2013 年 30 巻 p. 144-163
2013 年 30 巻 p. 144-163
Micronutrients and nutraceuticals such as vitamins, carotenoids, polyunsaturated fatty acids and polyphenols are classes of food ingredients that are essential for human health and well-being. These compounds are rarely added purely to the targeted food application but rather in encapsulated, solid, dry product forms with added functionalities such as improved stability, bioavailability or handling. This review presents some of the industrially most relevant particle technologies, as well as emerging ones, for synthesis and formulation of micronutrients and nutraceuticals. The influence of technological process parameters on product particle physicochemical properties such as size, morphology and structure are highlighted as well as their importance for end-use applications.
In 1912, the Polish biochemist Casimir Funk (1884–1967) coined the term vitamins. The discovery of vitamins as essential factors in the diet was a scientific breakthrough that changed the world! Already in 1906, Frederick Gowland Hopkins indicated that “no animal can live on a mixture of pure protein, fat and carbohydrate” – this started the search for “growth factors” in food. The Dutch physician Christian Eijkman found that a constituent of rice bran can prevent a beriberi-like disease in chickens and Gerret Gryns was the first scientist to adopt the deficiency theory for the etiology of this disease. He stated that the disease breaks out when a substance necessary for the metabolism is lacking in the food.
In 1912, Casimir Funk isolated a bioactive substance from rice bran which was at first given the name “vita-amine”. Funk realized that this substance could cure chickens and human patients from beriberi. He published a landmark paper “The etiology of the deficiency diseases” and stated that all “deficiency diseases can be prevented and cured by the addition of certain preventive substances, the deficient substances”, for which he proposed the name “vitamines”1). Two years later in 1916, the American biochemist Elmer V. McCollum introduced capital letters to differentiate between vitamin A, vitamin B, vitamin C and vitamin D. Later, vitamin E and vitamin K were added and it was realized that a food containing vitamin B can contain more than one factor and a further differentiation into vitamin B1, vitamin B2 and so on was applied.
These observations and findings facilitated the experimental research in the following years enormously. The next three decades were full of scientific breakthroughs in the understanding of the role of vitamins and by 1941, all 13 vitamins had been discovered and characterized. These are now classified as either water- (e.g. vitamin C) or fat-soluble (e.g. vitamin A), as listed in Table 1. The scientific breakthroughs were honored with twelve Nobel Awards to 20 Nobel Prize winners. The Nobel Award in Chemistry 1928 was given to Adolf Windaus for his studies on the constitutions of the sterols and their connection with the vitamins. This was followed by the Nobel Prize in Medicine and Physiology in 1929 jointly to Christiaan Eijkman for the discovery of the anti-neuritic vitamin and to Sir Frederick Gowland Hopkins for the discovery of the growth-stimulating vitamins.
| Micronutrients & Nutraceuticals | Major formulation challenge |
|---|---|
| Water-soluble vitamins | |
| Vitamin B1 (thiamine), vitamin B2 (riboflavin), vitamin B3 (niacin), vitamin B5 (pantothenic acid), vitamin B6 (pyridoxine), vitamin B7 (biotin), vitamin B9 (folate), vitamin B12 (cyanocobalamin), vitamin C (ascorbic acid) | chemical stability (oxidation, hydrolysis, light, temperature, pH), sensory (taste), discoloration |
| Fat-soluble vitamins2) | |
| Vitamin A (retinol, retinyl esters, retinal, retinoic acid), carotenoids, vitamin D (ergocalciferol, cholecalciferol), vitamin E (α-tocopherol, tocotrienol), vitamin K (phylloquinone, menaquinone) | chemical stability (oxidation, hydrolysis, light, temperature, pH), solubility in water, bioavailability |
| Polyunsaturated fatty acids (PUFAs) | |
| e.g. omega-3 fatty acids such as docosahexaenoic acid (DHA) | chemical stability (oxidation),sensory (smell, taste) |
| Polyphenols3, 4) | |
| e.g. epigallocatechin gallate (EGCG), resveratrol | chemical stability (temperature, oxygen, light, pH), bioavailability, sensory (taste) |
| Minerals | |
| e.g. Fe, Zn, Ca, Mg | sensory (taste) |
| Plant extracts5, 6) | chemical stability, sensory |
Already in the 1940s, authorities had started to establish dietary standards and nutrient requirements (recommended daily allowance) for the optimal intake of vitamins depending on age, gender and risk groups. In order to secure a sufficient intake of vitamins for the full population, a number of countries implemented fortification programs of staple food; today food fortification is established in more than 60 countries. Examples are the fortification of flour or sugar with vitamin A especially in low-income countries, the fortification of flour with folic acid in the US, Canada and Latin American countries, the fortification of margarine with vitamin A and D or the fortification of milk and juices with vitamin D. The World Bank’s assessment of fortification was: “probably no other technology available today offers as large an opportunity to improve lives and accelerate development at such low cost and in such a short time”. Today, there is a wide consensus amongst scientists about adequate vitamin intake and the relation to health and healthy aging. Science continues to provide new approaches and insights on the role of vitamins and to demonstrate that “the identification of the role of vitamins was one of the most important contributions of science to mankind”.
