2013 年 30 巻 p. 47-68
2013 年 30 巻 p. 47-68
Hollow particle is a promising material with the special properties of low densities, thermal insulation and distinct optical activity. Due to their potential promising applications in the fields of drug delivery, catalysis and optics, a great effort has been devoted to develop new preparation methods which are collected and reviewed in this paper. All these methods are classified into three groups, namely sacrificed template method, in-situ template method and device-based method based on the characteristics of the methods. The advantage and disadvantage of each method are compared and the trends for preparation are pointed out. In light of the wide applications of hollow particles, the later part of this paper focuses on their potential applications in industry. Their applications are not limited in the fields of papermaking, rubber processing and plastic improvement, but also expanded to electronic, catalytic and biological areas.
Hollow particle is a kind of powder contained interior hollow structure. And the hollow structure is usually covered by a solid shell. Hollow particles with dimensions from nanometer to micrometer are becoming a focus in nanoscience and nanotechnology. In particular, hollow nanostructures made of metals are intriguing to be investigated due to their special plasmonic properties1) and catalytic activities2) totally different from their solid counterparts. For example, gold nanoshells have been synthesized for use as photothermal triggers for drug release and as a contrast-enhancing reagent in optical imaging3–5). Owing to the special properties, such as low density, thermal insulation and prominent optical activities, hollow particles have been applied in many areas which include drug delivery1,2), bioencapsulation6,7), medical diagnostics8), catalysis2), plasmonics1) and composite electronic and structural materials9,10).
Various methods have been developed for preparation of hollow particles. A general route for the preparation of hollow particle is to coat /deposit desired materials on the surface of templates followed by the removal of template. The templates usually used include organic spheres11–17), inorganic particles18) and metal crystals19). All these templates have to be removed after the coating process. The method involved these kinds of templates are called scarified template method20,21). In addition, a great effort has been devoted to simply the preparation of hollow particles and developed in-situ template method which uses the reactant or product as template. The desired materials coat on the surface of reactants/product which are consumed in the later reaction, leading to the formation of hollow particles. The advantage of the new approaches is to save the process of template removal and to simplify the two-step process to onestep process. Besides, some device-based approaches are developed for the preparation of hollow particles, such as nozzle-based method. All these methods are reviewed in this paper and the comparison and analysis are elaborated.
In this paper, based on the different kinds of templates, the methods to prepare hollow particles were reviewed and classified into three groups, namely sacrificed template methods, in-situ template methods and device-based methods. Each group includes several methods with similar characteristics.
2.1 Sacrificed template methodThis kind of methods were characterized with the preparation of hollow particles by coating a template (such as polymer bead, emulsion and inorganic colloids) with a thin layer of the precursor through sol-gel process or layer-by-layer (LBL) self-assembly technique22) and subsequent removal of the template via a thermal decomposition or a chemical dissolution23).
2.1.1 Organic bead template methodThis method is designed to prepare hollow particles by using some organic beads (for example polystyrene spheres) as templates which are coated by desired materials (such as silica (SiO2)24), zirconia25), zeolite26), manganese oxide (MgO)27), magnetite28), zinc sulfide29), cadmium sulphide (CdS)30)) via deposition or electrostatic attraction31). After coating, the organic cores are removed by calcinations or decomposition to solvent, forming hollow particles. Fig. 1 is the scheme illustration of this method.
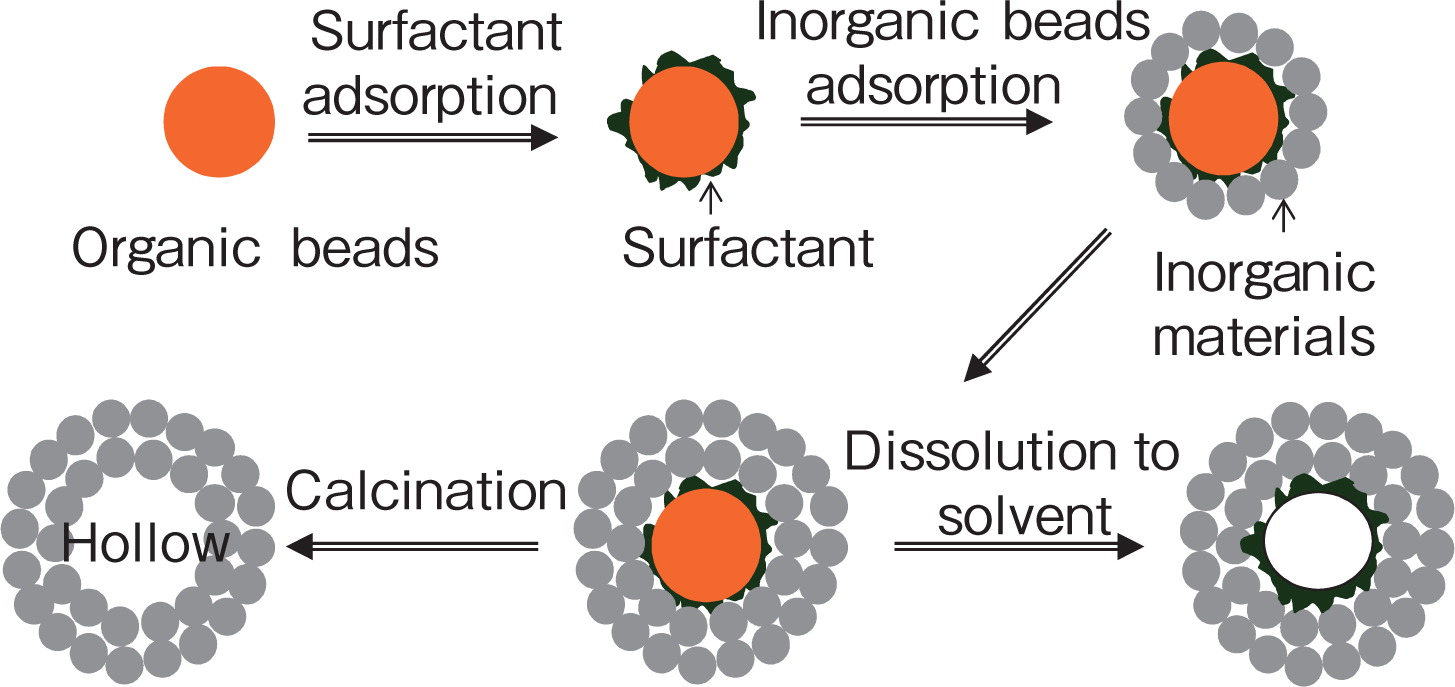
Illustration for preparing inorganic hollow spheres with organic bead template24).
F. Caruso and his colleagues have done many interesting works in this area24,28,32). They introduced a LBL self-assembly technique to this method. The LBL technique is a process to fabricate films on the surface of flat or spherical substance by alternating depositions of oppositely charged species based on electrostatic interaction or hydrogen bonding33–39). Caruso and co-workers have reported on the fabrication of hollow inorganic silica via the electrostatic LBL self-assembly of SiO2 nanoparticle on the surface of PS latex particles. The PS particles with diameter of 640nm were firstly coated with polymer films to provide a smooth and positively charge surface. Then SiO2 nanoparticles with negatively charged surface were coated on the surface of modified PS particles. For multi-coating, the polymer and silica were alternate adsorption on the PS template. The PS templates were removed by calcinations at 500°C. Then the hollow silica particles were prepared, as shown in Fig. 2. The advantage of this method is the readily permitted control of film thickness by variation of coating cycles.
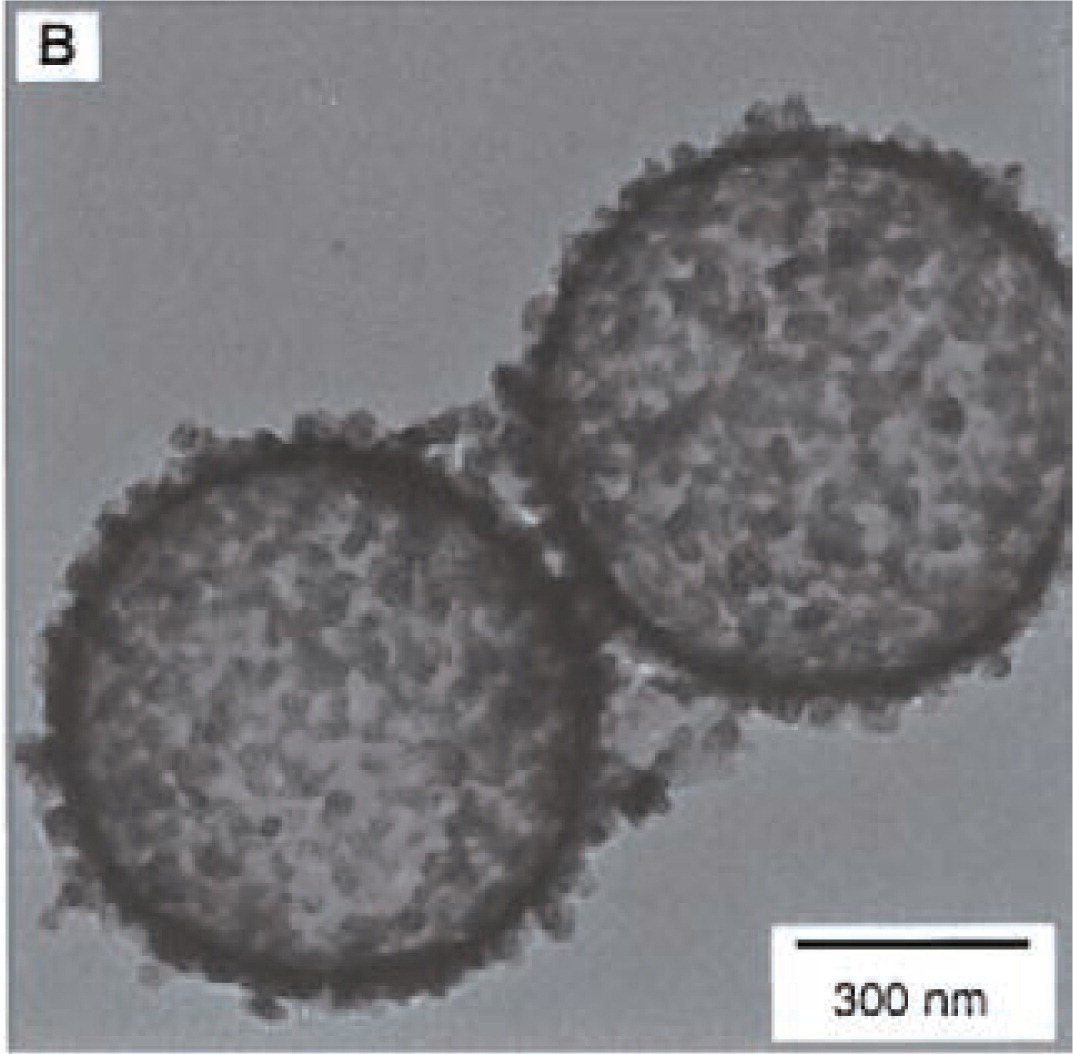
TEM micrograph of hollow SiO2 particles prepared by organic bead template24).
Shell microstructures including shell thickness are important factors to realize functionalities of hollow particles. Fuji and his co-workers have prepared hollow silica nanoparticles through fabrication of core-shell particles whose shell was derived from sol-gel reaction of silicon alkoxide (tetraethoxysilane, TEOS). In this method, fine SiO2 particles from hydrolysis and condensation of TEOS adsorb at the template surface to form a thin and dense SiO2 layer. According to Iler, sol-gel reacting conditions such as pH, reaction time, and temperature strongly affect size and morphology of the formed fine SiO2 particles40). Based on this theory, preparation technique to control shell microstructure was developed. They also proposed that the shell microstructure difference can be organized by apparent shell density which is defined by specific surface area and shell thickness. The apparent shell density of the obtained hollow SiO2 nanoparticles has been controlled within the range between 1.14 and 2.2 g/cm3 41).
Beside hollow silica, hollow magnetite (Fe3O4) have been prepared by coating submicrometer-sized PS templates with magnetite (Fe3O4) layers alternately adsorbed with polyelectrolyte (PE)28). For the precursor sensitive to water and readily hydrolyzing and condensing upon direct contact with water, they proposed a new route that combines LBL colloid templating and in-situ sol-gel processes by exploiting polyelectrolyte multilayer-coated PS particles as templates. The use of PE-coated spheres as templates overcomes the common problem of particle aggregation by localizing the reactions within the thin PE coatings on the colloids32).
