2013 年 30 巻 p. 119-124
2013 年 30 巻 p. 119-124
In this research, nanocrystalline ZnO flakes were synthesized via a simple reaction process by using zinc acetate dihydrate, diethyl amine and sodium hydroxide. It is confirmed by scanning electron microscopy analysis that nanocrystalline ZnO flakes are flat, irregular in shape, have a high thickness and their average dimension is about 300 nm. The X-ray diffraction (XRD) and FT-IR results indicated that the synthesized ZnO product has the pure wurtzite structure with lattice parameters a and c of 3.253 and 5.210 Å, respectively. The average crystallite size of the ZnO nanostructures deduced from the Scherrer formula is ∼31 nm. Raman scattering exhibits a sharp and strong E2H mode at 438 cm−1 which further confirms the good crystallinity and wurtzite hexagonal phase of the prepared ZnO nanostructures. The synthesized powder exhibited the UV absorption at around 370 nm with the estimated direct band gap energy of 3.278 eV. The particle size was also deduced by using the Brus equation and the estimated band gap energy of the ZnO nanopowder sample. The obtained value is in agreement with the calculated one from the Scherrer formula. The strain in the ZnO nanoparticles was also calculated.
Recently, nanostructured ZnO materials have attracted a great deal of attention due to their potential applications in ultraviolet (UV) lasers, solar cells, sensors and photocatalysts, etc.1–4). Nano-scale ZnO structures with a high surface area have been produced to enhance the physicochemical and electrochemical properties of the above applications. In fact, when the size of a nanostructure approaches the Bohr radius of excitation, optical, electronic and structural properties are greatly affected by the quantum confinement effect. Therefore, nanosized materials are very different from their bulks5–7). As a consequence, semiconductor nanoparticles are viewed as promising candidates for many important future technological applications.
The properties of ZnO nanostructures depend greatly on their size, orientation, morphology, respect ratio, and density of crystal8–10). The size and morphology control of ZnO nanoparticles is necessary to study their size-dependent properties and explore their applications in the diverse areas of nanotechnology. ZnO is readily produced in a large variety of one-dimensional and two-dimensional forms such as nanorods, nanoneedles, nanosheets, and nano-flakes11–14). ZnO nanoflake structures possess lots of interesting unique properties such as porous structures and large surface areas. It is reported that sensors based on ZnO nanoflake structures have an improved performance and a higher sensitivity compared to ZnO nanorods/nanowires15). To synthesize uniform nanosized ZnO particles and control their sizes and morphology, a variety of techniques have been used such as thermal oxidation of metallic zinc, hydrothermal methods, plasma chemical synthesis, laser ablation and vapor condensation16–18). Among these various fabrication techniques, the wet chemical route promises to be simpler, less energy intensive, less expensive – which is more profitable for large-scale production19).
In this research, we investigated nanocrystalline ZnO flakes prepared by a simple solution process using zinc acetate and diethylamine under refluxing at 85°C for 4 h. The structural, morphological, vibrational and optical properties of the as-synthesized ZnO nanoparticles are characterized by various techniques.
Nanocrystalline ZnO flakes were synthesized at low temperature by a simple solution process. The starting materials for the synthesis of ZnO flakes were zinc acetate dihydrate, diethyl amine and sodium hydroxide. All the reagents were purchased from Sigma-Aldrich and were used as received without further purification.
For the synthesis of nanocrystalline ZnO flakes in a typical reaction process, 0.1 M zinc acetate dihydrate, made in 50 ml of deionized water was mixed with an aqueous solution of 0.1 M diethyl amine (50 ml) under vigorous stirring at room temperature. To maintain the pH = 13 of the reaction mixture, several drops of NaOH was added under continuous stirring. The obtained solution was then refluxed at 85°C for 3 h. During refluxing, the solution temperature was controlled by manually inserting an adjustable thermocouple in the refluxing pot. After refluxing, white-colored precipitate was obtained which was washed with methanol and acetone several times and dried at room temperature. The synthesized ZnO flakes were characterized in terms of their structural, morphological and optical properties. The characterization techniques used in this work are field emission scanning electron microscopy (FE-SEM) equipped with energy dispersive spectroscopy (EDS), X-ray diffraction (XRD), FT-IR, UV-Visible and Raman scattering. The Raman spectrum was performed at room temperature with a Labram system equipped with a microscope in back-scattering configuration. The excitation line was at 514.5 nm from an Ar+ laser.