The understanding that micronutrients are essential for human and animal growth and health7) and that they have to be part of the diet was a major stimulus for nutrition science. Research was extended also to nutraceuticals and health ingredients such as polyunsaturated fatty acids, oily plant extracts and fruit powders that provide certain health benefits (but are not essential)8). Subsequent to the recognition of the vitamins and the discovery of their function it became clear that breakthroughs in the production, formulation and application would have to be achieved in order to allow them to be used by humans and animals. This inspired scientists in pharmaceutical companies in Europe and the US to develop synthetic routes and formulation technologies. The first production of a vitamin on a technical scale was achieved by Hoffmann-La Roche in 1934 for vitamin C based on a combined fermentation and chemical process developed by Tadeus Reichstein. In the following years all vitamins became available via chemical synthesis, fermentation or extraction from natural materials. Industrial production was not the complete solution yet, and new challenges arose with the incorporation of micronutrient and nutraceuticals in end-user applications such as tablets, vitamin waters, beverages, yoghurts and other foodstuffs.
Especially lipids and fat-soluble vitamins2) are difficult to add to food products (e.g. to a hydrophilic environment such as a beverage), and are often chemically unstable to, e.g. oxidation, hydrolysis, light and heat9, 10). New technologies had to be explored and again know-how and formulation competencies of pharmaceutical companies gave the basis for the development of vitamin forms. This offered opportunities to provide vitamins for humans and animals for optimal growth and health. Therefore, micronutrients and nutraceuticals are nowadays rarely sold in pure form and are mostly delivered in microencapsulated product forms to protect the active ingredient from the surrounding environment11–14). Thereby the stability and shelf-life of the compounds are prolonged and they can be released in a controlled and tailored manner. Further benefits of microencapsulation processes include easier handling, improved sensory properties with respect to appearance and taste and uniform dispersion of low-concentrated actives15). Innovation in colloid and nanosciences facilitates the development of product forms for different applications. Progress in formulation results from close cooperation between basic science carried out at universities and applied science in industry. However, the knowledge of formulation is often a special expertise of companies, protected by patents and not available in textbooks.
Particle properties in these dry product forms are of the utmost importance to achieve the desired performance in target applications and have to be carefully controlled during their synthesis. For example, particle size can govern the optical appearance and sensory perception of the product as well as the dissolution rate and bioavailability of the active. Particle morphology and shape influence flowability, ease of handling and processing of the powders. For example, during the preparation of multivitamin-multimineral tablets, several nutraceutical components and excipients are blended. The mixture must be uniform (no segregation) and free-flowing for accurate dosing in the tabletting equipment. Encapsulates are traditionally classified as reservoir (core-shell) or matrix-type spherical particles16), however, other shapes and morphologies such as rods or irregular shapes as well as aggregates and agglomerates also have to be considered. The internal structure and composition such as porosity, crystallinity and glass transition temperature would affect the stability and shelf-life of the products. Controlled release and stability of encapsulated active compounds can be achieved by the proper selection of the matrix material and its concentration17–20). Common encapsulation materials in the food industry are listed in Table 2. One of the central issues in the design of an optimal encapsulation material is the molecular mobility through the matrix as this affects the stability of the active ingredient. It has been shown that small quantities of low-molecular-weight compounds such as glycerol, sucrose or maltodextrin, in combination with high-molecular-weight carbohydrates improve storage stability21). The molecular mobility can be understood with the concepts of glass transition of amorphous biopolymers as well as the molecular free volume present in their disordered matrix. The oxygen diffusivity in a glassy carbohydrate matrix depends exponentially on the radius of the free volume element22). Therefore, already small differences in free volume can have a substantial impact on degradation of the encapsulated bioactive substance via chemical reactions, e.g. fish-oil lipid oxidation.
| Animal | Plant | Marine | Microbial | |
|---|---|---|---|---|
Carbohydrate |
chitosan | tree exudates: gum arabic, gum karaya, gum ghatti, gum tragacanth plants: starch, cellulose extracts: guar gum, pectin, galactomannans polysaccharides: soluble soy polysaccharide, maltodextrins |
carrageenan agar alginate |
xanthan gellan dextran curdlan pullulan |
Protein |
gelatin casein whey egg white lactoglobulin |
gluten soy pea rice sorghum lupine zein |
||
Lipid |
fatty acids glycerides / waxes phospholipids Shellac |
fatty acids glycerides waxes phospholipids |
Here, the synthesis and formulation technologies (not solely limited to microencapsulation) of micronutrients and nutraceuticals are reviewed that result in solid physical morphology. Emphasis is placed on resulting particle properties such as size and shape and their influence on performance in target applications. Industrially most relevant microencapsulation technologies such as spray drying and extrusion will be covered. Novel technologies that are not yet applied on an industrial scale and mostly yield nanoscale particles will also be highlighted. Although the application of nano-sized delivery systems in foods is still under some debate, the worldwide sales of nano-food products has grown and nanotechnologies are applied by several major food companies25). Emulsion technologies that are often part of encapsulation processes in the liquid state especially of lipophilic compounds will not be described here, and the reader is kindly referred to recent reviews focused on this topic26–29). However, emulsions are often converted from the liquid state to dry powders3) by technologies such as spray- or freeze-drying that are covered in the following text. Encapsulation technologies such as inclusion complexation30, 31) and liposomes32–34) will also not be discussed as their use in foods is limited due to regulatory, cost or performance issues16). Finally, a focus is placed on recent advances, and therefore primarily literature published within the past 5 years is reviewed.
