enhancement) for anatomical localization, attenuation correction, and diagnostic purposes. The patients were scanned using a Siemens BioGraph 40/64 TruePoint hybrid PET/CT scanner (Siemens Medical Solutions, Malvern PA, USA). Several emission scans were recorded at seven bed positions with the following parameters: duration method, step and shoot (from 1 August 2012 to 31 July 2014, BioGraph 64 used continuous bed motion); scan duration/bed, variable based on body weight (<50 kg: 60 s, 50–70 kg: 90 s, >70 kg: 120 s) until 31 August 2016 and uniformly set to 135 s from 1 September 2016. The collected data were reconstructed using Syngo (Siemens Medical Solutions) with the Iterative+Time-of-Flight method. The processing parameters were as follows: three iterations/21 subsets, Gaussian-Filter, relative accuracy of the scatter correction method, and 256 × 256-pixel image size.
MeasurementsWe measured various SUV-based parameters such as maximum SUV (SUVmax), mean SUV (SUVmean), peak SUV (SUVpeak),17 the standard deviation of SUVmean (SUVmean_sd), SUVpeak based on qPET (SUVqpeak),18 the average of the SUVqpeak (MeanQ), the average of Wahl’s SUVpeak19 using qPET (MeanW), and MTV on the PET/CT viewer.20 These parameters were based on the distributions of Fiji21/ImageJ.22 We manually set volumes of interest (VOIs) and defined targets using the following thresholds:
We evaluated all SUV thresholds and selected the best representative value. We measured SUVs (maximum, mean, and peak) based on the 18F-FDG-PET/CT: European Association of Nuclear Medicine procedure guidelines for tumor imaging, version 2.0.17 SUVqPeak was proposed by Hasenclever et al.18 for lymphoma cases. Average PET (MeanW) and average Q-PET (MeanQ) were calculated as the average SUVs for each slice including the delimited VOI of SUVpeak19 and SUVqpeak.18 Accordingly, we evaluated the following parameters: SUVmax, SUVpeak, SUVqpeak, SUVmean, MeanW, and MeanQ. We tested three patterns of the mean based on SUVpeak in addition to SUVmean for MeanW and MeanQ. The parameters also included MTV and MTV*mean, with three patterns of the mean for MeanW and MeanQ tested based on SUVpeak in addition to SUVmean. MTV*SUVmean represented total lesion glycolysis.
SUV measurement and highest SUVmax detectionWe detected the highest SUVmax using a VOI of 1 cm3 for each organ (esophagus, heart, liver, spleen, multifidus muscle, abdominal aorta, colon, and jejunum), because the SUVmax of the contoured organs could not be derived directly on CT. Subsequently, we measured the average and peak values for the organs based on the arranged VOI.
Parameter correction (method)We corrected SUV values using several parameters,23 including ideal body weight (IBW),24 predicted normal weight (PNW),25 body surface area (BSA),26 fat-free mass (FFM),27 two types of basal metabolic rate (BMR),28,29 and two types of lean body mass (LBM).30,31Table 1 shows the formulae used to calculate each parameter. In addition to Janmahasatian’s estimate (LBM.j)31 for SUV corrected by LBM (SUL) in the PET/CT viewer, we calculated LBM using Green and Duffull’s formula (LBM.gd).30
| Parameter | Method | Sex | Function | Purpose |
|---|---|---|---|---|
| SUVcorr | - | Both |
|
For use in each SUV correction formula |
| BMI | - | Both |
|
For use in each correction formula |
| BSA | DuBois’ formula | Both |
|
Indicator of metabolism used in chemotherapy |
| IBW | - | Male |
|
Index that is not affected by age and weight |
| Female |
|
|||
| PNW | - | Male |
|
LBM related indicators |
| Female |
|
|||
| FFM | - | Male |
|
LBM related indicators |
| Female |
|
|||
| LBM | Janmahasatian’s estimate (LBM.j) |
Male |
|
As basic parameters |
| Female |
|
|||
| Green and Duffull’s formula (LBM.gd) | Male |
|
||
| Female |
|
|||
| BMR | Roza’s formula (BMR.r) | Male |
|
Indicators affected by sex, age, and weight |
| Female |
|
|||
| Mifflin-St Jeor estimate (BMR.ms) |
Male |
|
||
| Female |
|
SUVcorr, SUV correction; Bw, body weight; Ht, height; BMI, body mass index; BSA, body surface area; IBW, ideal body weight; PNW, predicted normal weight; FFM, fat-free mass; LBM, lean body mass; BMR, basal metabolic rate; Cimg, image-derived radioactivity concentration; Doseinj, injected radioactivity dose.