Hollow calcium carbonate (CaCO3) particles have also been fabricated by the organic bead template method. Fig. 3 showed the hollow porous shells of crystalline CaCO3 prepared by using micrometer-sized PS beads as the templates42). Monodisperse PS spheres with a uniform diameter of 1.09 μm were covered by a film of microemulsions which contained tetradecane and supersaturated aqueous calcium bicarbonate (Ca(HCO3)2) solution, and washed repeatedly in hot hexane. Loss of carbon dioxide (CO2) at the air-water interface resulted in aragonite crystallization within the continuous aqueous layer surrounding the oil droplets. Removal of the surfactants and oil leaded to the formation of continuous cellular film of aragonite over the surface of polymer beads, followed by heat treatment of the mineral shell at 400°C, produced microshells of porous aragonite. The hollow shells were uniform in size with an external diameter and wall thickness of 1.35 μm and 125 nm, respectively. Kojima and Yasue43) also synthesized hollow CaCO3 via the organic template method.
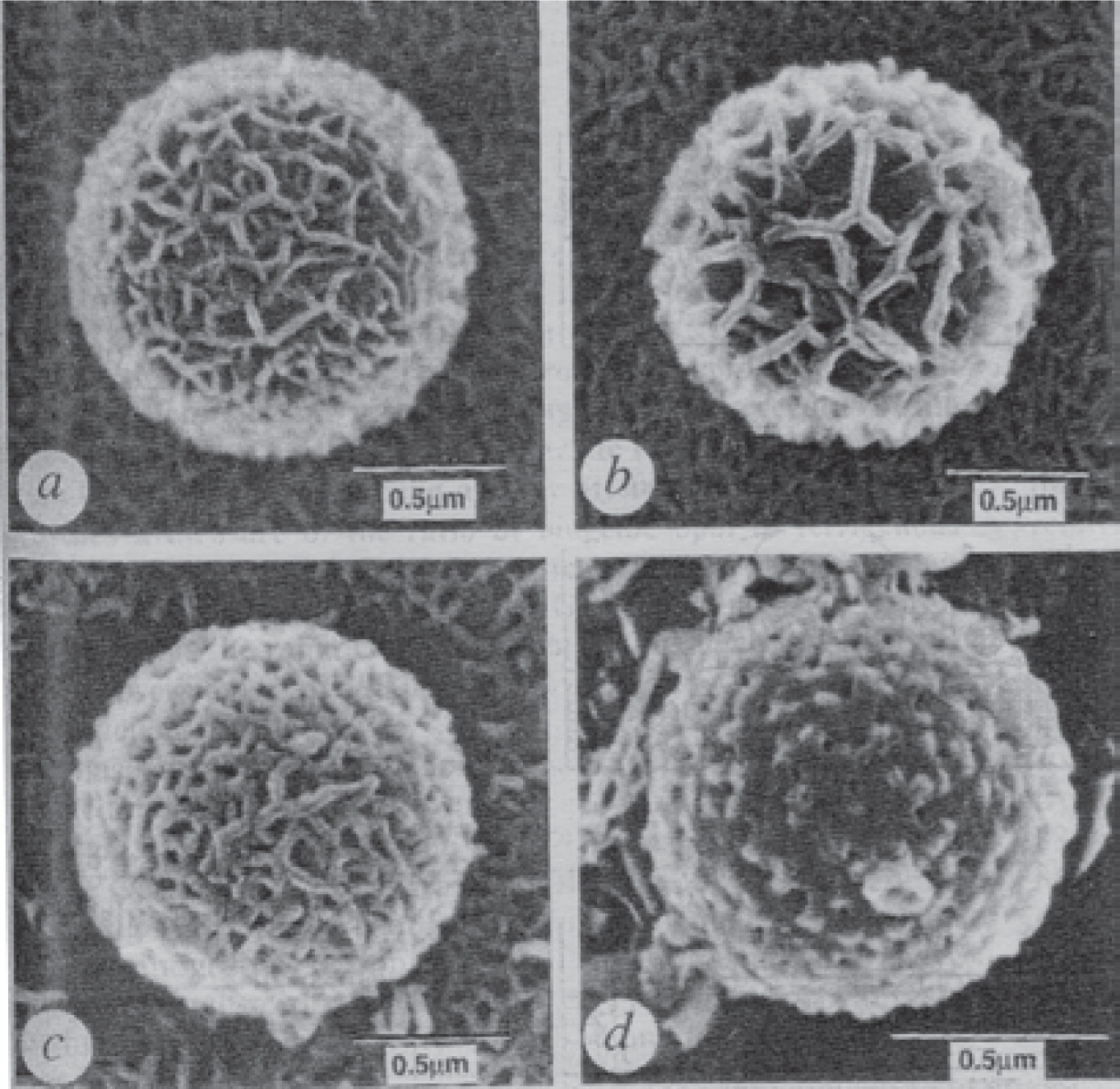
SEM images of the synthetic aragonite shells42).
Other hollow particles prepared by organic bead template method with the combination of LBL techniques include hollow MgO particles44), hollow zeolite particle45), hollow clay particles46), hollow zinc oxide (ZnO)47,48), hollow copper and copper compound particles49).
One more interesting report is that X. Zhang and co-worker50) have prepared cadmium selenide (CdSe) hollow spheres via an in-situ polymerization template under ultraviolet irradiation since the organic monomer have a tendency to rapid cross-link polymerization and form networks under high-energy irradiation. The obtained polymer from random coils restrains the appearance of special morphology and can provide the template to control the morphologies of CdSe. Based on the same idea, Y. Hu and coworkers51) have prepared hollow nickel sulfide (NiS) sphere by using in-situ polymerization template.
2.1.2 Emulsion template methodAn emulsion is defined as a heterogeneous system, consisting of at least two immiscible liquid or phase. Since emulsion is thermodynamically unstable, rearrangement from droplet into two bulks liquid is ready to occur with a net reduction of interfacial area, which is energetically favourable. So the surfactants or polymer, which can stabilize emulsion are necessary to use in the emulsion system. The emulsion template method is a process to fabricate hollow particles by localizing the reaction on the surface of emulsion and forming a solid shell. After removal of the emulsion template by calcination or solvent dissolution, hollow particles are obtained. The emulsion template is generally classified into two types. One is water-in-oil (w/o) templates which have oil continuous phase with water droplets. Another is oil-in-water (o/w) templates which have water continuous phase with oil droplets. The formation of these two kinds of emulsion is determined by the weight ratio of water and oil. High weight ratio of oil lead to the formation of w/o templates while high weight ratio of water result in the formation of o/w templates. The choice of which kinds of templates is determined by the reactants and reactions.
The emulsion template method has been successfully used to prepare hollow SiO2 particle. J. H. Park and co-workers52,53) have prepared hollow SiO2 particles in the w/o emulsion with the presence of surfactants. Their preparation procedures can be briefly described as following: First, an external oil phase was prepared by dissolving hydroxyl propyl cellulose (HPC) in octanol. Here HPC is a stabilizer of emulsion structure. Second, polyvinylpyrrolidone (PVPC) which was used to control the viscosity of water droplet was dissolved into water. Third, the water phase was added to an external oil phase at a stirring rate of 12000 rpm to form a stable emulsion. Then, the reagent TEOS was added into w/o emulsions. TEOS is soluble in the continuous oil phase, but it because water-soluble after its hydrolysis. After hydrolysis, the samples were dried and calcined to form hollow structure. The hollow SiO2 particles produced by this method are shown in Fig. 4.
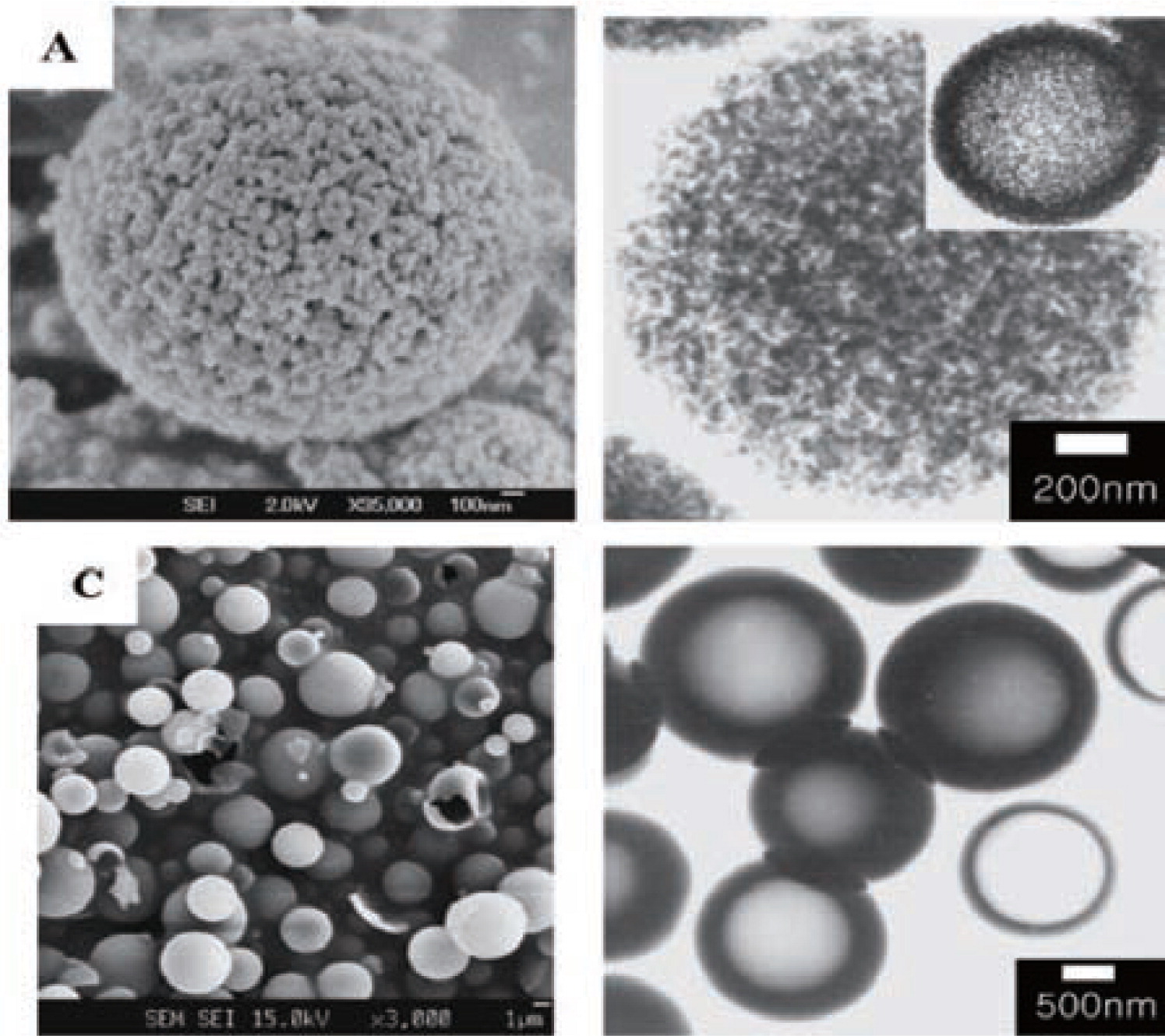
SEM (A,C) and TEM (B,D) of hollow SiO2 particles53).
M. Jafelicci Jr. and co-worker54) also prepared hollow SiO2 particles by the w/o emulsion template method. They added a fixed volume of aqueous phase (acid solution) to the heptane solution with surfactants, and then sodium silicate (Na2SiO3) diluted solution was added to the previously obtained microemulsion and sonicated. As hydrolysis and condensation of TEOS are pH sensitive reactions, they progress in acid medium, such as that of microemulsion interface region where is chemically adequate for a precipitation reaction to take place and form a SiO2 inner shell, consuming Na2SiO3 and hydroxonium ion (H3O+). Fig. 5 shows the model of microemulsion reactor.