Particle technologies applied for the synthesis and formulation of nutraceuticals in industry or academia span several length scales from nano to macro sizes (Table 3). Product properties are to a large extent governed by the particle size. Therefore, performance in the final application can be modulated by the proper choice of particle synthesis and formulation technology (Table 3), which are typically classified as either ‘top-down’ or ‘bottom-up’ approaches8). The former involves the break-up of larger particles or droplets to smaller ones using mechanical energy in classic processes such as milling35) or emulsification26–28). In the case of emulsions, the smaller droplets formed are then typically stabilized by surface-active compounds, so-called emulsifiers, such as low-molecular-weight surfactants, proteins or polysaccharides. These emulsifiers adsorb at the droplet surface thereby reducing the interfacial energy and facilitate droplet disruption during processing and/or prevent droplet coalescence28). The final microencapsulated product particle is then formed during the drying step, e.g. by spray drying36), or by directly cooling the emulsion to form solid lipid nanoparticles26). In the ‘bottom-up’ approach, individual components such as molecules, monomers or ions are assembled into larger structures or particles using a physical or chemical process8) such as flame spray pyrolysis or precipitation/crystallization. These processes mostly yield particles of the pure compounds in the nanometer size range. On the other hand, e.g. extrusion technology can produce macro-sized particles with incorporated nutraceuticals that can be directly applied in the final food application.
| Technology | Particle Morphology | Size (μm) | Active | Processing aids Raw materials | Solubility |
|---|---|---|---|---|---|
| FSP |
 |
0.01 – 0.1 | minerals | organic solvents | hydrophilic |
| SLN NLC |
 |
0.1 – 100 | carotenoids lipophilic vitamins | triglycerides fatty acids waxes | lipophilic |
| Precipitation |
 |
0.1 – 100 | carotenoids polyphenols | supercritical fluids | hydrophilic lipophilic |
| Spray Drying |
 |
10 – 100 | PUFA oily extracts | maltodextrin starch gum arabic | hydrophilic |
| Spray Chilling |
 |
10 – 1000 | hydrophilic vitamins minerals | waxes | lipophilic |
| Complex Coacervation |
 |
10 – 1000 | carotenoids lipophilic actives | gelatin & gum arabic | (hydrophilic) |
| Microbeads |
 |
10 – 5000 | polyphenols lipophilic actives | alginates | hydrophilic |
| Extrusion |
 |
500 – 5000 | minerals, hydro- & lipophilic actives | maltodextrin starch waxes | hydrophilic lipophilic |
 lipophilic active
lipophilic active
 hydrophilic active
hydrophilic active
 hydrophilic matrix
hydrophilic matrix
 hydrophilic gel
hydrophilic gel
 lipophilic matrix
lipophilic matrix
Industrially most relevant as well as emerging novel technologies for the synthesis and formulation of nutraceuticals will be described in the order of the resulting particle sizes from nano to macro, though a certain overlap between the various technologies exists (Table 3).
3.1 Flame spray pyrolysisFlame spray pyrolysis (FSP) is a bottom-up approach for the synthesis of nanostructured materials37). Traditionally and on an industrial scale, vapor-fed flame reactors have been utilized for the synthesis of metal oxide nanoparticles such as SiO2 or TiO2. The emergence of FSP technology, where a liquid precursor solution, emulsion or slurry is sprayed and ignited, enabled the development of functional nano-structured materials with a first break-through in the synthesis of highly active supported noble metal catalysts38). In recent years, this has been followed up by research on bio-related compounds for dental, orthopedic and even nutritional applications.
Rohner et al.39) produced amorphous FePO4 by FSP with closely controlled particle characteristics (Fig. 1). Water-soluble, highly bioavailable Fe compounds such as FeSO4 often cause unacceptable changes in the color or taste of foods, whereas poorly water-soluble Fe compounds such as FePO4, although more stable in foods, tend to have a low bioavailability. The absorption of the latter in the human body can be improved by decreasing their size to the nanoscale40), e.g. by a bottom-up approach such as FSP. An organic Fe-containing precursor solution is atomized through conventional air-assist or ultrasonic nozzles during FSP synthesis37). The thus formed spray is ignited by rather small pilot flames and the organic solution is combusted. Particles are formed by nucleation from the gas phase and grow by surface reaction and/or coagulation and subsequent coalescence into larger particles37). The size of the as-produced FePO4 nanoparticles (Fig. 1) can be precisely controlled by FSP process parameters such as precursor composition or precursor feed rate to the reactor. The chemical composition of FSP-made particles can also be tailored to produce multicomponent compounds37). Combined Fe/Zn-containing nanoparticles41) as well as Mg- or Ca-doped Fe2O342) have been produced by FSP for iron food fortification in foodstuffs such as rice, flour or dairy products. The nano-sized particles have high specific surface areas (SSA; up to 200 m2/g)41) that ensure their high solubility and bioavailability41, 43). Each primary, single, constituent particle is dense and spherical39). However, during synthesis, multiple particles can assemble into larger fractal-like structures, so-called aggregates or agglomerates held together by chemical or physical bonds, respectively37). The presence of agglomerates can actually facilitate the powder handling and they can also easily be broken up to their constituent aggregates and/or primary particles, e.g. by ultrasonication or high-pressure dispersion44).
Transmission electron microscope (TEM) images of commercial (a: SSA = 33 m2/g) and FSP-made (b,c: 69 and 195 m2/g, respectively) FePO4 particles. The FSP-made FePO4 particles were dense and spherical, while the commercial ones exhibited an irregular and highly porous structure. The particle size calculated from specific surface area measurements corresponds to 11 nm for the small, FSP-made particles (c). The SAED (selected area electron diffraction) patterns in the insets are characteristic of an amorphous substance for all three compounds39).