We verified the correlation between overall survival and disease-free survival (OS-DFS) for the PET values in the following three groups: Pre alone (Prealone), difference between Pre and Post (DiffPrePost), and quotient of Pre and Post (QuotPrePost). This process is ongoing, and useful parameters and their cutoffs have been identified.
Visual evaluationA single doctor visually evaluated various organs using the 0–4-point method. However, the entire brain was not imaged or evaluated in multiple cases. For myocardial assessment, we could not reproduce the reconstruction of the vertical long-axis/horizontal long-axis/short-axis images. Therefore, we associated 17 segments of the fusion image with three-way maximum intensity projection images [anterior to posterior: AP; lateral (left to right): LR; left anterior oblique: LAO].
Statistical analysisWe analyzed the data using R software (version 3.1.0, R Foundation)32 and three additional packages: pROC,33 survival,34 and MASS.35 We computed and evaluated the correlation coefficients between each parameter and event using Cox regression analysis (Cox proportional hazards model). We also evaluated each parameter using stepwise regression36 on Akaike’s information criterion37 using the MASS package. Receiver operating characteristic curve analysis was conducted to derive the optimal cut-off value for each parameter to predict 2-year survival using the Youden index.38 Survival curves were estimated using Kaplan–Meier methods based on the survival package (R software). Generalized logistic regression analysis of binary data was performed using the glm function in the R software package. We also used the logistf package39 with Firth’s penalized likelihood method40 to verify the exact logistic regression because of the small number of cases. The history of the program files was managed by Git41 (a free, open-source system) and GitHub (provides hosting for software development and version control using Git). All P values except those for hazard ratio (HR; obtained from Cox regression model), likelihood ratio (obtained from likelihood test), and log-rank test were calculated using the Wilcoxon rank-sum exact test or Wilcoxon rank-sum test. A two-sided P value of <0.05 was considered statistically significant.
Amongst the 112 patients, 44 received neoadjuvant chemotherapy and 35 did not undergo 18F-FDG-PET/CT (in most cases, post-CCRT PET was not performed). Moreover, the images of 4 patients could not be technically evaluated because of corrupted or unreadable data. Finally, 29 patients who met the inclusion criteria were examined. The observations for 13 patients were right-censored, and the time of death was recorded for all other patients. At the final follow-up on 26 June 2021, the median overall survival was 21.4 months (range, 3.6–100.9 months), with 8 survivors. Table 2 presents the clinical characteristics of the study population. Following CCRT, the initial evaluation revealed a complete response in 12 patients, a partial response in 8 patients, stable disease in 4 patients, and progressive disease in 5 patients. Surgery (n = 6), endoscopic submucosal dissection (n = 2), chemotherapy (n = 7), and radiotherapy (n = 3) were also used as salvage therapies.
| Characteristic | n =29 |
|---|---|
| Initial age (years) | 66 [61–76] |
| Height (cm) | 168 [160–171] |
| Diabetes | |
| Yes | 2 (6.9%) |
| No | 27 (93%) |
| Tobacco experience | |
| Yes | 26 (90%) |
| No | 2 (6.9%) |
| Unknown | 1 (3.4%) |
| Tobacco (pack-year) | 28 [12–35] |
| Alcohol experience | |
| Social | 28 (97%) |
| Never | 1 (3.4%) |
| Alcohol face flush | |
| Yes | 15 (52%) |
| Yes (former) | 3 (10%) |
| No | 6 (21%) |
| No (Unknown for previous) | 2 (6.9%) |
| Unknown | 3 (10%) |
| Alcohol (drinks/day)a | 4.40 [3.89–6.60] |
| Initial stage (UICC 8th) | |
| I | 5 (17%) |
| II | 4 (14%) |
| III | 7 (24%) |
| IV | 13 (45%) |
| Pathology | |
| SCC | 28 (97%) |
| ADC | 1 (3.4%) |
| RTx dose (Gy) | |
| <50 | 3 (10%) |
| ≥50 | 26 (90%) |
| CTx regimen | |
| FP1000/75b | 23 (79%) |
| FP700/70c | 4 (14%) |
| 5-FU1000d | 1 (3.4%) |
| PTX30e | 1 (3.4%) |
Data given as median [25%–75%] or number (percentage).