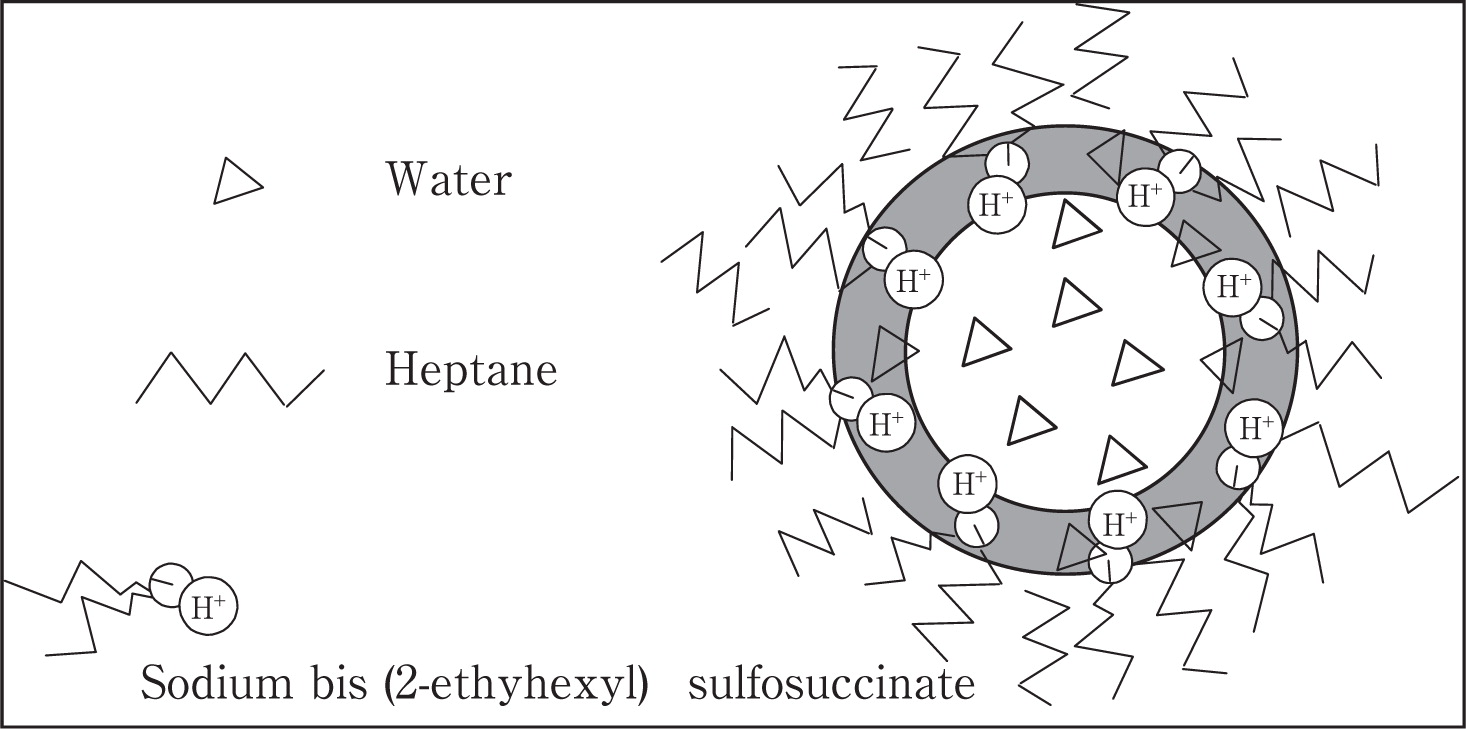
Schematic representation of microemulsion model54).
The reports on preparation of hollow SiO2 particles by emulsion template method also include reference55) to reference 58).
Except hollow SiO2, hollow CaCO3 particles have also been prepared by w/o emulsion template method. For example, D. Walsh and co-workers59) reported a facile and high yield approach to produce spherical particles of crystalline CaCO3 (vaterite) with an elaborate sponge-like micro architecture. J. A. Thomas and co-workers60) also prepared CaCO3 microcapsules for the encapsulation of organic and inorganic substrates using a liquid emulsion system. They prepared the emulsion by mixing a solution of 3.0 M sodium carbonate (Na2CO3) which contains the materials to be encapsulated within the oil membrane phase. The two phases were mixed together until a homogeneous macro-emulsion was formed. This w/o emulsion was then added to a solution of calcium chloride (CaCl2) or calcium nitrate (Ca(NO3)2). Based on work by Nakahara61), this method allows calcium ions (Ca2+) to diffuse across the oil membrane into internal aqueous droplets that contains the materials to be encapsulated. Carbonate ions (CO32−) in the internal aqueous phase react with the diffused Ca2+ at the o/w interface and solid CaCO3 in the metastable vaterite form precipitates at the interface, encapsulating the materials dispersed in this phase. The vaterite spheres are prone to transformation to the more stable calcite polymorphy, which would destroy the capsules. In order to prevent this transformation and stabilize the vaterite, L-glutamic acid, a known inhibitor of calcite crystallization was added to the aqueous phase prior to precipitation. After decantation and centrifugation, hollow vaterite spheres inside with desired substance were obtained.
In addition to w/o emulsion template, the o/w template had also been applied to fabricate hollow particles. A. D. Dinsmone and co-worker62) reported a flexible approach to produce hollow elastic capsules, with size ranging from micrometers to millimeters. Their fabrication process used controlled self-assembly in three steps. First, aqueous solution is added to oil containing colloid particles which were then adsorbed on the surface of emulsion droplets. Second, particles are adsorbed automatically onto the surface of the droplet to reduce the total surface area. After the droplet surface is completely covered by particles, these particles are subsequently locked together by addition of polycations. Third, if required, the capsules are transferred to water by centrifugation. The resultant structures, which are called “colloidosome”, are hollow, elastic shells whose permeability and elasticity can be precisely controlled.
M. M. Wu and co-workers63) reported to prepare hollow bead titanate particles via an o/w emulsion template. The reactants titanium butoxide is just hydrolyzed at the o/w interface where the shells are formed through condensation. The morphology of lead titanate shells reflects the shape of oil spheres at the micrometer scale, indicating the reaction happening at the interface of emulsion. Hollow latex particles have also been prepared via o/w emulsion template by controlling the charged colloids adsorption around the emulsion droplets64).
Besides, T. Nakashima and N. Kimizuka65) have prepared hollow titania (TiO2) particles in ionic liquids based on the limited miscibility of toluene with the ionic liquids. Microsized droplets are formed in the ionic liquid under vigorous stirring and the Ti(OBt)4 molecules in the microdroplets are hydrolyzed selectively at the interface, resulting in the formation of smooth and hollow TiO2 particles.
2.1.3 Surfactant vesicle template methodAmphiphilic block copolymers in water can self-assemble into various ordered mesospheres66–70). For example, low molecular weight macromolecular surfactants produced spheres which are readily dispersed in water at low concentration as spherical vesicles with the polar head group assemble together, creating a water core that is typically 10–100 nm in diameter71,72). F. Caruso and co-workers73,74) have developed a technique for the preparation of monodisperse vesicles comprising asymmetric lipid bilayers supported on colloidal particles. The monodisperse asymmetric lipid-coated colloids were prepared by depositing two lipids which can form the inner and outer monolayers. Following formation of the lipid bilayer membranes, the core templates were removed by acid treatment to obtain vesicular particles.
The spherical vesicles can be used as template for preparation of hollow particles based on the hydrolysis and cross-linking of inorganic precursors at the surface of supramolecular surfactant assemblies. P. T. Tanev and T. J. Pinnavaia75) have prepared hollow SiO2 using surfactants vesicles as templates. Their approach is based on the hydrolysis of an inorganic alkoxide precursor in the interlayered regions of multilamellar vesiculars of surfactant that contains two polar head groups linked by a hydrophobic alkyl chain. The multilamellar regions of the vesicles are composed of closed packed layers of surfactant separated by water layers. The additions of TEOS penetrate the vesicle interface, diffuse into the multilamellar regions, and participate in hydrogen-bonding interactions with the lone electron pairs on the surfactant head groups. The simultaneous growth of the parallel SiO2 layer leads to the formation of hollow particles.
T. Z. Ren and co-workers76) prepared hollow TiO2 microspheres with mesoporous crystalline shells with the assistance of non-ionic poly (alkylene oxide) surfactant molecules. The preparation of hollow TiO2 microspheres was performed in a ethanol-surfactant system using decaoxyethylene cetyl ether (C16(EO)10) surfactant as the template. M. S. Wong and co-worker77) have reported a room temperature and wet chemical-based synthesis route in which SiO2 and gold (Au) nanoparticles are cooperatively assembled with lysine-cysteine diblock copolypeptides into robust hollow spheres. Key determinants in the formation of the Au/SiO2 hollow sphere (as shown in Fig. 6) are the ability of the sulfydryl group to form inter and intrachair disulfide bonds and the formation of the Authiolate bonds. Hollow sphere was formed only when the copolypetide was reacted with n-Au prior to reacting with n-SiO2. The n-Au particles were thus found copolypeptide chains into the microsized aggregated around which n-SiO2 attached. The scheme illustration of the assembly process is shown in Fig. 6.
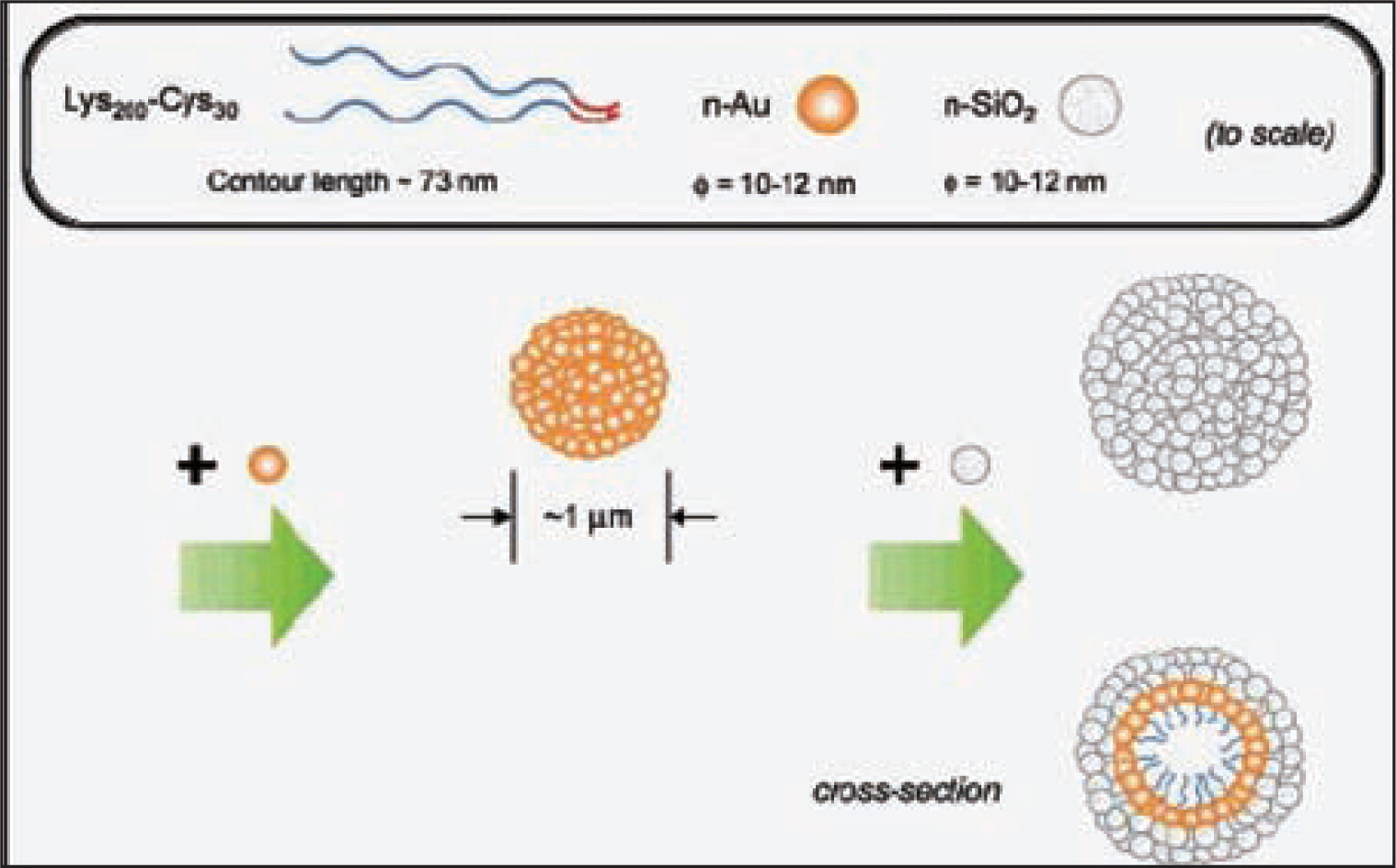
Scheme of self-assembly of Au and SiO2 nanoparticles77).
Other hollow particles prepared by surfactants vesicles templates method include hollow CaCO378), hollow zirconium oxide (ZrO2)79), hollow PS80), and hollow aluminosilicate81).
2.1.4 CaCO3 template methodRecently, some researchers proposed to use nano-sized CaCO3 as the sacrificed templates to prepare hollow particles82–85). M. Fuji and his colleagues firstly reported the preparation of nano-sized hollow SiO2 particle using fine CaCO3 particles as templates18,87,88). The hollow SiO2 particles were prepared by coating SiO2 layer on the surface of CaCO3 particles via sol-gel process, followed by the removal of CaCO3 template through acid etching. Then hollow particles with different morphologies (such as spherical, cubic and tube) were prepared by choosing the different crystal templates (vaterite, calcite and aragonite)88). They also showed that “skeletal” SiO2 nanoparticles which consist of twelve SiO2 nanoframe and six square windows using cubic-shaped calcite template whose surface was covered by organic acid. By control affinity between the organic acid and reaction solvent, skeletal SiO2 nanoparticles can be obtained completely separately from hollow SiO2 nanoparticles89).