The in vivo bioavailability of these FSP-made Fe and Fe/Zn compounds in rats was comparable to the golden standard FeSO4, and no tissue accumulation could be detected43). Thus the nanosizing of such poorly water-soluble Fe compounds is a promising approach to increase their absorption in the human body and their nutritional value, though further toxicological studies also in humans are required before this technology can be applied industrially40). From a technological point of view, the scale-up of FSP reactors from laboratory to pilot scale is feasible and has already been realized in industrial-oriented settings45).
3.2 Solid lipid nanoparticles (SLN) and nano-structured lipid carriers (NLC)An emerging type of encapsulation system are lipid nanoparticles. The first and second generations are termed solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC), respectively46–50). Lipid nanoparticles have been widely studied for pharmaceutical and cosmetic applications where nanotechnologies can be implemented more readily. Their potential in food and agricultural industries has recently also been highlighted51, 52). In general, lipid nanoparticles can be compared with conventional liquid emulsions in the sense that lipid droplets stabilized by a surfactant are dispersed in an aqueous continuous phase. However, in the case of SLN or NLC, the lipid phase is either fully or partially solidified26, 50). The lipids used to prepare both SLN and NLC are typically triglycerides, fatty acids, waxes and partial glycerides50). Some of the main advantages associated with SLN are high encapsulation efficiencies, good physical stability, no organic solvents required during synthesis, easy large-scale production with cheap raw materials and a controlled release profile of the active substance due to the solid matrix50, 52). SLN typcially crystallize with imperfections in their crystal lattice after preparation, which also allows the incorporation of active substances. However, during storage, a polymorphic transition to lower-energy crystal lattice arrangements can occur resulting in the expulsion of the incorporated active substance. In order to overcome this drawback of SLN, NLC were developed that exhibit a less ordered crystal lattice arrangement by blending solid and liquid lipids. The imperfections in NLC also allow for a higher loading of active substances compared to SLN50, 53).
Lipid nanoparticles are commonly prepared by forming an oil-in-water emulsion (at a temperature above the melting point of the lipid phase) and then cooling the emulsion so that the dispersed lipid phase completely or partially solidifies50). The preparation of a fine emulsion is crucial in order to obtain particle sizes in the nano range with preferably narrow poly-dispersity for the product SLN or NLC. Production techniques include, e.g. melt homogenization, melt microencapsulation and cold homogenization, which have all been reviewed elsewhere51).
Mainly lipophilic nutraceuticals have been incorporated into lipid nanoparticles, as they can enhance the chemical stability of these active substances that are sensitive to light, oxidation and hydrolysis50). Several studies have demonstrated the incorporation of carotenoids such as lutein53, 54) or β-carotene55–57) into NLC. Liu et al.53) developed lutein-loaded NLC by optimizing the concentration of lipophilic and hydrophilic surfactants during their preparation. The lutein-loaded NLC had an imperfect crystalline lattice and a spherical morphology with particle sizes as small as 130 nm. However, the particle size increased with increasing lutein load due to entrapment of the active substance in the matrix of the nanoparticles. The encapsulated lutein was effectively protected in the presence of a simulated gastric fluid, and slowly released lutein in vitro into simulated intestinal fluid53). The particle sizes of NLC loaded with β-carotene were stable and did not exhibit polymorphic transitions after 7 months of storage at 4–8°C, which could make them feasible in beverage applications57). Thrandur et al.56) emphasized the proper choice of surfactants during NLC manufacture to improve the protection of β-carotene against chemical degradation. The role of the surfactant is dual, as it controls the formation of the crystal lattice structure during synthesis and stabilizes it during storage.
Lipophilic α-tocopherol55), vitamins K158) and A59–61) have also been incorporated into NLC. The reported concentration of encapsulated active substance is typically quite low, e.g. 2% for vitamin A palmitate59) and <5% for vitamin K158). Similarly to lutein-loaded NLC53), the SLN size increased with increasing vitamin K1 content (132 and 212 nm for 0.25 and 5.0% vitamin K1, respectively) and furthermore the entrapment efficiency decreased (Fig. 2)58). Vitamin-K1-loaded SLN were stable after 4 months with respect to particle size and vitamin content if stored at temperatures < 25°C. The stability of NLC loaded with vitamin A palmitate was improved by freeze-drying61). Cryoprotectants such as glucose or mannitol were added during freeze-drying in order to prevent particle aggregation resulting in the sizes 200 – 250 nm in the dry product form61).