UICC, Union for International Cancer Control; SCC, squamous cell carcinoma; ADC, adenocarcinoma; RTx, radiation therapy; CTx, chemotherapy.
a One drink =10 g ethanol; b Fluorouracil 1000 mg/m2 + cisplatin 75 mg/m2; c Fluorouracil 700 mg/m2 + cisplatin 70 mg/m2; d Fluorouracil 1000 mg/m2; e Paclitaxel 30 mg/m2.
Fig. 1 shows plots of each SUV correction parameter for examining the effects. The correction parameters differed only in the IBW derived from height; however, the other values increased with height and weight. LBM.j, LBM.gd, and PNW showed similar trends for men and women and for weight and height; however, LBM.gd and PNW decreased more with short stature and increased weight. In addition, Roza’s BMR (BMR.r) and BMR.ms accounted for the age-related decline. Considering the age distribution of the patients, the weight/height graph for BMRs narrowed between the ages of 40 and 90 years. The height was fixed at 165 cm for all parameters because it represented the mean patient height rounded down to the nearest whole number, and a three-dimensional plot would be too complex to interpret. The correlations of body weight and age with correction parameters are shown in Supplement Figures 1 and 2. BMR.r and BMR.ms decreased with age; however, BMR.ms was fundamentally similar to FFM. BMR.r had different slopes for men and women. The difference in the SUV correction value for BMR.r between men and women was almost constant, regardless of age and weight. However, for BMR.ms, the slope for men was steeper than that for women, and SUV was reversed.

Correlation between male and female weight, height, and weight correction parameters.
Correlation of weight, height, and weight correction parameters between males and females. The basal metabolic rate values for each sex are displayed with a gradient. BW, body weight; BMI, body mass index; BSA, body surface area; IBW, ideal body weight; PNWT, predicted normal weight; FFM, fat-free mass; LBM.j, lean body mass (Janmahasatian’s estimate); LBM.gd, lean body mass (Green and Duffull’s formula); BMR.r, basal metabolic rate (Roza’s formula); BMR.ms, basal metabolic rate (Mifflin-St’s estimate).
18F-FDG-PET/CT images of all patients allowed evaluation from the neck to the pelvis. Among the patients, one had different bed movement settings and four had different imaging timings for pre-treatment and post-treatment scans, although none had both. Table 3 and Table 4 show the SUV-correlated body parameters based on sex.
| Characteristic | Male | P | Female | P | Overall | P | |||
| Pre (n =24) | Post (n =24) | Pre (n =5) | Post (n =5) | Pre (n =29) | Post (n =29) | ||||
| FDG dose (MBq) | 238 [216–259] | 232 [204–244] | 0.31 | 153 [139–180] | 165 [144–172] | >0.99 | 236 [180–256] | 227 [191–236] | 0.37 |
| BW adapted RA (MBq/kg)a | 3.89 [3.69–3.99] | 3.93 [3.69–4.06] | 0.77 | 3.98 [3.87–4.30] | 4.08 [3.88–4.18] | >0.99 | 3.90 [3.69–4.05] | 3.93 [3.71–4.09] | 0.71 |
| Interval (days) | 15 [10–20] | 34 [27–52] | <0.001 | 15 [14–21] | 29 [27–35] | 0.008 | 15 [11–21] | 33 [27–52] | <0.001 |
Data given as median [25%–75%]; P values from Wilcoxon rank-sum exact test.
a Radioactivity by body weight.