J. F. Chen and co-workers90,91) have also done many interesting work in the area. They fabricated porous hollow SiO2 particles by using CaCO3 as templates. In their experiments, CaCO3 nanoparticles were firstly modified by surfactants. Then the silica precursor (NaSiO3•9H2O) was added into the solution. After the reaction, the core-shell composite with CaCO3 as the core and SiO2 as shell were prepared. The CaCO3 cores were finally removed by acid etching (in HCl solution). The preparation procedure is illustrated as Fig. 7.
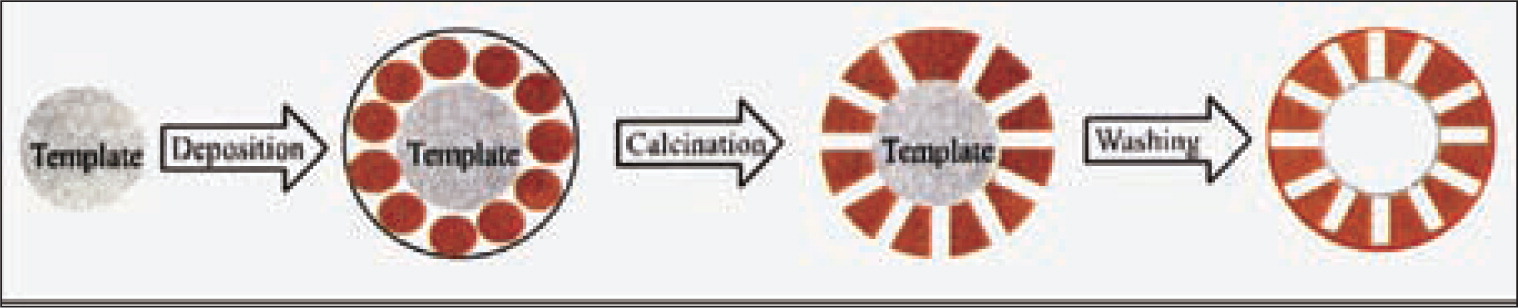
Illustration on preparation of hollow porous SiO2 particles prepared by CaCO3 template method91).
As further research, J. F. Chen developed a double-template method in which the CaCO3 nanoparticles served as templates and the cetyltrimethylammonium bromide (CTAB) as shell structure directing agents. The prepared hollow particles have an average external diameter of about 85 nm with disordered mesopores shells92).
Other carbonate templates include manganese carbonate (MnCO3) which has been used to prepare hollow particles for encapsulation of deoxyribonucleic acid (DNA)93). MnCO3 particles with diameter of 4 μm were used as template. The water insoluble DNA/sperimidine (Sp) complex was precipitated on the surface of MnCO3 template. Further alternated LBL assembly of biocompatible poly[β-glucuronic acid-(1, 3)-N-acetyl-β-galactosamine-6-sulfate-(1, 4)] (PG)/poly(-L-arginine) (PA) shell was carried out. At the final step, MnCO3 template particles were dissolved in HCl solution. As a result, biocompatible PG/PA capsules containing DNA/SP complex were obtained.
2.1.5 SiO2 template methodSiO2 particles with size ranging from nanometer to micrometer can be used as templates for preparation of hollow particles since they are easily decomposed into silyl tetrafluoride by a hydrofluoric acid (HF) solution. For example, Y. Itoh and co-workers94) have prepared biodegradable hollow nanocapsules by an alternate LBL assembly of cationic chitosan (CT) and anionic dextran sulphate (Dex) on the surface of SiO2 template. The assembly of the ultrathin polymer films on the SiO2 nanoparticles was confirmed by microelectrophoresis. After coating, the SiO2 templates were etched by HF solution, and hollow capsules were obtained. The particles size of prepared hollow capsules can be easily controlled from a nanometer to micrometer, depending on the size of the SiO2 template.
F. Caruso and co-workers have reported to use mesoporous SiO2 (MS) spheres as sacrificial template for both enzyme immobilization and PE multilayer capsule formation by PE coating on the surface of the catalase-adsorbed MS through LBL technique95,96). The MS templates were removed by exposure to HF. Their results indicated that the catalase encapsulated in biocompatible microcapsules can be released by pH- or salt- induced permeability changes.
S. B. Yoon and co-workers97) have prepared hollow carbon particles by using SiO2 spheres with solid core and mesoporous shell (SCMS) structures as templates. Phenol and formaldehyde were incorporated into the mesoporous shell, further carbonized to obtain carbon/aluminosilicate nanoparticles. The dissolution of the aluminosilicate template using either sodium hydroxide (NaOH) or HF solution generated hollow core and mesoporous shell carbon capsules. T. K. Mandal and co-workers98) have prepared uniform hollow polymer microspheres by coating SiO2 microsphere template with poly(benzyl methacrylate) using surface-initiated control radical polymerization and subsequently removing the core by HF etching. Shell thickness was controlled by varying the polymerization time. Y. Xia and coworkers99) have synthesized polymer hollow spheres inside with a movable Au nanoparticle by using SiO2 template. First, they coated the Au nanoparticle with uniform shells of amorphous SiO2 derived from TEOS precursor. Second, uniform polymer shell with controllable thickness were formed on the SiO2 surface. Finally, the SiO2 shell sandwiched between Au core and the polymer shell was selectively dissolved using aqueous HF to generate hollow particles containing movable Au nanoparticle. The encapsulated u nanoparticle provides an optical probe for monitoring the diffusion of chemical reagents into and out of the polymer shells. Besides, hollow TiO2100) and hollow silicon carbide (SiC)101) have also been prepared by using SiO2 as template.
In this method, the choice of SiO2 template is very important in producing efficient hollow particles because the properties of the templates, such as size, shape and ease of removal, govern the properties of the resultant hollow particles.
2.1.6 Hydroxyapatite (HAp) template methodHAp (Ca10(PO4)6(OH)2) is low cost materials that can be prepared in nanoparticles with high level of monodispersity and reproducibility. Synthetic HAp particles were prepared by the wet method in which molar ratio of Ca2+ and phosphate ions (PO43−) was adjusted. The HAp surface possesses several kinds of P-OH group act as adsorption sites for various molecules, which are influenced by synthetic process like calcined temperature, additives, and so on. The number of surface P-OH groups determines various surface properties, for instance, acidity and basicity, affinity and reactivity to molecules and catalytic activity. Besides, HAp templates can be easily dissociated at mild acid concentrations and no special equipment is needed to recycle the dissolved HAp particles102).
When using HAp as an inorganic template for hollow particle, such good affinity between HAp surface and shell species can be expected to form core-shell particle. The synthetic processes also provide various shapes to obtained HAp particles. Green and his co-workers synthesized hollow SiO2 nanoparticles with HAp-replicated shapes such as rod-like and spherical103). Mesoporous SiO2 shell can be obtained by using CTAB as a structure directing agent103) while shell with micro-pores formed without any agent104).
2.2 In-situ template methodRecently, some new and interesting results have been reported to fabricate hollow particles without using the additional template, which was called in-situ template method. The in-situ template method includes in-situ reactant template method and in-situ product template method. For in-situ reactant template method, hollow particles are formed by directly coating/precipitation nanocrystals on the surface of reactants that would be consumed in the following reactions. After reaction, the templates are consumed and hollow particles are directly obtained without the further treatment to remove the templates. For in-situ product templates method, hollow particles were formed on the surface of interim product which can be removed after reaction.
2.2.1 In-situ reactant template methodIn such a process, one of reactants acts as the template for the formation of hollow particles. The advantage of utilizing such a template is that the template is automatically removed at the end of the reaction, which avoids the step of removing templates to obtain hollow products.
Y. Xia and his colleagues have done many interesting researches in this area. They have reported a procedure based on replacement reaction to generate nanoshells from various metals19). The mechanism of this process was illuminated with the Au/silver (Ag) combination as an example. Because the standard reaction potential of AuCl4−/Au redox pair (0.99V vs. the standard hydrogen electrode (SHE)) is higher than that of the Ag+/Ag redox pair (0.80V vs. SHE), Ag nanoparticles are immediately oxidized to Ag+ when mixed with an aqueous chloroauric acid (HAuCl4) solution105) as shown in following chemical reaction (1).
| (1) |
The elemental Au should be mainly confined to the vicinity of the template surface. Once the concentration of Au atoms has reached a critical value, they will nucleate and grow into small clusters and eventually evolve into a shell-like structure around the Ag template.
The major steps involved in this process are shown in Fig. 8106). (A) Initiation of replacement reaction at a specific spot with relatively high surface energy; (B) Continuation of the replacement reaction between Ag and HAuCl4 and the formation of a partially hollow nanostructure; (C) Formation of nanoboxes with a uniform, smooth, homogeneous wall composed of Au-Ag alloy; (D) Initiation of dealloying and morphological reconstruction of Au-Ag nanobox; (E, F) Continuation of dealloying, together with the formation of pores in the wall; and (G) Fragmentation of the porous Au nanobox. The cross-sectional views correspond to the plane along dashed lines.
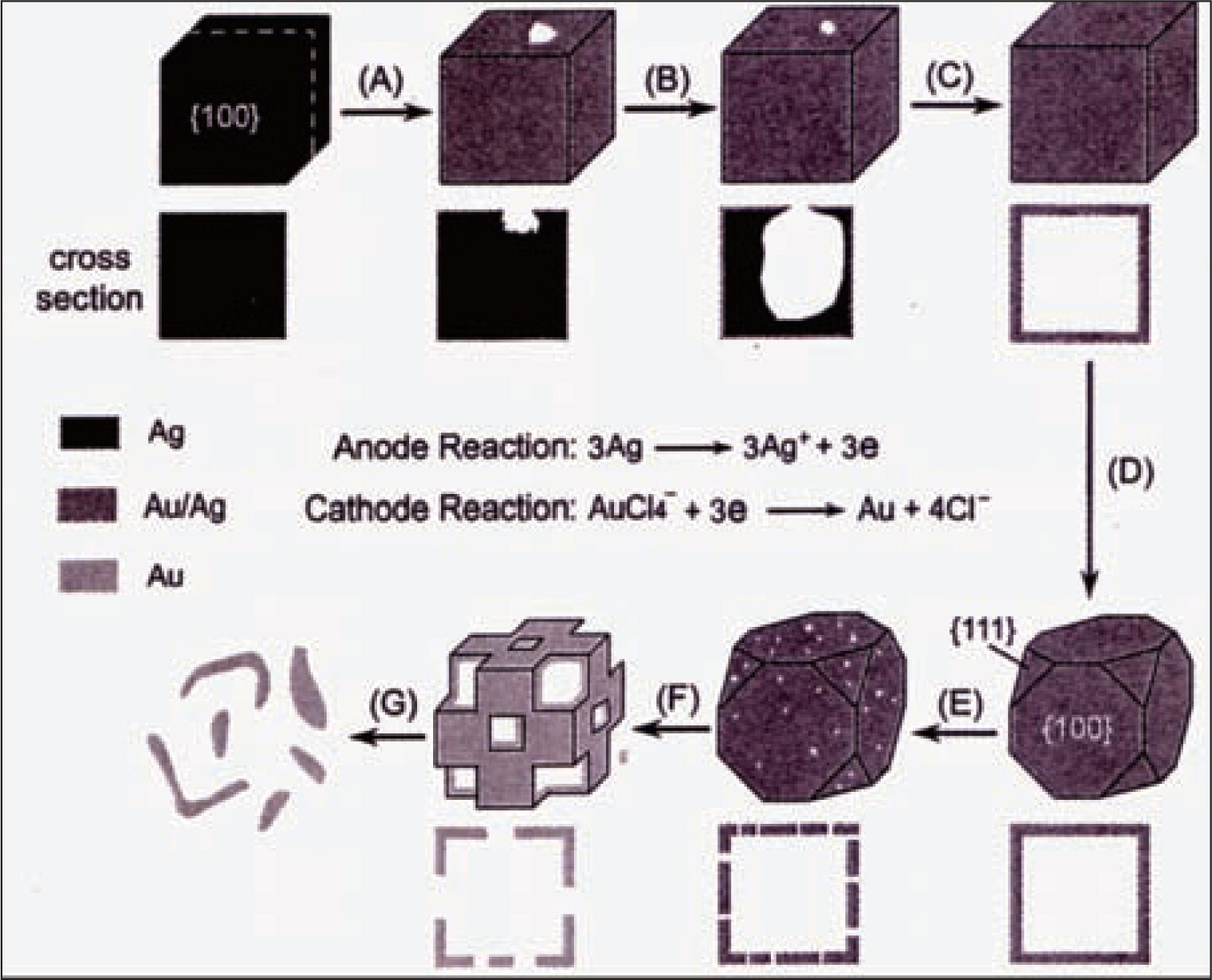
Schematic illustrations on the preparation of porous Au nanoshell by templating again Ag nanoparticles106).