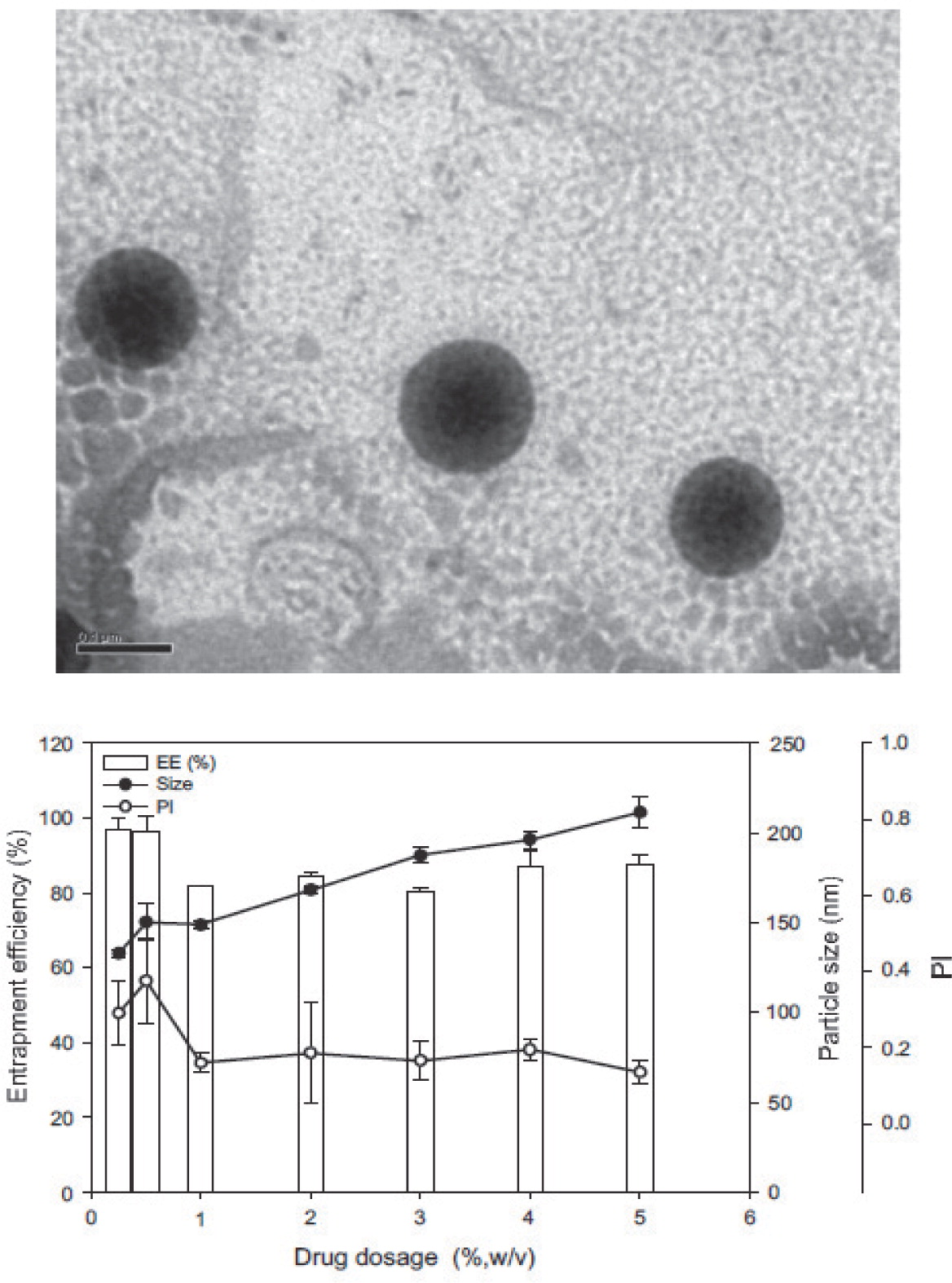
TEM image (scale bar: 100 nm) of vitamin-K1-loaded SLN (left) and the effect of the vitamin K1load on the particle size, entrapment efficiency (EE) and polydispersity index (PI) (right). The incorporation of more vitamin K1 into the SLN increased the particle size due to incorporation of the vitamin into the matrix of the lipid nanoparticles58).
Precipitation or crystallization is a bottom-up approach for the synthesis of nano- to micron-sized particles. It was mainly developed in the pharmaceutical industries to increase the aqueous solubility and oral bioavailability of poorly water-soluble drugs62, 63). Product properties such as particle size, morphology and surface properties can be more closely controlled by precipitation methods compared to classic top-down approaches such as milling64). The nucleation and growth kinetics dictate the final particle size and are controlled via supersaturation63). However, the precipitation process is more challenging compared to a relatively simple milling method. The high surface area formed by precipitation needs to be rapidly stabilized to avoid aggregation/agglomeration, recrystallization and Ostwald ripening62, 63). For example, the Ostwald ripening of β-carotene nanoparticles has been investigated both numerically and experimentally65). It was shown that the particle dispersions are more stable the narrower the initial size distribution, as the difference in solubility between small and large particles decreases65).