| Group | Characteristic | Male | P | Female | P | Overall | P | |||
| Pre (n =24) | Post (n =24) | Pre (n =5) | Post (n =5) | Pre (n =29) | Post (n =29) | |||||
| Patients | Age | 67 [65–77] | 68 [65–78] | 0.72 | 59 [56–61] | 60 [57–61] | 0.69 | 66 [61–76] | 67 [61–77] | 0.75 |
| Correction parameters | BW | 61 [54–65] | 59 [53–64] | 0.35 | 45 [35–46] | 41 [33–44] | 0.69 | 60 [50–63] | 57 [47–62] | 0.35 |
| BMI | 21.4 [19.9–24.1] | 20.8 [18.2–23.0] | 0.35 | 16.8 [14.0–20.4] | 14.9 [14.0–20.2] | 0.84 | 21.0 [18.9–24.1] | 20.2 [17.6–23.0] | 0.38 | |
| BSA | 1.67 [1.62–1.82] | 1.66 [1.55–1.77] | 0.57 | 1.36 [1.28–1.50] | 1.35 [1.25–1.42] | 0.84 | 1.67 [1.50–1.74] | 1.62 [1.50–1.71] | 0.56 | |
| IBW | 65.3 [57.6–67.5] | 65.3 [57.6–67.5] | >0.99 | 50.4 [44.0–51.7] | 50.4 [44.0–51.7] | >0.99 | 64 [56–66] | 64 [56–66] | >0.99 | |
| PNW | 60 [56–68] | 59 [53–65] | 0.46 | 44 [37–50] | 44 [35–45] | 0.84 | 59 [50–63] | 57 [49–62] | 0.45 | |
| FFM | 51.4 [48.3–55.5] | 51.0 [47.3–54.0] | 0.68 | 34.4 [34.4–40.3] | 34.3 [33.9–38.8] | 0.69 | 51 [44–54] | 50 [44–53] | 0.70 | |
| LBM.j | 49 [47–55] | 48 [44–53] | 0.52 | 30.3 [26.6–33.5] | 30.1 [25.7–30.8] | 0.84 | 49 [41–51] | 47 [40–51] | 0.59 | |
| LBM.gd | 50 [47–55] | 48 [44–53] | 0.46 | 34.6 [30.2–38.2] | 34.3 [29.1–35.0] | 0.84 | 49 [43–52] | 47 [42–51] | 0.53 | |
| BMR.r | 1291 [1223–1443] | 1294 [1161–1407] | 0.51 | 1067 [928–1149] | 1061 [943–1098] | 0.84 | 1255 [1149–1432] | 1261 [1121–1399] | 0.56 | |
| BMR.ms | 1308 [1238–1439] | 1301 [1195–1411] | 0.53 | 925 [791–1067] | 919 [798–1011] | 0.84 | 1281 [1145–1430] | 1267 [1137–1407] | 0.59 | |
Data given as median [25%–75%]; P values from Wilcoxon rank-sum exact test.
BW, body weight; BMI, body mass index; BSA, body surface area; IBW, ideal body weight; PNW, predicted normal weight; FFM, fat-free mass; LBM.j, lean body mass (Janmahasatian's estimate); LBM.gd, lean body mass (Green and Duffull's formula); BMR.r, basal metabolic rate (Roza's formula); BMR.ms, basal metabolic rate (Mifflin-St Jeor estimate).
Fig. 2 shows the SUVmean values based on the pre-and post-treatment 18F-FDG accumulation in various organs. Supplement Figure 3 shows the pre-and post-treatment data plots for the corresponding data. A significant difference was observed in the distribution of the myocardium and multifidus before and after treatment (P = 0.008 and P = 0.001, respectively). In multiple cases, the accumulation of 18F-FDG in the myocardium decreased or recovered significantly before and after treatment. The myocardium was also visually evaluated by applying each segment model based on the 17-segment model. (Supplement Figure 4).

Paired data plot of standardized uptake values for pre- and post-treatment accumulation.
Paired data plot for pre-and post-treatment fluorodeoxyglucose accumulation in the abdominal aorta, colon, jejunum, myocardium (left ventricle), liver, spleen, and multifidus muscle are shown. P values are based on the Wilcoxon signed-rank test. Left ventricular accumulation was measured as myocardial accumulation. The liver and spleen were assessed with a 1-cm3 VOI, but the total volume accumulation could not be assessed. Pre, pre-treatment; Post, post-treatment.