The XRD patterns of the ZnO nanocrystals measured with Cu-Kα radiations (λ = 1.54178 Å) in the range of 20 – 80° with 6°/min scanning speed are shown in Fig. 1. The XRD spectrum indicates that these nanosized ZnO particles are highly crystallized in structure and the entire diffraction peaks match well with Bragg reflections of the standard wurtzite-type ZnO structure20, 21). Diffraction peaks related to the impurities were not observed in the XRD spectrum, confirming the high purity of the synthesized nanocrystals with cell constants of a = 3.253 Å and c = 5.210 Å. Furthermore, it could be seen that the diffraction peaks shown in Fig. 1 were more intensive and narrower, implying a good crystalline nature of the synthesized ZnO product. The average crystallite size of the ZnO nanoparticles (d) calculated from the widths of the major diffraction peaks observed in Fig. 1 through the Scherrer formula are given in Table 122). The average crystallite size is ∼31 nm and is consistent with the value of nanosized ZnO particles reported in literature23). Fig. 2 shows FE-SEM images of the ZnO nanopowder sample synthesized by a simple solution process. The ZnO nanoparticles look like flakes in shape and are synthesized in a very large quantity as confirmed by the low resolution image (Fig. 2(a)). The nanocrystalline ZnO flakes are flat and irregular in shape (Fig. 2(b)). They have a high thickness (∼30 ±10 nm) and an average dimension of 300 ±100 nm.
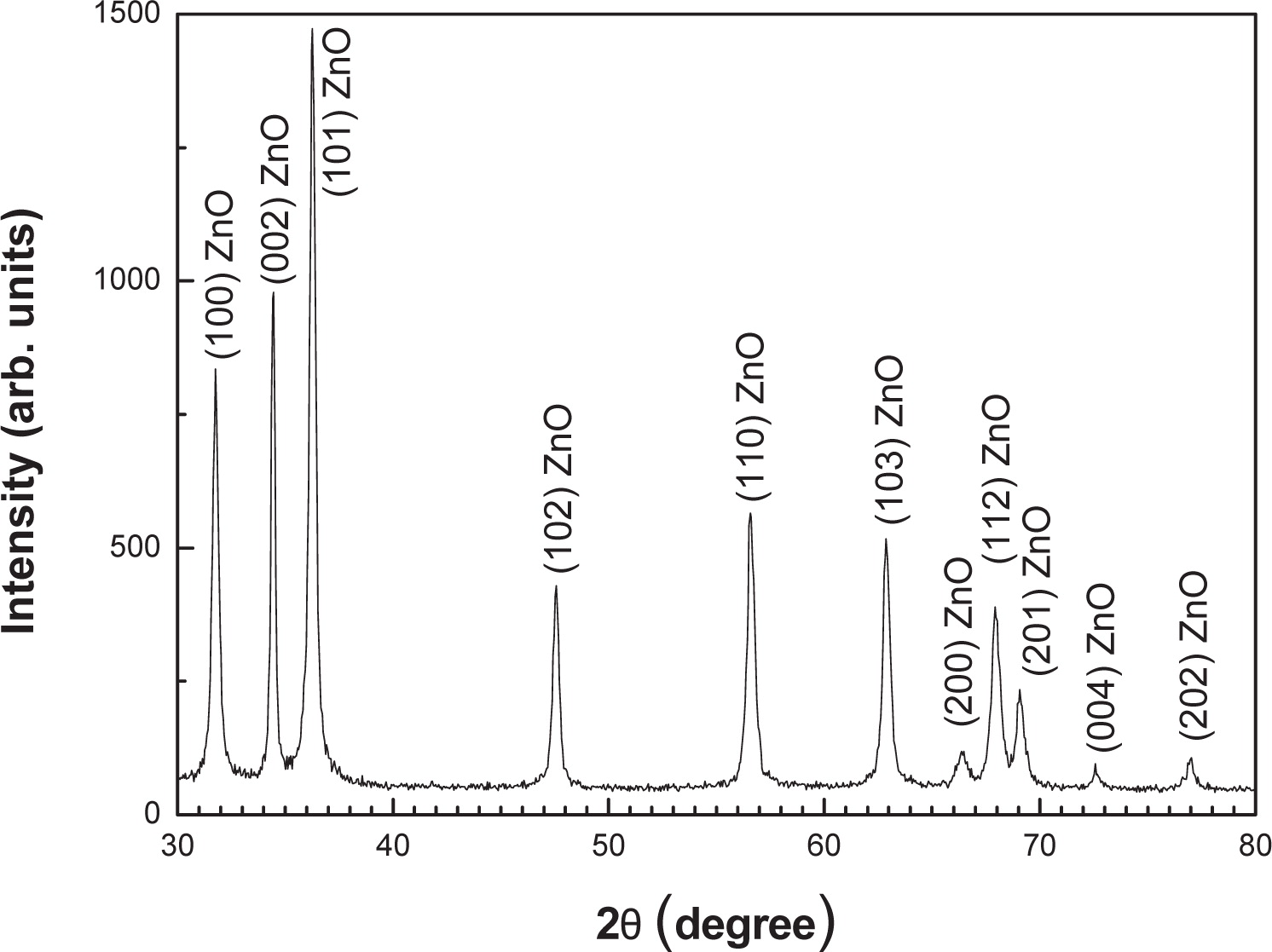
XRD data of the nanocrystalline ZnO flakes synthesized via a solution process using zinc acetate and diethylamine under refluxing at 85°C for 4 h.
| (101) | (002) | (100) | Average crystallite size dav (nm) | |||