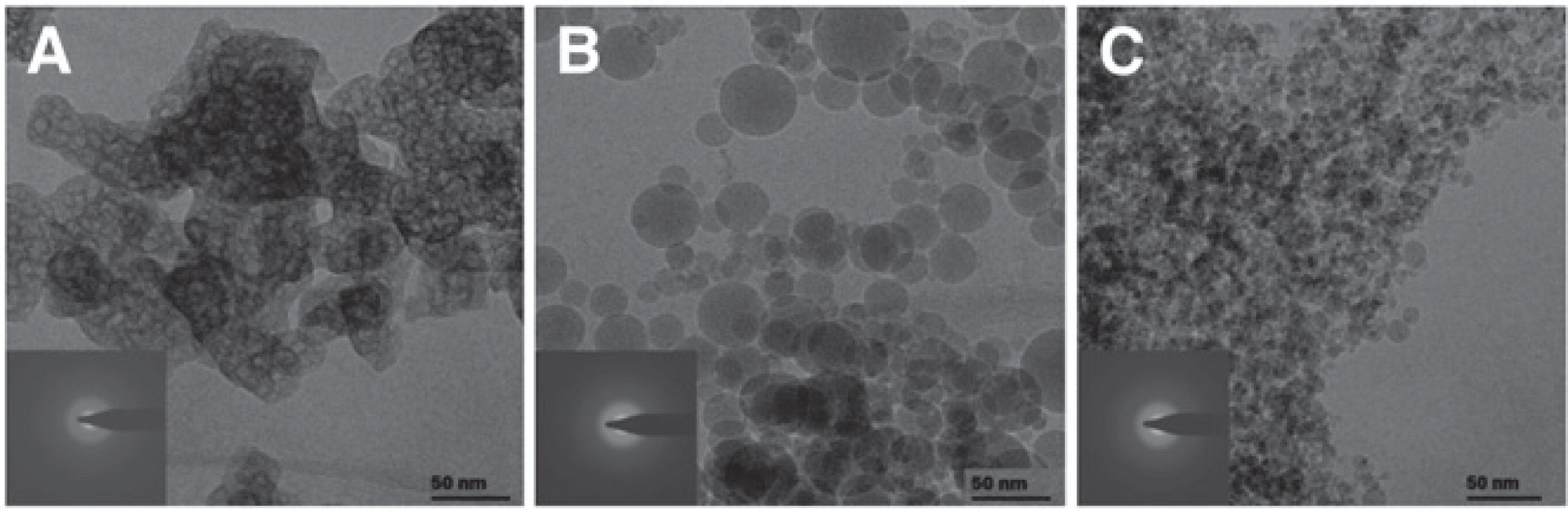
Precipitation approaches have mainly been applied to carotenoids66–73) and polyphenols3) amongst the micronutrient and nutraceutical compounds, and are mostly carried out with supercritical fluid technologies. In the supercritical gas phase, carbon dioxide is most commonly used as it is inert, non-toxic and has a low supercritical temperature ideal for heat-sensitive materials64). Carotenoids have a low solubility in supercritical CO2 and therefore the CO2 is used as an antisolvent in such precipitation processes70). Particle size and morphology can be controlled by antisolvent precipitation process parameters such as temperature and pressure, generally resulting in mean sizes between 1 and 200 μm or even smaller70). For example, the mean particle size of trans-resveratrol was reduced from 560 to 230 nm by decreasing the precipitation temperature from 25 to 5°C74). The trans-resveratrol particles were stabilized by adsorption of hydroxypropyl methylcellulose (HPMC) on the particle surface (Fig. 3). The commercial trans-resveratrol particles were needle-shaped and had particle sizes ranging between 20 and 100 μm (Fig. 3a). Precipitation without HPMC resulted in irregularly shaped particles several microns in size (Fig. 3b), while aggregates of almost spherical particles ∼240 nm in size were formed in the presence of HPMC (Fig. 3c)74). The saturation solubility and dissolution rate of precipitate trans-resveratrol nanoparticles were significantly higher compared to the commercial starting material and could be attributed both to the decrease in particle size as well as to crystallinity74).

Scanning electron microscopy (SEM) images of commercial trans-resveratrol particles (a) as well as after precipitation without (b) and with (c) HPMC74).
Active compounds can also be directly encapsulated by co-precipitating them simultaneously with a second component such as a biopolymer or a sugar, forming either a polymer coating around the active compound, polymer particles with the active compound incorporated or co-crystals with the individual components connected by non-covalent interactions3, 69, 70, 73, 75). For example, the solubility of pterostilbene can be dramatically increased by forming co-crystals with compounds of a significant aqueous solubility75, 76). The concentration ratio between the active compound and the biopolymer during co-precipitation has a great influence on the final particle morphology, encapsulation efficiency and yield69, 70). Green-tea poly-phenols have been co-precipitated and encapsulated with a biodegradable polymer poly-ε-caprolactone77). The product particles were rather spherical, 3 – 5 μm in size with a narrow particle size distribution but a high degree of agglomeration. A high fraction of the polyphenols was entrapped in the crystalline domains of the polymer and can thus only be released by matrix degradation, a process that may take months77).
3.4 Spray dryingSpray drying as an encapsulation process was introduced in the 1930s9, 10) and is still the most frequently used technology in the food industry4). The process is relatively cheap, straightforward and flexible, and produces stable particles of high quality9, 18, 36, 78). It has been used to encapsulate and protect a range of lipophilic micronutrient and nutraceutical compounds such as fish oil, vitamins and polyphenols15, 19, 79–82). For many years, the spray drying of heat-sensitive active compounds was a challenge due to high process inlet temperatures. However, process optimization and control of the temperature has now also enabled the spray drying of sensitive active compounds such as lycopene, anthocyanins and β-carotene36, 81, 83, 84).
In a spray drying process, a liquid product is atomized in a hot gas current to instantaneously evaporate water and obtain a dry powder. The initial liquid feed can be a solution, an emulsion or a suspension3, 15). The three main steps of microencapsulation by spray drying are preparation of the dispersion or emulsion, homogenization, and finally atomization of the mass into the drying chamber9). The particle size and shape of spray-dried products depend to a large extent on the initial feed properties (e.g. solution viscosity, matrix materials and their concentration in the solution) as well as on the process parameters (e.g. nozzle geometry, tower height, inlet temperature). Typically, spray-dried products are rather fine powders of spherical particles 10 - 100 μm in size. However, with an adequate set of process parameters available, large-sized particles (2 – 3 mm) can also be obtained3, 9). Wide size distributions are not uncommon due to the droplet size distribution in the liquid spray.