Fig. 3 displays representative examples of changes in 18F-FDG accumulation. SUV levels were insignificant and stable in the multifidus muscles; however, they were uneven in the liver and spleen, often showing changes before and after treatment.

Time course of 18F-FDG accumulation in three patients.
All three patients underwent imaging once before treatment and twice after treatment. Arrows indicate the primary tumor, and arrowheads indicate the myocardial area.

Frequency distribution of each weight correction parameter for SUVmean, SUVmeanW, and SUVmeanQ.
SUV before treatment is shown on the left side, and SUV after treatment is shown on the right side (P <0.001). SUVmeanW, average PET; SUVmeanQ, average Q-PET; BW, body weight; IBW, ideal body weight; BSA, body surface area; FFM, fat-free mass; PNW, predicted normal weight;LBM.j, lean body mass (Janmahasatian’s estimate); LBM.gd, lean body mass (Green and Duffull’s formula); BMR.r, basal metabolic rate (Roza’s formula); BMR.ms, basal metabolic rate (Mifflin-St Jeor estimate).

Histogram of each weight correction parameter for MTV, Vol.mean,Vol.meanQ, and Vol.meanW.
The left side shows each count before treatment, and the right side shows each count after treatment. Vol.mean, MTV*SUVmean; Vol.meanQ, MTV*SUVmeanQ; Vol.meanW, MTV*SUVmeanW; BW, body weight; IBW, ideal body weight; BSA, body surface area; FFM, fat-free mass; PNW, predicted normal weight;LBM.j, lean body mass (Janmahasatian’s estimate); LBM.gd, lean body mass (Green and Duffull’s formula); BMR.r, basal metabolic rate (Roza’s formula); BMR.ms, basal metabolic rate (Mifflin-St Jeor estimate).
Fig. 4 and Fig. 5 show the distribution of SUVs and SUV*Volumes by weight corrections for SUVmeans (SUVmean, SUVmeanW, and SUVmeanQ). Vol.means (Vol.mean, Vol.meanW, and Vol.meanQ) had higher correlation coefficients for MTV than the SUVmeans (SUVmean, SUVmeanW, or SUVmeanQ) for all body weight corrections before and after treatment, when the threshold was fixed. Table 5 presents the detailed characteristics of the corrected and measured data from the 18F-FDG-PET scans. In the relative cutoff, the maximum correlation coefficient for MTV to Vol.means was observed when SUVmean was corrected using IBW and the Mifflin-St Jeor estimate (BMR.ms). The detailed parameters are provided in Supplementary tables. Supplementary Table S1 presents the top 20 correlation coefficients between Vol.means (Table S1–1) and SUVmeans (Table S1–2).
| Characteristic | Male | P | Female | P | Overall | P | |||
| Pre (n =24) | Post (n =24) | Pre (n =5) | Post (n =5) | Pre (n =29) | Post (n =29) | ||||
| Vol.ml | 22 [12–33] | 17 [9–25] | 0.21 | 78 [40–113] | 36 [15–44] | 0.22 | 23 [12–40] | 18 [10–27] | 0.13 |
| Vol.mean | 99 [47–185] | 41 [20–63] | 0.001 | 641 [191–1,079] | 112 [27–117] | 0.10 | 142 [49–191] | 41 [21–73] | <0.001 |
| SUVMean | 4.53 [2.75–6.97] | 2.25 [1.97–2.58] | <0.001 | 7.68 [4.82–8.26] | 2.67 [1.98–3.03] | 0.056 | 4.82 [2.76–7.62] | 2.28 [1.98–2.67] | <0.001 |
| SUVPeak | 9.9 [5.3–12.7] | 3.4 [2.8–4.1] | <0.001 | 13.5 [10.6–13.8] | 4.7 [2.9–4.8] | 0.056 | 10.6 [5.4–12.9] | 3.5 [2.9–4.7] | <0.001 |
| MeanWahl | 4.76 [3.16–7.14] | 2.62 [2.32–3.14] | <0.001 | 7.37 [5.50–10.32] | 3.18 [2.26–3.46] | 0.056 | 5.40 [3.23–7.86] | 2.65 [2.26–3.20] | <0.001 |
| SUVqPeak | 11.5 [6.4–13.8] | 4.0 [3.2–4.9] | <0.001 | 14.2 [12.8–15.4] | 5.4 [3.2–5.8] | 0.056 | 12.8 [6.5–14.3] | 4.1 [3.2–5.4] | <0.001 |
| MeanQpet | 5.30 [3.49–7.73] | 2.83 [2.51–3.45] | <0.001 | 7.82 [6.10–11.07] | 3.48 [2.47–3.75] | 0.056 | 5.89 [3.56–8.52] | 2.87 [2.47–3.55] | <0.001 |
| SUVMax | 11.8 [6.4–14.0] | 4.1 [3.2–5.1] | <0.001 | 14.3 [12.9–15.6] | 5.4 [3.2–5.8] | 0.056 | 12.9 [6.6–14.7] | 4.2 [3.2–5.4] | <0.001 |
Data given as median [25%–75%]; P values from Wilcoxon rank-sum exact test.