Dr. Xia and other group have successfully prepared Ag nanostructures exhibiting a range of morphologies that include spheres, triangular plate, cubes, rods and wires96–101). By reaction these Ag nanostructures with aqueous HAuCl4 solution, Dr. Xia have generated hollow structures of Au with various morphologies, similar those of the Ag templates. Besides, Dr. Xia and co-workers107) have prepared hollow platinum (Pt) with controllable dimensions using trigonal selenium (t-Se) as templates. A little difference is that the t-Se core are not fully consumed during the reduction of Pt2+ and the t-Se template were completely removed by soaking the sample in pure hydrazine monohydrate liquid after the formation of Pt shell. The galvanic displace reaction has also been exploited by other researchers108) to fabricate hollow cobalt-platinum (CoPt) nanostructures using Co nanoparticles as templates.
Y. Yin and co-worker109) also prepared hollow structure nanocrystals (such as cobalt sulphide (CoS)) by using cobalt nanocrystal as templates. The hollow CoS nanospheres were synthesized by immediate injection of a solution of sulphur (S) into hot Co nanocrystal dispersion. The reaction between Co and S results in the formation CoS shell at the surface of Co. After Co was consumed, hollow particles were obtained. But they attributed the formation of hollow particles to Kirkendall effect in which the mutual diffusion rates of vacancies and Co in a diffusion couple differ by a considerable amount, resulting in the void formation inside. As the reaction start, Co atoms diffuse out to the shell and the accompanying transport of vacancies leads to the formation of void inside. The authors have pointed out the formation of hollow particles by galvanic displace reactions may also involves this phenomenon.
Besides, Y. Qian and co-workers have also prepared some hollow particles by in-situ template method. For example, they prepared hollow bismuth telluride iodide (BiTeI) using bismuth telluride (Bi2Te3) as template110), hollow carbides using sodium as templates111) hollow CdS using carbon disulfide (CS2) as template112). J. J. Zhu an co-workers113) prepared hollow CdSe using the reactant cadmium hydroxide (Cd(OH)2) as templates with the assistance of sonochemistry. The use of sonication provided some active spots on the surface of Cd(OH)2 for the formation of CdSe nuclei.
2.2.2 In-situ product template methodThis method refers to the method to prepare hollow particles by using interim product or by product as template. After reaction, the templates are removed by selective etching or thermal decomposition, resulting in the formation of hollow particles. Y. Qian and co-workers114) have prepared hollow titanium carbide (TiC) spheres by using titanium tetrachloride (TiCl4) nanotube and sodium as starting materials. They attributed the formation of hollow TiC particles to the small vesicles templates which were generated by gasification of TiCl4 reactant. G. Hu and co-workers115) has prepared hollow carbon spheres using hexachlorobenzene (HCB) and sodium as starting materials. However, they ascribed the templates to sodium chloride (NaCl) particles generated in the start of reactions. In addition, Y. Xie116) also attributed the formation of their prepared hollow boron nitride (BN) particles to the templates of sodium bromide (NaBr) which is a by-product of the reaction. Even though their reaction and results are similar, their explanations on the formation of hollow particles are different. Further investigations are necessary to discover the truth of this mechanism.
Some researchers proposed to prepare hollow particles by using metal nanocrystals as templates which are generated in the reactions. The metal templates are removed by selective dissolution or sublimation after the formation of solid shell. For example, Z. Wang and co-workers117) have prepared ZnO cages and shells by using solidified zinc (Zn) nanocrystals as templates via a process comprised of solidification of liquid Zn droplets, surface oxidation, and sublimation. B. Liu and H. C. Zhang118) also fabricated hollow ZnO particles by using metallic Zn particles as templates via a modified Kirkendall process. While A. M. Herring119) prepared hollow carbon nanospheres from cellulose chars by using a reduction product of nickel (Ni) nanocrystals as templates. The Ni templates were removed by acid etching.
Besides, some researchers120–124) have reported that they have prepared hollow particles without using templates. Actually, even they did not add template at the start of reaction, the formation of hollow particles also involved the templates which were formed during reactions. For example, H. J. Hah and coworkers120) have prepared hollow SiO2 particles without using templates. Their preparation procedure included two steps. In the first step, the hydrolysis of phenyltrimethoxysilane (PTMS) was performed under acid conditions. In the second step, the condensation of the silane progressed under basic conditions. When PTMS was added to the acid solution in the first step, PTMS is immiscible with the aqueous solution and phase separation occurs in the mixed solution. Under stirring conditions, droplets of PTMS were formed and became smaller gradually due to miscibility of hydrolyzed PTMS with aqueous solution as the hydrolysis progresses. In the second step, after the addition of NaOH, the condensation is commenced immediately. The production of methanol caused by hydrolysis has an effect on the solubility of the underhydrolyzed PTMS, existent in the interior of droplets in the aqueous solution. The release of the unhydrolyzed PTMS with methanol from the droplets coincides with the formation of hollow SiO2 particles.
2.2.3 Bubble template methodBubble template method is novel process to prepare hollow particles by using bubbles as templates, proposed by M. Takahashi and his colleague125) in Nagoya Institute of Technology, Japan. In this method, bubble is not only the template for hollow particles, but also one of reactants. Part of bubble dissolve into solution and reacted with the species in solution, forming nanocrystals. Owing to the high special surface areas, the newly-formed nanocrystals tend to assemble on the surface of left bubble and form a solid shell. After separation, the pure and clean hollow particles are obtained.
To test the feasibility of bubble template, a single bubble was used as a template with the purpose to observe a direct evidence of crystal nucleation on the surface of bubble113). A single ammonia (NH3) bubble generated by a capillary tube was passed into a SiO2 sol. After the bubble was passed into sol, the sol started to gel on the surface of bubble and bubble was completely coated by gel at last. This result indicated that it is possible for nanocrystals nucleated or coated on the surface of bubbles. It was shown that bubble surface in aqueous solution would maintain chemical compositions distinct from those of the bulk liquid. The presence of a chemical heterogeneity at the bubble liquid interface would lower the surface energy which would promote the assembling of crystals on the surface of bubbles.
In the following, hollow CaCO3 particles have been successfully prepared by bubble template method via bubbling CO2/nitrogen (N2) mixed gas into CaCl2 solution. When the mixed gases were passed into CaCl2 solution, the CO2 gas dissolve into solution and react with Ca2+, forming CaCO3 precipitates which are not stable and ready to attach on the surface of left bubbles to reach a minimum of total surface free energy. With the progress of reaction, the bubbles were completely coated by precipitates and formed a solid shell. After filtering and drying, the hollow CaCO3 particles were obtained, as shown in Fig. 9125,126).

Hollow CaCO3 particles of (a) broken and (b) cross-section areas which were prepared by bubble template method. Scale bars are 1μm.
The results of x-ray diffraction (XRD) measurements showed that the hollow particles were mainly composed of vaterite. In the case of calcium carbonate, three polymorphs, namely calcite, vaterite and aragonite are often found in crystallization from aqueous solution. The formation of specific form depends on the crystallization conditions (such as temperature, pH and supersaturation)127–131). Generally speaking, spherical vaterite is helpful to form stable hollow structures compared with other two polymorphs. Therefore, it is an important step to stabilize vaterite for the formation of hollow particles in this process. J. A. Thomas and co-workers have reported that stabilization of the vaterite by the addition of L-glutamic acid surfactant60). Besides, investigation of B. D. Chen and co-workers132) indicated that the addition of surfactants leaded to the stability of bubbles and induced template nucleation on the surface of bubbles. This result inspired us to further improve the formation of hollow particles by using some surfactants. The surfactants are expected to have two functions. One is to stable the bubbles in solution. Second is to make the nanocrystals ready to assemble on the surface of bubble. Cationic, anionic and non-ionic surfactants have been tried in experiments. The results indicated that cationic surfactants play a positive effect on the formation of hollow particles.
Recently, bubble template method has been used to prepare hollow ZnO by passing ammonia bubbles into zinc chloride (ZnCl2) solution at 90°C. It was found that the prepared samples are ZnO tube with a diameter of 300–500 nm and average length of 5μm. The formation of ZnO nanotubes is attributed to the precipitation of ZnO on the surface of floating bubbles133,134).
Detail mechanism of hollow structure formation using bubble is still on the way of investigation. Recently, Fuji and his co-workers reported how CO2 gas bubbles affected formation of hollow calcium carbonate microspheres from stepwise microscopic analysis. When CO2 gas dissolves in the starting solution, primary amorphous CaCO3 particles firstly form and gather to be microsized secondary particles. With gradual decrease pH derived from further CO2 dissolution, only primary particles which exist at outer shell of the secondary particle dissolve once and transfer to vaterite. Subsequently, amorphous CaCO3 particles which just inside the outer shell start to transfer to vaterite crystal. During repeat of these dissolution and re-precipitation process, the outer CaCO3 shell completes to form with columnar structure135).
Even though the bubble template is a new process and still on the way of investigation, it was indicated that this method is a facile and promising process to prepare hollow particles with the characteristic of low cost and high production. One more advantage of this method is its universal property, which can be used in other system involved gas reactions.
2.3 Device-based methodsDevice-based methods include the method to prepare hollow particles by using some equipment, such as spray pyrolysis, nozzle process and so on.
2.3.1 Spray pyrolysisSpray pyrolysis is a powerful tool to synthesize a variety of materials in power form including metals, metal oxides, superconducting materials, fullerenes, and nanophase materials. G. L. Messing136) has reviewed the formation of particles with different morphology using a variety of spray pyrolysis techniques. The report showed that the spray pyrolysis techniques allow to produce dense (solid), hollow, porous or fibrous particles and even to deposit thin film, but the formation of hollow spherical particle is the most typical.
Particles synthesis by spray pyrolysis involves the atomization of a precursor solution into discrete droplets. These droplets are subsequently transported through a furnace where the solvent is evaporated from particulate. Spray pyrolysis has a number of advantages including the followings: (1) the particles produced are spherical, (2) the distribution of their diameter is uniform and controllable from nanometer to micrometer, (3) the purity of the product is high, and (4) the process is continuous. Fig. 10 showed the sample of hollow spherical particles prepared by K. T. Wojciechowski137) using spray pyrolysis method. The microscopic observations have shown that the hollow particles have the diameter ranging from 100 to 300 nm with shell thickness of 8–50 nm. Some big hollow particles also prepared by spray pyrolysis, as shown in Fig. 11138).

TEM image of hollow particles prepared by spray pyrolysis137). Scale bar is 1 μm.
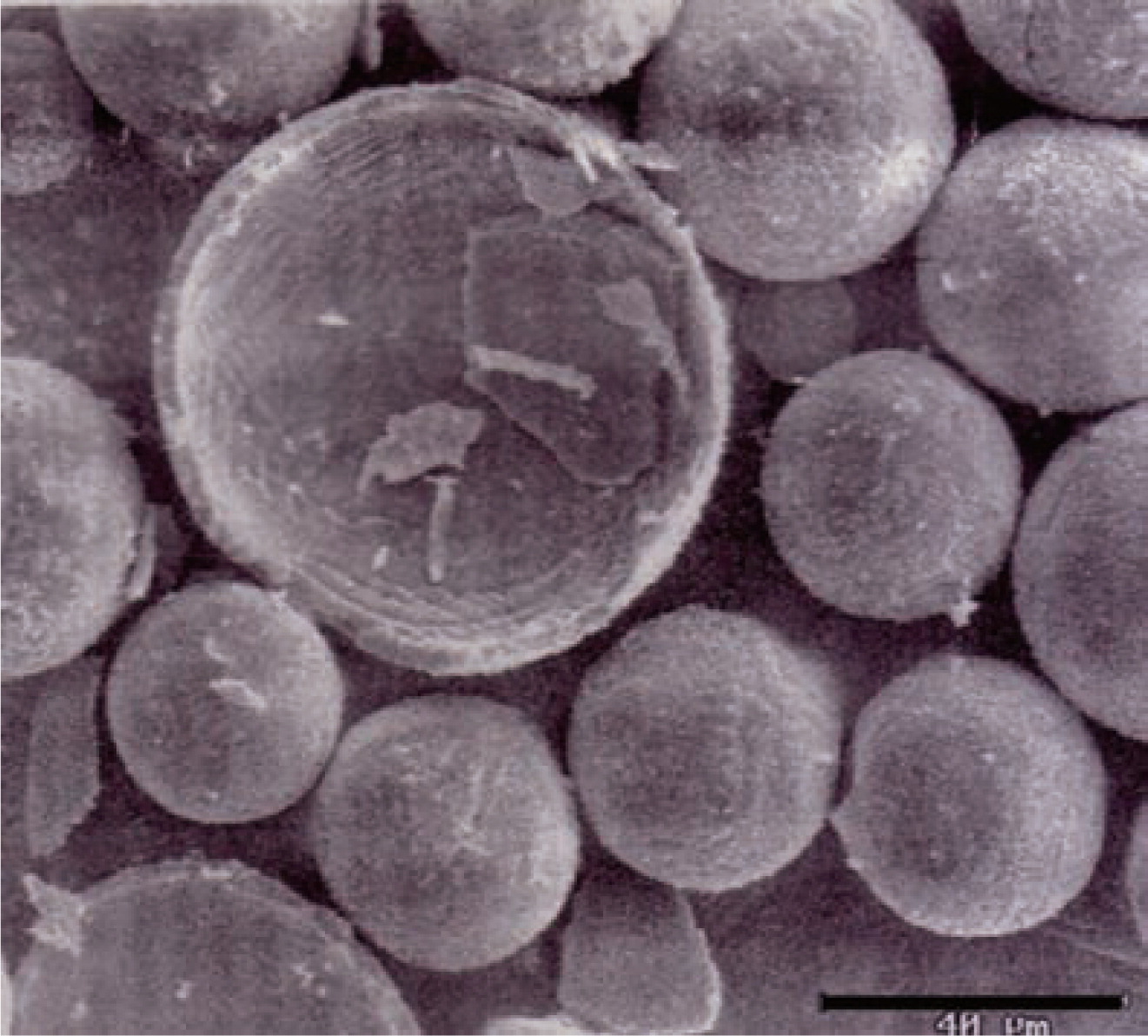
SEM image of hollow Al2O3 particles prepared by spray pyrolysis138). Scale bar is 40 μm.