The influence of process parameters on product particle properties has been investigated in several studies9, 78, 85, 86). The oil droplet size in the emulsion should be rather small and the viscosity should be low enough to prevent air inclusion and the formation of elongated, large particles78). Furthermore, a low emulsion droplet size results in a lower amount of unprotected (fish) oil on the surface of the particles that is essential for the shelf-life of the product87). A low inlet temperature facilitates the formation of dense particles with a spherical shape (Fig. 4), smooth surfaces and a uniform particle size distribution78, 86). At higher temperatures, the water evaporation rates increase and yield irregular particle shapes due to cycles of rapid wall solidification, inflation and collapse78). However, the choice of too low an inlet temperature results in inefficient drying and powder agglomeration78, 86, 88).
Scanning electron micrographs of spray-dried fish oil microcapsules. A higher inlet/outlet temperature results in lower-density powders as the particles are elongated with large volumes of included air (a: 210/90°C; b: 160/60°C)78).
Matrix materials used for spray drying must be soluble in water at an acceptable level and available for food applications, this narrows down the available choices15). Maltodextrins were the most widely applied matrix materials for spray drying in the past3). Nowadays, research focuses on combinations of mainly plant-based materials to obtain particles with improved protective barriers. Jafari et al.87) spray-dried fish oil with either whey protein concentrate (WPC) or modified food starch as surface-active bio-polymers. The particles produced with modified food starch had more surface dents and shrinkage compared to WPC (Fig. 5) that could be attributed to a slower skin formation during drying87). Therefore, the fish oil was better entrapped in the modified food starch formulation. Similarly, Drusch et al.89) found that a higher protein (caseinate) content in fish oil microcapsules leads to an increase of lipid oxidation during storage. Furthermore, an excess of protein increased the free volume which could negatively affect oxygen diffusion into the capsules89). Alginate, a marine carbohydrate, has been combined with starch as wall materials for the spray drying of fish oil microcapsules90). The addition of alginate resulted in a higher oil load capacity and in quite spherical particles with a high protection of the fish oil against oxidation. Microcapsules with up to 50% fish oil loading and > 90% encapsulation efficiency have been produced with sugar beet pectin17, 88). Nevertheless, residual metal ions in sugar beet pectin accelerate the fish oil oxidation, resulting in poor storage stability. Blends of maltodextrin and apple pectin have also been investigated as encapsulation materials for polyphenols91). The combination of pectin with maltodextrin improved the storage stability and sensory properties of the product microcapsules compared to maltodextrin alone.
Scanning electron microscope images of fish oil microcapsules spray-dried with WPC (a,b) or modified food starch (c,d)87).
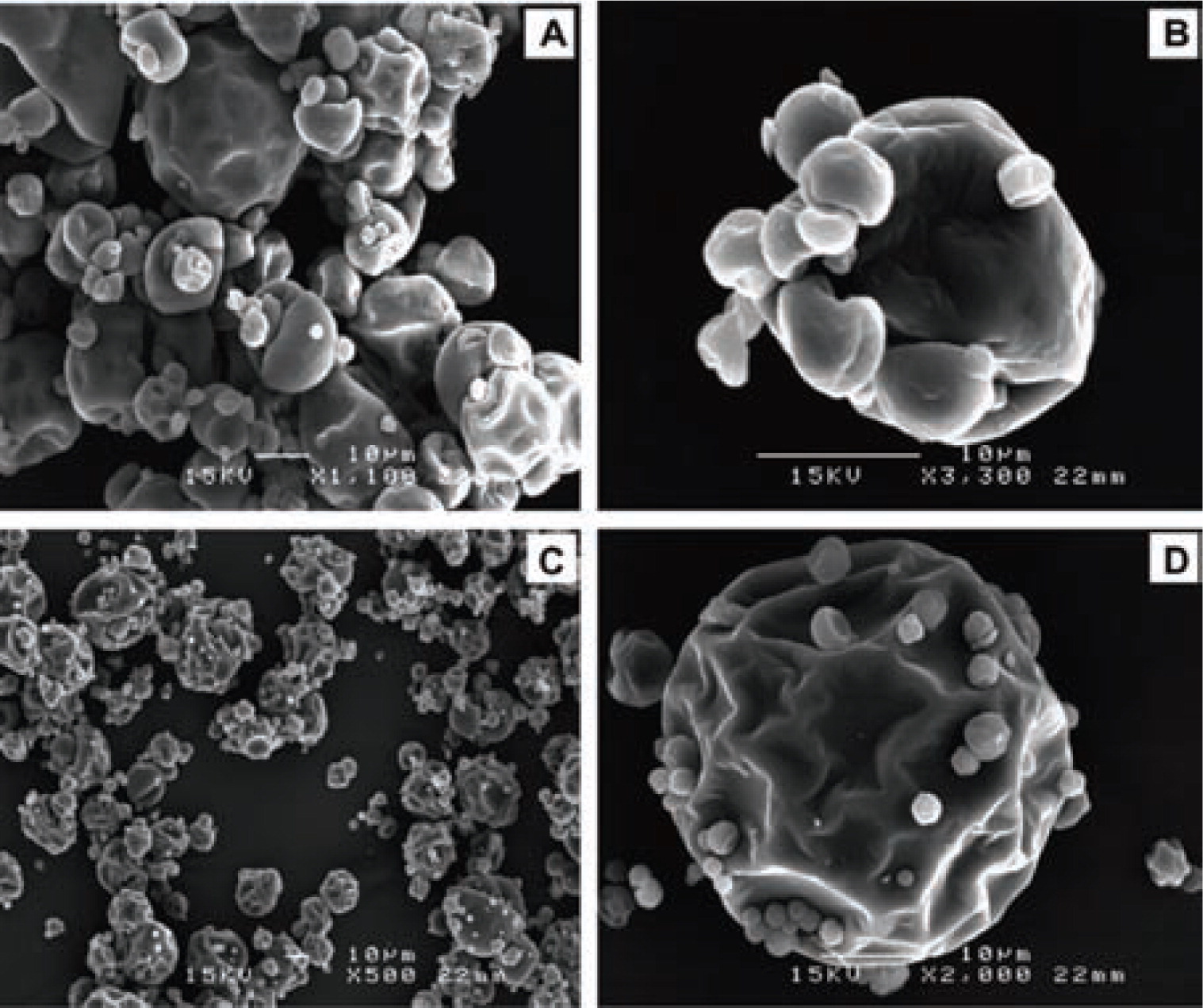
Freeze drying is considered an alternative technology to encapsulate heat-sensitive compounds, as the active compound does not experience high temperatures during processing92). Although spray drying is considered an energy-intensive process due to its poor heat utilization, freeze drying remains a considerably more expensive technology9). Therefore, at least on an industrial level, freeze drying cannot yet be considered an alternative to spray drying and requires further research. Anwar et al.93) made a comparative study of spray drying, spray granulation and freeze drying for the encapsulation of fish oil (Fig. 6). The microcapsule product stability decreased in the order spray granulation > spray drying > freeze drying. This could be attributed to the process drying temperature: a longer residence time at a lower temperature was found favorable for a lower oxidation of the fish oil. Although the temperature during freeze drying is very low, the resulting particle morphology is porous, irregular and flake-like, which allows oxygen diffusion93).