Vol.ml, volume only (= MTV); Vol.mean, volume × SUVmean (= total region glycolysis); SUVMean, mean standardized uptake value; SUVPeak, peak standardized uptake value; MeanWahl, average of SUVs in each slice that include the delimited VOI of SUVpeak; SUVqPeak, method for lymphoma as proposed by Hasenclever et al.18; MeanQpet, average of SUVs in each slice that include the delimited VOI of SUVqpeak; SUVMax, maximum standardized uptake value.
The correlation coefficient between SUVmean and Vol.means decreased as the cutoff increased and reached its maximum at PNW, with a cut-off value of 2.0. With an SUV cut-off value of 3.0, a negative correlation coefficient was observed between survival time and MTV and Vol.means. Furthermore, the correlation coefficient between Vol.MeanW and Vol.meanQ was higher than that between Vol.meanW and Vol.mean. The highest correlation coefficient was observed when Vol.meanW was corrected for body weight. The top 20 results are summarized in Supplementary Table S2.
Cox regression analysisTable 6 presents the top three groups in the Cox regression analysis. Pre-treatment MTV alone was selected as the best parameter for calculating results based on each parameter using the difference and quotient. This was followed by pre- and post-treatment Vol.meanQ and pre-treatment Vol.mean. No values exceeded the obtained PET values. Supplementary Table S3–1 shows the top 20 parameters by stepwise regression analysis, and Supplementary Table S3–2 provides detailed thresholds and cut-off values for Table 6.
| Group | Calculation | SUVs | Thresholds | Correction parameters | Hazard ratio | c-statistic | Likelihood ratio | |||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | P | 95% CI | LR | P | ||||||
| 1 | Prealone | Vol.ml | 3.0 | - | 39.4 | <0.01 | 4.6–339.3 | 0.81 | 21.5 | <0.01 |
| 2 | DiffPrePost | Vol.meanQ | 2.5 | BMR.ms | 38.5 | <0.01 | 4.4–333.3 | 0.80 | 20.9 | <0.01 |
| 3 | Prealone | Vol.mean | 2.5, 3.0 | - | 38.1 | <0.01 | 4.4–330.9 | 0.80 | 20.7 | <0.01 |
| DiffPrePost | Vol.meanW | 2.5, 3.0 | BMR.ms, BMR.r, BSA, BW, FFM, IBW, LBM.gd, PNW | 38.1 | <0.01 | 4.4–330.9 | 0.80 | 20.7 | <0.01 | |
| Vol.meanW | 3.0 | LBM.j | 38.1 | <0.01 | 4.4–330.9 | 0.80 | 20.7 | <0.01 | ||
| Vol.meanQ | 2.5, 3.0 | BMR.r, BSA, BW, FFM, LBM.gd, PNW | 38.1 | <0.01 | 4.4–330.9 | 0.80 | 20.7 | <0.01 | ||
| Vol.meanQ | 3.0 | BMR.ms, IBW | 38.1 | <0.01 | 4.4–330.9 | 0.80 | 20.7 | <0.01 | ||
SUVs, standard uptake values; HR, hazard ratio; CI, confidence interval; LR, likelihood ratio; Prealone, only pre-treatment data are used, with no post-treatment data included; DiffPrePost, difference between pre-treatment and post-treatment tests; Vol.mean, volume × SUV mean (= total region glycolysis); Vol.meanQ, volume × SUV meanQ; Vol.meanW, volume × SUV meanW; BW, body weight; BMI, body mass index; BSA, body surface area; IBW, ideal body weight; PNW, predicted normal weight; FFM, fat-free mass; LBM.j, lean body mass (Janmahasatian's estimate); LBM.gd, lean body mass (Green and Duffull's formula); BMR.r, basal metabolic rate (Roza's formula); BMR.ms, basal metabolic rate (Mifflin-St Jeor estimate).