The most apparent theory for the formation of hollow particles in spray pyrolysis is the surface precipitation induced by rapid drying rate of droplet in thermal condition. The formation of hollow particles can be controlled by changing aerosol decomposition parameters. For example, Lenggoro139) have demonstrated experimentally that hollow ZrO2 particle is formed if the reactor temperature is high and initial solute concentration is low. Furthermore, the results of his simulation indicated that hollow particles are formed if the initial droplets are large and the droplet number concentration is low.
2.3.2 Nozzle processNozzle process is developed to prepare big hollow particles by using inert bubbles as templates, which involves a coaxial nozzle, as shown schematically in Fig. 12.
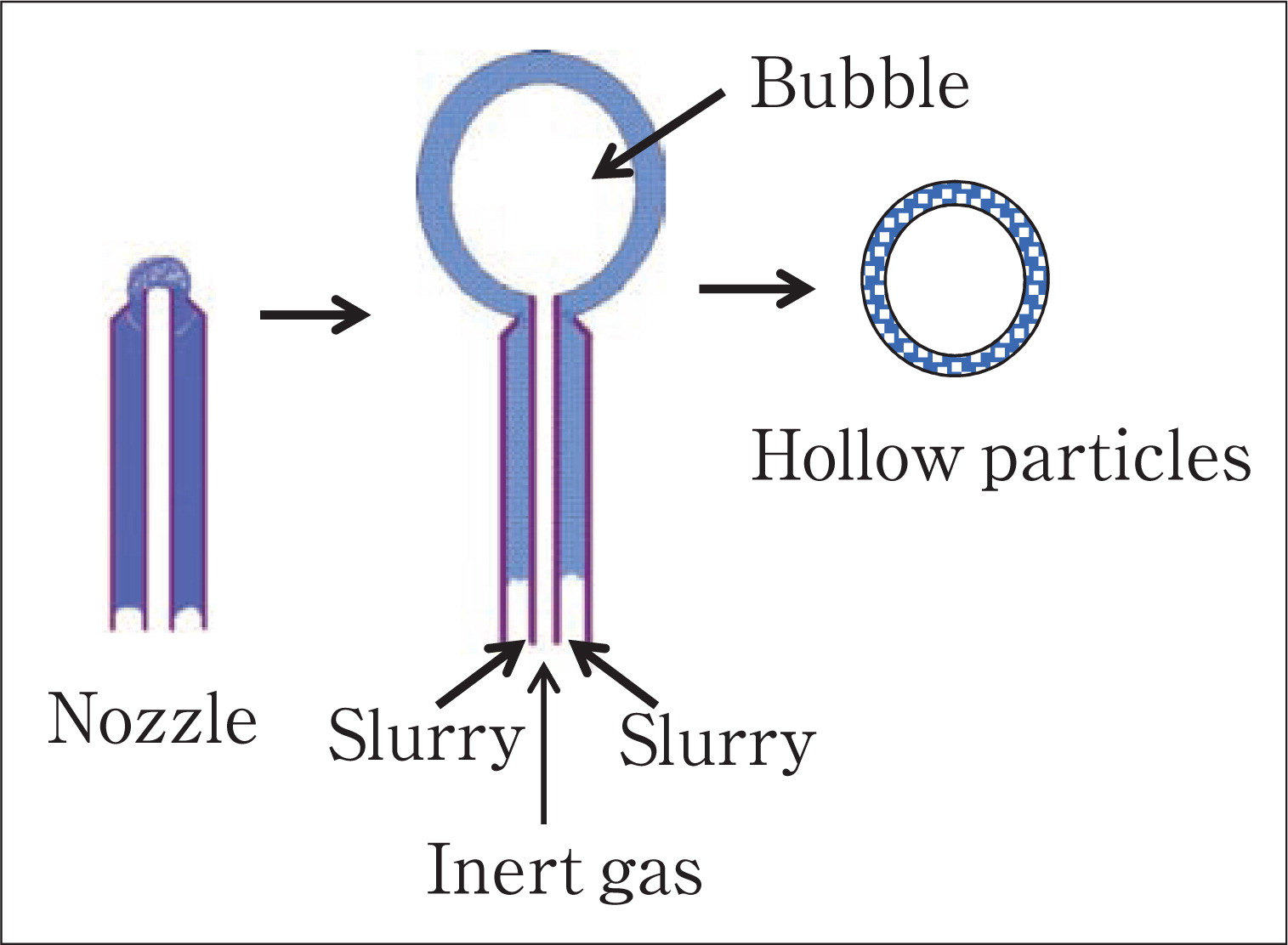
Illustration of nozzle process to prepare hollow particles.
In nozzle process, a non-reactive gas is fed through the inner nozzle and a slurry, made up of dispersed solid particles (which can be metal, ceramic or a mixture), a binder and a continuous volatile liquid phase, is fed through the outer nozzle. The slurry exits the nozzle in the form of hollow cylinder that close at a constant diameter due to surface tension and hydrostatic forces, forming a hollow sphere. The monosized spheres are hardened in flight by the binder as the solvent evaporates. After forming, the spheres are sintered to dense and strength the walls. Due to the high rates of spherical production and low cost fabrication, nozzle process is economical. Torobin140–144) has done many interesting research in this area. R Meyer Jr.145) has prepared hollow lead zirconate titanate (PZT) particles by using nozzle process. These hollow particles have potential applications in medical ultrasound, non-destructive testing and low density transducer arrays. I. G. Loscertales et al.146) have combined nozzle process with electro-hydrodynamically (EHD) process and generate a liquid-filled hollow fibre. The thickness and diameter of fibres are easily to be controlled by adjusting the applied voltage.
Besides, some researchers147) proposed to prepare hollow particles using the electrostatic atomization combined with alcohol solidification. The nozzle is composed of a stainless-steel hypodermic needle. The ground electrode is an aluminum plate with a hole at its center for the produced droplets to pass through. High voltage is applied between the nozzle and the ground electrode using a dc high voltage supply. The material in the sample vessel is pressurized by gas, and flows through the nozzle. The nozzle can control the particle size by adjusting voltage. The produced droplets immediately fall into an alcohol bath to dehydrate their surface. After filtered and dried, the hollow silica particles were produced.
One of the disadvantages of recycled paper when compared to paper from virgin pulp is difficult to gain high brightness. To improve brightness, papermakers make efforts to develop recycling process such as introducing a strong surfactant in flotation and intensive bleaching. But, these process demand more water and energy, causing adversity to environment. Another possible idea is to hide gray appearance with an efficient coating or internal loading for high opacity. Hollow spheres made of CaCO3 could be suitable for this purpose because hollow structure scatters more light resulting in higher brightness and opacity.
T. Enomae and K. Tsujino148) have reported the preparation of spherical particles made of CaCO3 by emulsion template method. Prepared spherical particles were tried to be applied to papermaking as a filler and were compared with some kinds of ground CaCO3 that are all of the commercial grades and of a similar particles size. Spherical particles were observed to disperse evenly while there were, though rarely, large particles 10 μm in diameter for the commercial CaCO3. The density of paper loaded with the spherical particles was lower than that of the unloaded paper. This is often the case with filler loading because of acting as obstruction to interfiber bonding. The spherical particles increased surface smoothness presumably because the spherical particle trended to align with a flat surface due to their shape and no irregularly large particles protruding above the surface. The specific light scattering coefficient also increased with the spherical particles due to their porous structure. This finding suggests that the spherical hollow particles are promising for coating use although attention must be paid to coating rheology. Besides, the possible thermal insulation due to the hollow structure is a unique feature to potentially enhance thermal wax-transfer printing. It was found that more addition of spherical hollow particles provided a larger area of wax ink transfer because efficient thermal insulation due to the porous structure maintained the temperature.
3.2 FoamSpherical hollow particles can be used to fabricate foam by sintering or bonding the packing of hollow particles. The foams produced by hollow particles have high porosities, low density and can be net-shape. Besides, some researchers have fabricated porous foam by using 3D colloid array as templates. For example, B. T. Holland and co-workers149) have prepared TiO2, ZrO2 and aluminum oxide (Al2O3) foam by permeating the monomeric alkoxide precursor into the arrays of bulk PS sphere and condense in air. The foams with open pore were obtained after calcinations of the inorganic component. J. E. G. J. Wijnhoven and W. L. Vos150) also synthesized TiO2 foam by filling the voids of artificial opals and subsequently removing the original opal materials by calcinations.
J. K. Cochran9) has made a review on the application of ceramic foam. For example, hollow ceramic spheres have been incorporated into metal and polymeric matrices to create syntactic foam which can be used as energy absorbing parts for occupant protection in automotive interiors and function well when the crush distance is limited. The polymer syntactic foam produced lower crush force and a squarer wave stress-strain behavior compared to the unfilled polymer foam. This translates to higher energy absorption at lower applied stress which is desirable for passenger protection. Besides, foam diffuser base made from mullite were shown to have excellent thermal shock resistance for radiant burners, in that the radiant surface could be heated to 1200°C in 10 seconds and cooled through more than 100 cycles without visible effects. Besides, Q. Gu and co-workers151) have prepared low density porous tin oxide (SnO2) foam with a potential application as target materials for laser induced extreme ultraviolet (EUV) emission.
3.3 Drug deliverySiO2 is an important drug delivery media owing to its non-toxic and biocompatible. The advantages of such materials have attracted many attentions for the controlled delivery of therapeutics152, 153).
J. F. Chen and co-workers84, 154) have prepared porous hollow SiO2 nanoparticles by using CaCO3 nano-particles as the inorganic template. The hollow particles were uniform spherical particles with a diameter of 60–70 nm, wall thickness of approximately 10 nm. The assynthesized hollow particles were subsequently employed as drug carrier to investigate in vitro release behavior of brilliant blue F (BB) in simulated body fluid. The preparation of drug carrying is illustrated as Fig. 13. BB release behavior from dense SiO2 particles and hollow particles were investigated in deionized water solution, as shown in Fig. 14. BB loaded on normal SiO2 nanoparticles exhibited a rapid release of 100 % within 10 min. However, BB loaded into the hollow particles showed a different release style: 60 % of BB was released in the first 10 min, while the other 40 % followed a typical sustained release pattern and was dissolved out slowly and evenly for a time period of 1140 min. This is because that through the BB loaded on the surface of hollow SiO2 is release quickly while the release rate of BB entrapped in the inner core of hollow particles is constrained. The above results suggested that the prepared porous hollow SiO2 nanoparticles could be applied as promising drug vehicles for controlled release systems92).
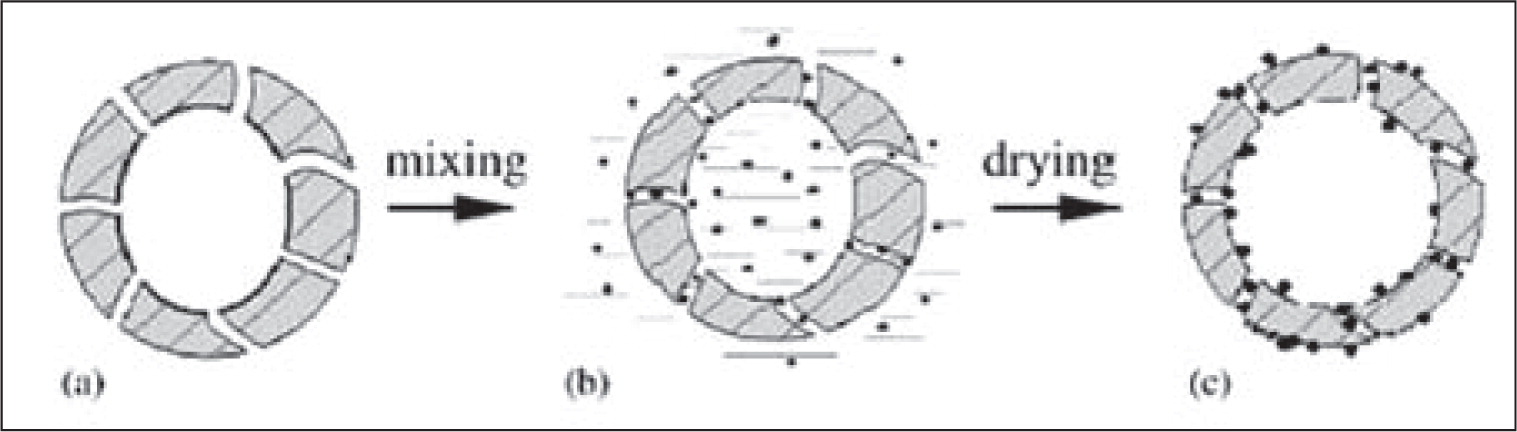
Preparation of drug carrying for porous hollow SiO2 particles. (a) hollow SiO2 particles; (b) suspension of BB and hollow particles; (c) BB entrapped in hollow particles154).