| FWHM (degree) | crystallite size d(nm) | FWHM (degree) | crystallite size d(nm) | FWHM (degree) | crystallite size d(nm) | |
| 0.302 | 27.40 | 0.206 | 39.80 | 0.318 | 25.64 | 30.94 |

FE-SEM images showing the morphology of the nanosized ZnO powdered sample. (a) low resolution and (b) high resolution.
The room-temperature Raman spectrum of nano-crystalline ZnO flakes synthesized by a simple solution process is reported in Fig. 3. At the region of low frequency, the peaks at ∼332, 384 and 409 cm−1 are attributed to 2E2(M), transverse optical (TO) A1 and E1 modes24), respectively. The phonons in a nanocrystal are confined in space and all the phonons over the entire Brillouin zone will contribute to the first-order Raman spectrum. The intense peak at ∼438 cm−1 is the E2(high) (E2H) mode, characteristic of the ZnO crystallinity25). Observation of these modes indicates that the ZnO product has the wurtzite structure as confirmed by XRD analysis. The Raman mode at around 583 cm−1, which falls just between the A1(LO) (574 cm−1) and E1(LO) (590 cm−1) modes26) of a single crystal of ZnO, may be interpreted as an LO quasi-mode with mixed A1 and E1 symmetry due to relaxation of symmetry properties in nanoparticles. Recent reports related the appearance of this mode to lattice defects, being either oxygen vacancies or zinc interstitials or their combination27, 28). The weak peak at ∼539 cm−1 can be attributed to 2B1(low) second-order mode26). At high frequency, the broad and relatively intense peak at 1153 cm−1, which is found between the doubled frequencies measured for the A1(LO) and E1(LO) modes, contains contributions of 2A1(LO) and 2E1(LO) modes at the Γ point of the Brillouin zone26).

Room-temperature Raman backscattering spectrum of the nanocrystalline ZnO flakes.