Fig. 6 shows the Kaplan–Meier plots for 2-year overall and progression-free survival in the top three groups based on HR. The selected parameters were considered good predictors of overall and progression-free survival.
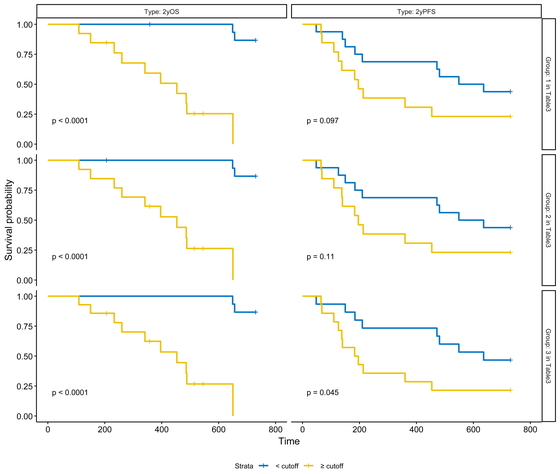
Kaplan–Meier plots of 2-year overall survival and 2-year progression-free survival for the top three groups.
Two-year overall and progression-free survival for the top three groups (each group corresponds to the groups in Table 6). Hazard ratios were used in the plots.
For pre- and post-treatment comparisons, the difference between pre-treatment and post-treatment (DiffPrePost) Vol*Means (MTV SUV threshold of 2.5) corrected for BMR.ms was selected as the best HR factor. Although the HR value was not superior to the Prealone MTV single factor, which is currently widely used, it was considered to be almost equivalent based on the c-statistic.
A report evaluating the parameters of pre-treatment 18F-FDG-PET found that MTV with an automatic threshold of 20% or 40% was useful (although a fixed value of 3.0 or 2.5 was more useful for the threshold in our study), consistent with our results.42,43,44,45 Kanoun et al.15 reported that the baseline MTV in patients with Hodgkin’s lymphoma was a favorable predictor, and that combination with the difference in SUV before and interim treatment provided a significant insight into the prognosis. These results were consistent with those of the present study.
Previous studies of squamous cell carcinoma on the oral side of the diaphragm have examined ratios and rates of change in MTV and SUV and reported a cutoff for their correlation with the prognosis.12,13,14 However, in our study the quotient did not show statistically significance, which did not support the usefulness in prognosis prediction.
Among the DiffPrePost, the difference in Vol.*Means was higher than the difference in MTV for both HR and likelihood ratio. Only Vol.*MeanQ, which used BMR.ms for correction, was different from the others. SUVmeanQ (tumor SUV normalized by liver SUVmean) using BMR.ms for correction was superior to MTV probably because of the small number of cases and the effect of body fat correction in men and women. FFM, PNW, and LBM evaluate metabolism by body mass, but BMR also considers age. It is suggested that not only sex differences but also age correction may be important for LBM. LBM.gd, PNW, and FFM also resembled BSA and body weight because the range of 160–170 cm, which was common in this case, was similar, as observed in Fig. 1.
In addition, in this study, the body weight of women was less than 50 kg both before and after treatment. It is possible that women’s metabolism was overestimated because of the following factors. First, PNW and LBM (especially PNW and LBM.gd) have smaller sex differences for patients with low weight. Second, the BMR.r slope tends to flatten for women. In particular, the values for men and women are reversed when the weight is less than 50 kg.