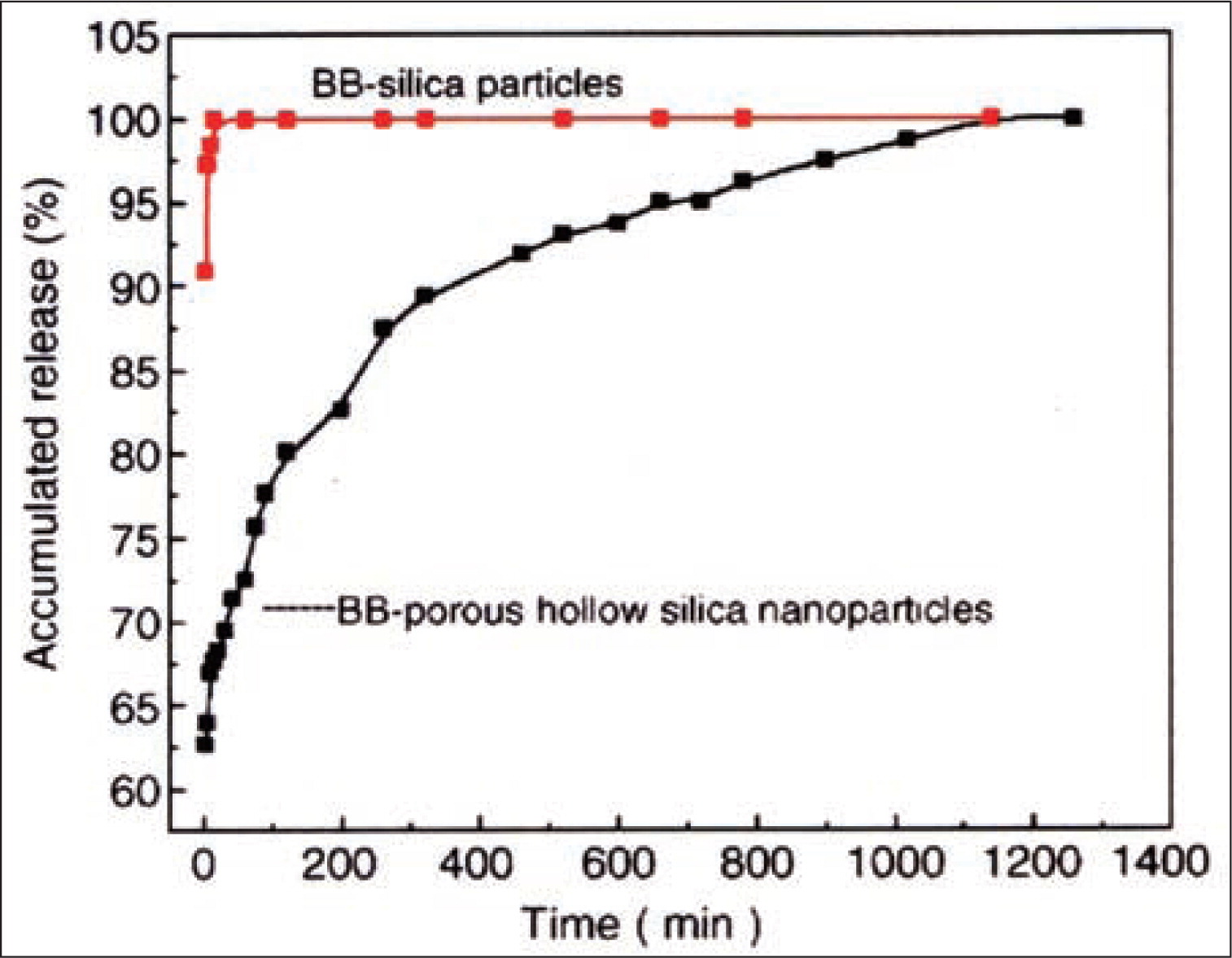
Release profile of BB form hollow SiO292).
Recently, the use of magnetic particle for the delivery of drugs or antibodies to the organs or tissues altered by disease has become an attractive field of research. The process of drug localization using magnetic delivery system is based on the competition between force exerted on the particles by flood compartment and magnetic force generated from the magnet, i.e. applied field. When the magnetic force exceeds the linear blood flow rates in arteries or capillaries, the magnetic particles are retained at the target site and maybe internalized by the endothelial cells of the target tissue. P. Tartaj and co-workers155) have reported the preparation of SiO2 coated Fe2O3 hollow spherical particles with an average size of 150 nm by aerosol pyrolysis of methanol solution containing iron ammonium citrate and TEOS at a total salt concentration of 0.25 M. It is worth mentioning that the small particles size of the composite renders these particles a potential candidate for their use in in vitro applications.
3.4 NanoshellN. J. Halas and co-workers1,156,157) have fabricated a new type of composite nanoparticles called nanoshells, consist of a dielectric or semiconducting core coated with a nanometer scale metallic shell. These nanoparticles manifest a strong optical resonance that is dependent on the relative thickness of the nanoshell. This sensitive dependence of the optical resonance frequency on the structure of metal nanoshells is illustrated in Fig. 15. As the shell thickness decreases, the optical absorption is shifted to longer wavelengths. It is theoretically possible to shift the resonant absorption to beyond 10 μm in the infrared as shown in Fig. 15b. In Fig. 15a, as the core radius-shell thickness ratio is varied among 3 to 12, the predicted resonances of the nanoparticles span a range of 300 nm in wavelength. If the order of these layers were inverted, that is, a metallic core and a dielectric shell, less than 20 nm optical resonance shift would be expected.
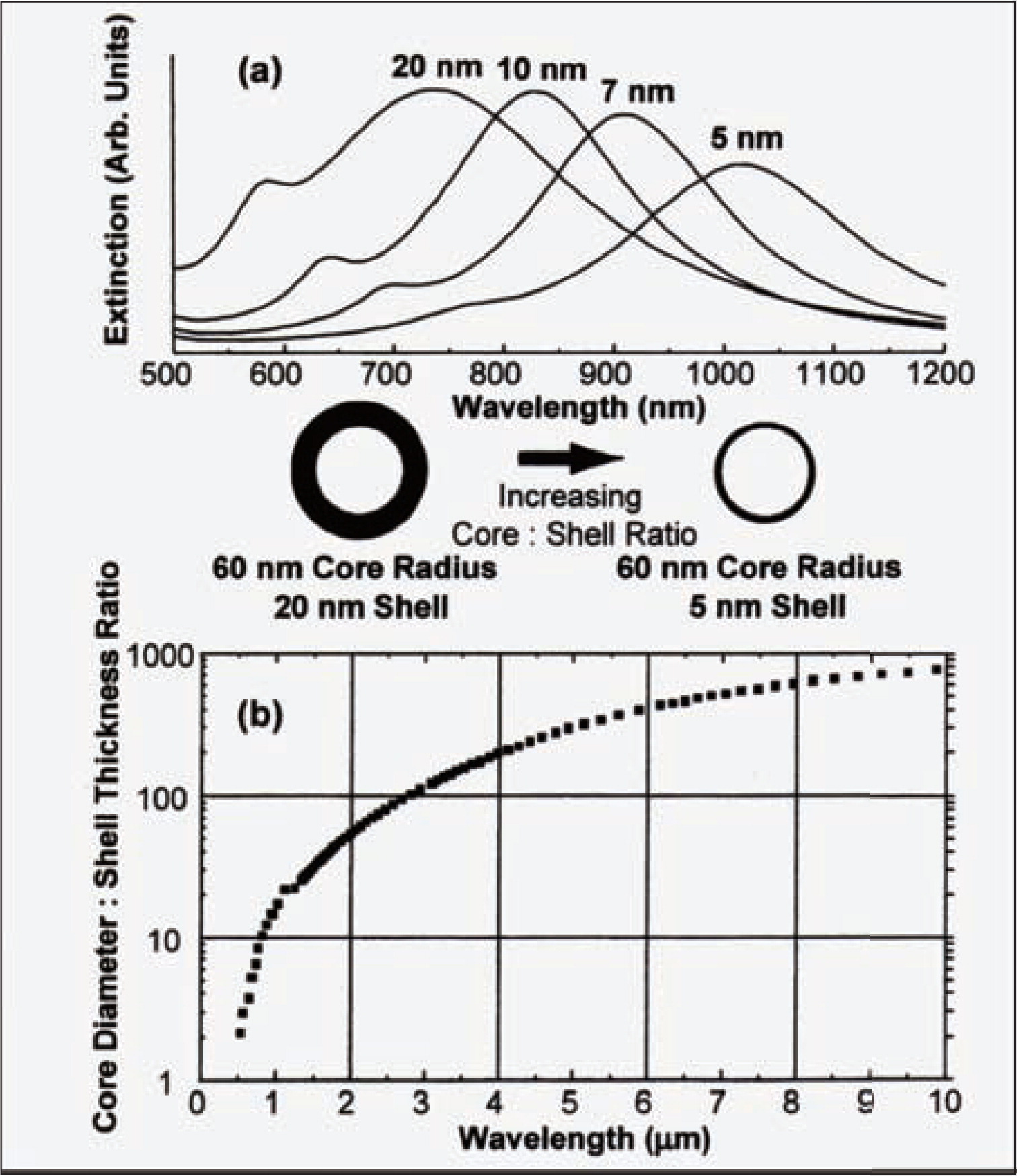
(a) Theoretically calculated optical resonance of metal nanoshells over a range of core radius/shell thickenss ratios, (b) calculation of optical resonance wavelength versus core radius/shell thickness ratio for metal nanoshells (SiO2 core, Au shell) 1).
SiO2-Au nanoshells offer enormous flexibility to tune the resonance frequency by varying the relative dimensions of the SiO2 core and Au shell. The resonance of SiO2-Au nanoshell particles can easily be positioned in the “water windows” in the near-infrared (800–1300 nm) ranges, where absorption by organism is low. Together with the high degree of biocompatibility of Au nanoshells, these results open the door to a wide variety of biological applications. Halas and West showed nanoshells can be used to enable fast whole blood immunoassays. For conventional blood immunoassays performed at visible wavelengths, the whole procedures need several hours or days owing to a purification step needs to be carried out to separate out a variety of unwanted biomaterials that absorb visible lights. In the immunoassay procedure proposed by Halas and West, nanoshells are conjugated with antibodies, which manifest a strong plasmon related absorption feature with a fast absorption measurement in the water window, circumventing the time-intensive purification step. Halas and West also showed how nanoshells can be incorporated into temperature-sensitive hydrogels to synthesize a new type of composite materials that collapses on laser irradiation. Owing to the tunable absorption feature of nanoshells, SiO2-Au nanoshells were designed to absorb light from laser irradiation and then transform the light to heat around nanoshell, which vaporized water and caused the collapse of hydrogel. Such a remotely addressable hydrogels may find applications in drug delivery and microfluidic values or pumps. F. Caruso and co-workers158, 159) have reported the fabrication of optically addressable nanostructured capsules comprising a PE multilayer shell doped with light-absorbing Au nanoparticles. Their results indicated both enzyme lysozyme and macromolecules can be encapsulated within the PE/Au shell and the encapsulated samples can be released on demand without significant loss of bioactivity following irradiation with short pulse of near infrared (NIR) laser light.
The most exciting prospect is that nanoshells could play a role in future cancer treatments. These particles are small enough to find their way through human circulatory system on injection. Bioactive molecules can be attached to the nanoshells surface to cause selective binding or accumulation of these particles within a tumor. Using NIR laser, carcinoma tissue can then be destroyed by local thermal heating around nanoshells based on an efficient light-to-heat conversion.
Besides, C. Graf and A. V. Blaaderen160) have prepared hollow Au shells by dissolving the SiO2 core in diluted HF. Their results indicated that removing the core would lead to a significant shift of the maximum of the extinction spectrum to shorter wavelengths, which is caused by change of the refractive index of the core from 1.45 (SiO2) to 1.33 (water). This result demonstrated that the dissolution of the SiO2 core allows a further adjustment of the optical properties of the Au nanoshell particles.