The room-temperature optical absorption spectrum for the ZnO powdered sample is shown in Fig. 4. This spectrum reveals that nanoparticles have low absorbance in the visible region, which is a characteristic of ZnO. The absorbance spectrum exhibits an excitonic absorption feature peaked at 367 nm. No other peaks were observed in the absorbance spectrum, which confirms the purity of the synthesized ZnO product. The absorption peak of the ZnO nanoparticles has a blue shift compared to that of the ZnO bulk (375 nm). It was demonstrated that this blue shift depends on the nanocrystal size29). Moreover, the local stain in the ZnO nanoparticles (3. 941×10−3), estimated from the Stokes-Wilson equation, can contribute to the blue shift of the absorption peak.
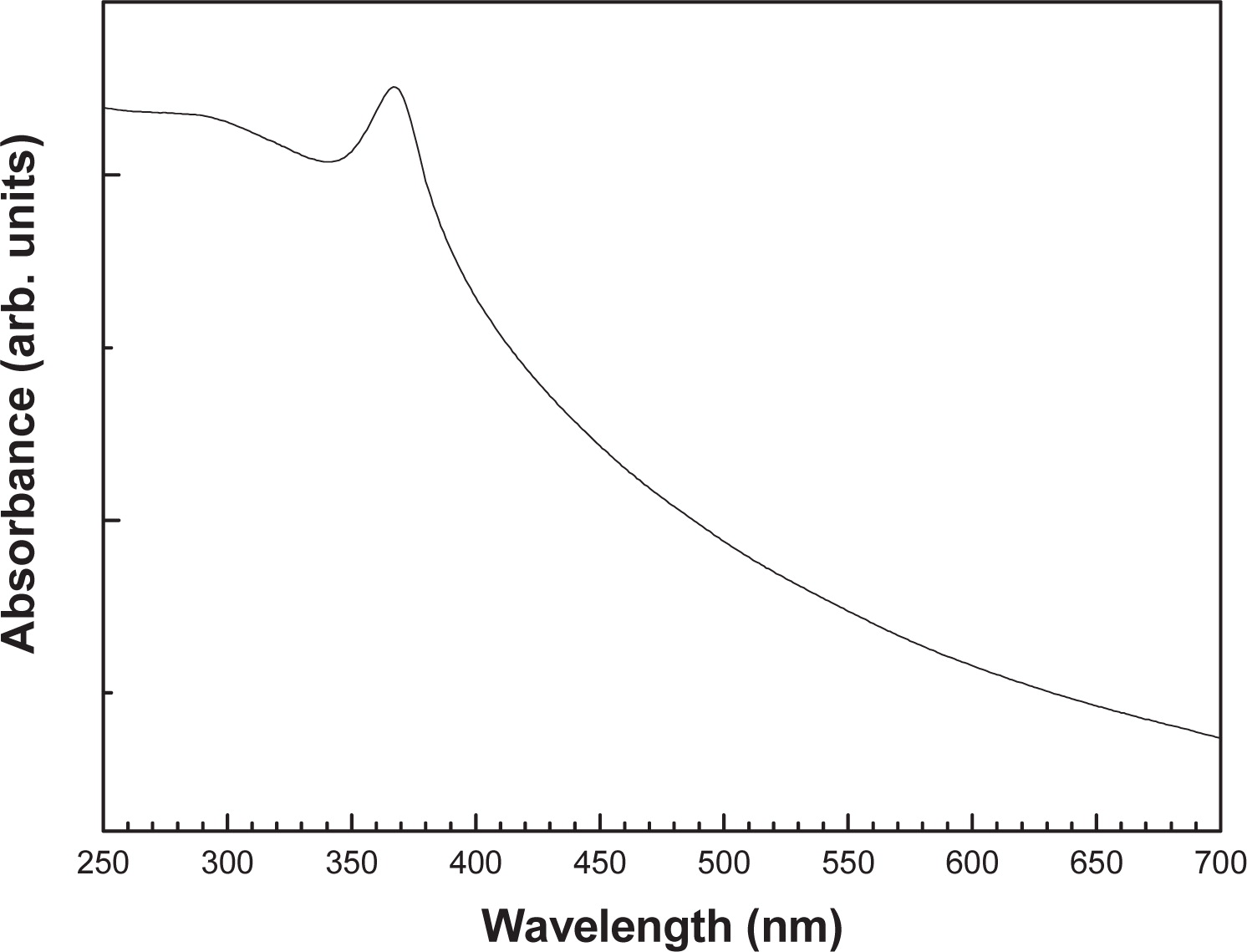
Room-temperature UV-Vis optical absorbance spectrum of the nanocrystalline ZnO sample.