Only SUVmeanQ and SUVmeanW were more useful in the differential analysis. This may reflect insufficient standardization of SUVmean or poor correction of adipose tissue. It has been reported that the estimated muscle mass from the estimation formula differs between Asians and other races.46 Regarding the evaluation of LBM, which is related to metabolism, it is considered necessary to at least consider the root genes (e.g., three races), sex, and age.
In addition to reconstructing the estimation formula, we think that it is one of the countermeasures to consider the prediction of body weight that contributes to metabolism according to resting energy expenditure by other methods, including indirect calorimetry.47 This includes Weir’s formula48 and its simplifications.49The Weir equation is as follows:
The simplified Weir equation is as follows:
Although there was a significant difference in the distribution of myocardial uptake and multifidus muscle uptake before and after treatment, there was no significant trend in the course of increase or decrease. Therefore, the effect of the glucose metabolism environment, such as the effect of fasting before the examination, was considered. In addition to the effect of glucose metabolism, the effects of alcohol50,51,52 and smoking53,54 on glucose metabolism have been reported.
Reactive oxygen species (ROS) induce glucose metabolic pathways that are distinct from the normal pathway (e.g., α7nAchR-NF-κB-IL-6 → JAK2/Akt crosstalk).55 α7-Nicotinic acetylcholine receptor (α7nAchR) is involved in these pathways and is upregulated by the nuclear factor-κB (NF-κB) pathway involving tumor necrosis factor-α and interleukin-6 (IL-6) induced by lipopolysaccharide (LPS) produced from intestinal bacteria,56,57 by ethanol,58 or by nitrosamine ketones derived from tobacco smoke extract and nicotine.59
Although the effects of alcohol withdrawal on LPS are unclear, even though LPS is also induced by alcohol, the dual effect observed on cytochrome P450 2E1 (CYP2E1) was short-lived, with no increase in activity after more than 8–10 days of abstinence.60,61 However, this does not happen after about 3 consecutive days,62 and it takes about 2 weeks to reduce the liver damage caused by chronic alcohol consumption63 and 3 months for normalization of the metabolic environment in the body.64
CYP2E1-mediated responses in the microsomal ethanol oxidizing system exhibit sex differences65 and are upregulated by chronic alcohol consumption.66 This fact may be more influential in some people, such as many Japanese and some Southeast Asians who have the ADH1B2 allele (also known as the alcohol dependence gene), which accelerates the metabolism of ethanol to acetaldehyde, and/or ALDH2*2, which slows down the metabolism of acetaldehyde to acetate. On the contrary, the effect of smoking on CYP2E1 regulation is unclear, as there are reports of both upregulation and downregulation, but the effect of nicotine on α7nAch receptors is known.67 Therefore, to reduce the effects of metabolic abnormalities on the test, abstinence from alcohol consumption (including smoking cessation if possible) for at least 10 days before each pre- and post-treatment test is desirable.
This study has some limitations. The patients examined and analyzed in this study could have received irradiation for submucosal tumors that had small volumes before and after treatment, and this may have underestimated SUV levels in one test because of the partial volume effect. Therefore, the availability of SUV values related to the amount or rate of change was a secondary goal. Second, because of the retrospective study design, the time period in which the patients underwent PET-CT was varied. Cases with post-treatment PET-CT within 1 month were included, and this included the effect of the post-treatment inflammatory response. Patients who received preoperative chemotherapy were excluded, limiting the number of cases.
Despite these limitations, the strength of this study was that it evaluated the strength and weakness of metabolism by examining changes in the intensity of SUVs rather than evaluating only the amount of SUV that accumulated above the cutoff value. With regard to the indirect evaluation of metabolism, the backgrounds of the original studies were different, and the metabolic formula used for standardization was examined in detail by comprehensive evaluation of the different distribution characteristics.
In conclusion, considering the effect of age-related decreases in LBM, the difference in the product of MTV and SUVmeanQ before and after CCRT may be a useful parameter to predict the prognosis of patients treated with CCRT for esophageal cancer. However, pre-treatment MTV remains a solid prognostic parameter.
The authors thank the late Dr. Shinichi Tominaga (who passed away on September 2, 2018) for his guidance and support.
The authors have declared that no conflict of interest exists.