3.5 E-inkParticle-based display systems are attractive for their optical and electronic properties. These beneficial properties result from the highly scattering and absorbing microparticles that contain pigments like TiO2 and carbon black. The general representations of particle based display are an electrophoretic image display system161) and a rotating bichromal microspheres system162). Despite many attractive features, however, particle-based display systems suffer from some shortcomings, including difficulty to achieve perfect rotation and short lifetime due to coagulation and agglomeration caused by colloidal instability. To overcome these shortcomings, J. Jacobson and co-workers163) created E-ink, a new display system utilizing microencapsulation techniques with a fusion of chemistry, physics, electronics and other technologies. Each microcapsule with about the diameter of a human hair contains millions of tiny pigment microparticles that are well dispersed in an organic solution with a low dielectric constant. When an electric field is applied between microcapsules, the microparticles move in the low dielectric constant solution toward the oppositely charged electrode in the phenomenon of electrophoretic migration. If the top transparent electrode is positively charged, white microparticles with negatively charges should move toward the top electrode, making the surface appear white at that spot. At the same time, an opposite electric field pulls the black particles to the bottom of the microcapsules. By reversing this process, the black microparticles appear at the top of the microcapsules, which makes the surface become black at that spot. This is how microencapsulated ink (E-ink) forms letters and pictures on the display. The benefit of microencapsulation in this case is the isolation of electrophoretic dispersion in discrete compartments. In other words, agglomeration of electrophoretic dispersion in microcapsules has no influence on electrophoretic dispersion in neighboring microcapsules.
Japan is one of the leading countries in microencapsulation technology. The Japanese Ministry of Economy, Trade and Industry (METI) has created a nation project concerning paper-like display that employs microencapsulation, called the Full Color Rewritable Paper Using Functional Capsules Project164).
3.6 CatalysisPalladium (Pd)-catalyzed cross-coupling reactions of aryl halides with arylboronic acid, often referred as Suzuki coupling reactions, are versatile method for synthesizing unsymmetrical biaryls. The Suzuki coupling reactions have been applied extensively in the synthesis of natural products, nucleoside analogues, and pharmaceuticals. Many Pd complexes have been used as homogeneous catalysis for these reactions. S. W. Kim and co-workers2) have reported the preparation of hollow Pd spheres by using the templates of uniform SiO2 spheres. They have investigated the application of these hollow spheres to heterogeneous Suzuki coupling reactions. The results showed that the Pd hollow spheres are highly active for this reaction, in addition, the catalyst can be recycled and reused seven times without losing its catalytic activity. The high surface area of Pd spheres resulting from the nanoparticle nature of the shell is responsible for the high catalytic activity. Earlier studies by other research groups reported the Pd nanoparticles used in Suzuki coupling reactions were agglomerated after one cycle, resulting in a loss of catalytic activity. Heterogeneous catalysts often suffer extensive leaching of the active metal species during reactions and eventually lose their catalytic activities even after seven recycles. Elemental analysis of the filtrate after the reaction demonstrated to no leaching of Pd from the hollow spheres, which is very important when Pd catalysts are used for pharmaceutical production.
TiO2 is a promising oxide that is able to degrade many kinds of organic pollutants in water with the production of hydrogen by photocatalysis. Although TiO2 has been intensively investigated, its application in photocatalysis is limited because of its low efficiency in energy conversion. To enhance the efficiency, the absorption spectrum of TiO2 is expected to extend to the visible range. Some researchers73,165) found that the hollow TiO2 particle has a smaller band gap and obvious absorption shift towards longer wavelength. Fig. 16 is the diffuse reflectance spectra of hollow TiO2 which was prepared by surfactant-assisted templating method. The onset wavelength (λonset) of the spectra recorded from anatase is about 420 nm, while the λonset of the hollow TiO2 shift toward longer wavelength region. The band gaps estimated from the spectra in Fig. 16 give an evident reduction of the band gap for hollow TiO2 compared with anatase, since a red shift of λonset was observed for hollow TiO2. This indicates that the present hollow TiO2 microspheres should be efficient in photocatalysis applications76).
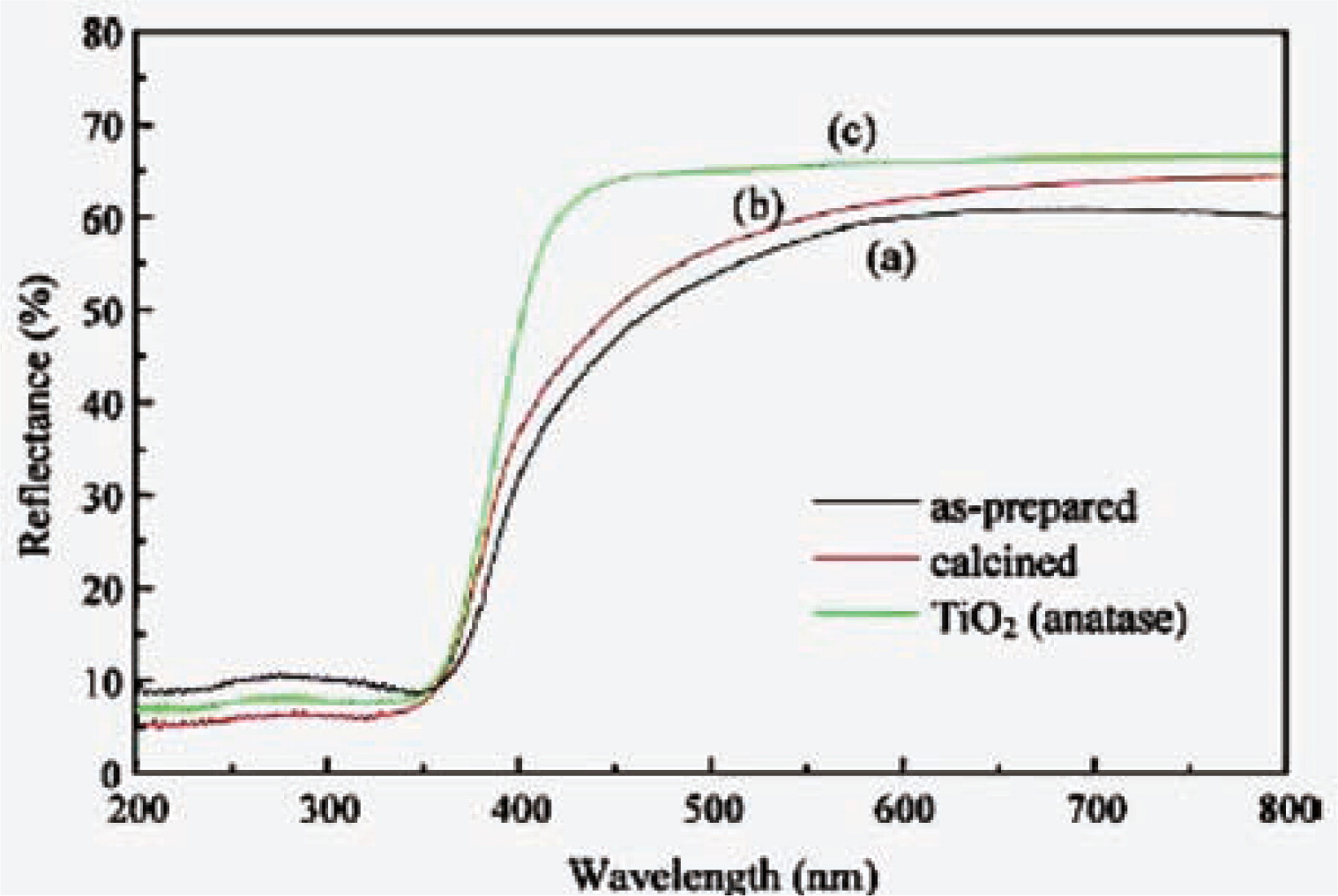
UV-vis diffuse reflectance spectra of (a) as prepared, and (b) calcined hollow mesoporous TiO2 microspheres, and (c) a well-crystalline TiO2 sample of anatase phase76).
However, some other researchers166–168) have reported a different conclusion that hollow structures leaded to a blue shift of wavelength, which was explained as the result of quantum size effect. This confliction would inspire a further research to discover the truth of the properties of hollow particles. Even though the exact mechanism is still on the way of investigation, it might be speculated that the thickness and microstructure of shell should play an important on the optical properties of hollow particles.
3.7 OthersFuji at Nagoya Institute of Technology, Japan, demonstrated superior thermal insulation of their synthesized hollow SiO2 nanoparticles (HSNPs). The composite film where HSNPs were dispersed in polymer matrix exhibited ten-time higher thermal insulation than original polymer film and also high transparency in visible region. This excellent performance has been achieved by nano-sized hollow interior which can be as quasi-vacuum state169).
Researchers at Pennsylvania State University, Led by Newnham9,145), are developing miniature spherical transducers based on PZT hollow spheres. Tiny hollow spheres used as piezoelectric sensor and actuator manifested an improved resolution and high powder density. Hydrostatic coefficient for the hollow sphere transducer were 1–2 orders of magnitude larger than those for bulk PZT and the hydrophone figure of merit (FOM) was three orders of magnitude higher than that of bulk PZT. The hollow sphere of PZT have good dielectric and piezoelectric properties, which suggests a number of possible applications, such as flow noise sensor and sonar arrays with the advantage of low density. Besides, the geometry of spheres allows them to be used as sensors that are independent of receiving direction. The omnidirectional characteristic of the sphere also allow it to be used as a transducer in non-destructive testing and in monitoring flow noise in energizing system.
Another example for the application of hollow particles involves the improved magnetic property. J. Bao and co-workers170) have prepared hollow Ni spheres with a typical coercivity of 32.3 Oe which is higher than that of the bulk Ni (around 0.7 Oe).
Besides, K. T. Lee and co-workers171) have tried to improve the cycle performance of nanosized Tin (Sn) particles for anode material in lithium secondary batteries by encapsulating Sn particles with spherical hollow carbon. The spherical hollow carbon plays several important roles in this preparation. First, the hollow carbon acts as a barrio to provides a void space where Sn metal particles experience a volume charge without a collapse of carbon shell. Third, the hollow carbon itself is an active material for additional lithium (Li) ion storage. Finally, the carbon particles are spherical in shape, which provides a high packing density in practical Li cells to allow a high volumetric energy density.
The development of hollow particles will continue focusing on the preparation of uniform products with controllable particles size and shell thickness. For virtually all the applications under consideration, properties will be enhanced by improvement in perfect of the particles geometry. The pore size and microstructure of solid shell are also significant when permeability of hollow particles is considered in their applications. Besides, new methods to prepare hollow particles with the characteristics of low cost and high production are warmly expected, especially in industrial production. In the applications of hollow particles, it is important to functionalize the interior of hollow particles to gain desired properties. And the applications of hollow particles in electronic and biologic areas promise us a golden and expecting future.
A part of this study was supported by Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for Scientific Research (B) (22310066), Grant-in-Aid for challenging Exploratory Research (24655191), and Japan Science and Technology Agency (JST)-Advanced Low Carbon Technology Research and Development Program (ALCA).
Masayoshi Fuji
Masayoshi Fuji is Professor of Advanced Ceramics Research Center, Nagoya Institute of Technology (NIT), Gifu, Japan. He received his Dr. Eng. from the Faculty of Engineering of Tokyo Metropolitan University in 1999. He joined the Dept. of Industrial Chemistry, Tokyo Metropolitan University, as a Research Associate in 1991. He was visiting Researcher at Engineering Research Center, University of Florida from 2000 to 2001. He joined NIT since 2002 and was promoted Professor in the Graduate School of Engineering in 2007. His research interests are surface chemistry, ceramic processing, and powder technology.
Yongsheng Han
Dr. Yongsheng Han got his bachelor degree from Changchun University and Science and Technology in 1997, master degree from Jilin University in 2000 and doctor degree from Tsinghua University in 2004 with the specialty of materials science and engineering. Immediately after his graduation, he went to Japan, Nagoya Institute of Technology and worked there as a postdoctoral researcher for more than 3 years. In 2007, he was awarded Alexander von Humboldt fellowship and went to Germany, Max Planck Institute of Colloids and Interfaces. In 2011, he succeeded in the application of Hundreds Talents Program and joined in the Institute of Process Engineering, Chinese Academy of Science. His current research interest is the controlled synthesis of catalysts and biomaterials.
Chika Takai
Chika Takai is a postdoctor of Advanced Ceramics Research Center, Nagoya Institute of Technology, Japan since 2010. She received B. S. from Department of Materials Engineering in 2001, M. S. from Graduate School of Engineering in 2003, and ph. D. from Graduate School of Engineering of Nagoya Institute of Technology in 2006. She joined Kurimoto Ltd. in 2007 which is cast-iron pipe company in Osaka and was involved in research and development. Her research interests are particle synthesis with structure control and control particle dispersion.