In order to extract the band gap energy (Eg) from the absorption spectrum, we use the point of inflection obtained from the first derivative of absorbance with respect to photon energy and find the maximum in the derivative spectrum30). The Eg is associated to the maximum in the spectrum, i.e. where the absorbance has a maximum increase with respect to photon energy. A derivative spectrum of the nanopowder sample is presented in Fig. 5 and the obtained band gap energy is 3.278 eV.

First derivative absorption spectrum of the nanocrystalline ZnO flakes.
The band gap energy including the quantum confinement as a function of the average particle size is obtained from the effective mass model given by the following equation31):
Fig. 6 illustrates the FT-IR spectrum of the nano-sized ZnO powder in the range 500–4000 cm−1. In the FT-IR spectrum there is a significant spectroscopic band at around 521 cm−1. This is the characteristic band of ZnO33). The peak at 885 cm−1 is probably due to the carbonate moieties which are generally observed when FT-IR samples are measured in air. Also, the characteristic absorption bands at about 3425, 1632 cm−1 are observed and correspond to the O-H stretching and bending modes of water absorbed on the surface of nanocrystals34), respectively. The EDS analysis confirms that the synthesized ZnO flakes are composed only of zinc and oxygen with a weight percentage of 41.08 and 58.92, respectively. It may be noted that the slightly higher oxygen content in the powdered sample is due to the ambient air where the ZnO powder reacts with atmospheric oxygen. This result is confirmed by FT-IR measurements where an O-H group is detected.
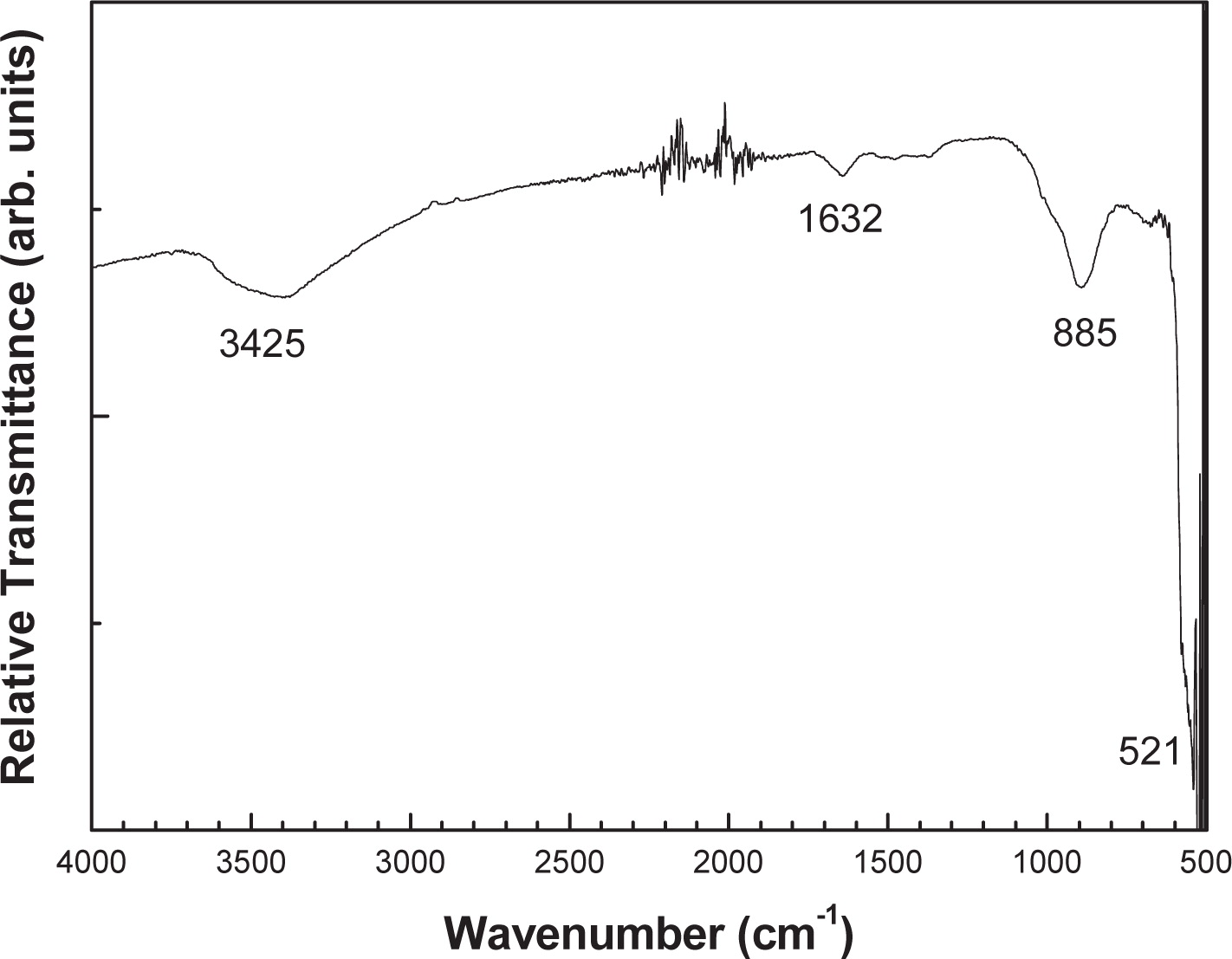
Room-temperature FT-IR spectrum of the nano-sized ZnO powdered sample.
In summary, ZnO nanoparticles with an average crystallite size of 31 nm and synthesized via a simple solution process using zinc acetate and diethylamine under refluxing at 85°C for 4 h were investigated by a variety of techniques. The XRD data indicate the high crystallinity of the ZnO nanoparticles in the hexagonal lattice with parameters a and c of 3.253 and 5.210 Å, respectively. The average size of the nanocrystalline ZnO flakes, estimated from FE-SEM, is about 300 nm. The nanocrystalline ZnO powder exhibited the direct optical band gap energy of 3.278 eV. The grain size deduced from XRD and the one calculated from the Brus equation show a good agreement. Raman scattering, EDS and FT-IR analysis confirmed the high purity of the nanocrystalline ZnO flakes prepared by the simple reaction process which is a low-cost method.
The author is grateful to Professor A. A. Al-Ghamdi for his support and constant interest in the present work, for help with the UV-Vis, FT-IR and FE-SEM measurements and for discussions.
Amor Sayari
Amor Sayari, born in 1964, received a bachelor’s degree in physics in 1989 from the University of Tunis, a master of science, and a PhD graduate degree in solid-state physics in 1991 and 1998, respectively, from the University of Tunis, Tunisia. He obtained the habilitation qualification in physics at the University of Tunis in July 2012. Dr. Sayari worked as an assistant at the University of Sfax from 1992 to 1998. After his PhD on “study of folding, confinement and interface effects in GaAs/AlAs superlattices and quantum wells by Raman spectroscopy”, Dr. Sayari became an assistant professor at the University of Tunis where he combined teaching activities with experimental research in the field of solid-state physics. He is a member of the Raman Group, Faculty of Science Tunis.
His research interest is vibrational, optical and structural properties, especially for III/V and II/VI semiconductor heterostructures.
In 2006, he joined the King Abdulaziz University, Saudi Arabia, where he became interested in ZnO nanoparticles and their applications in nanotechnology.