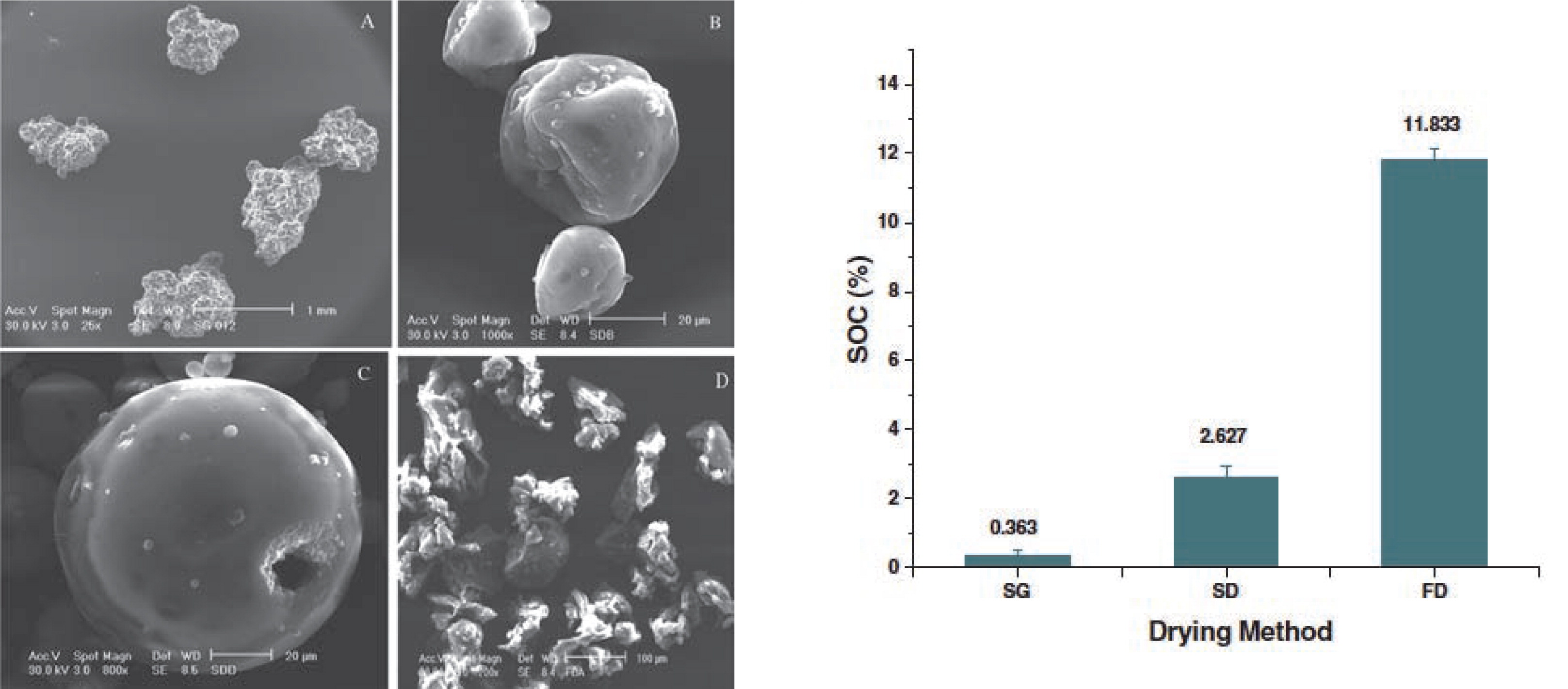
Scanning electron micrographs of fish oil microcapsules produced by spray granulation (SG; a), spray drying (SD; b,c) or freeze drying (FD; d). The lowest surface oil content (SOC) was found for the spray-granulated product93).
A similar process to spray drying is spray chilling or spray cooling where a molten liquid is atomized into cooled or chilled air. The resulting droplets solidify and form prills or powders that are solid at room temperature. The same processing equipment as for spray drying can be used94). Common spray-chilled carriers for encapsulation are fats, waxes, polyethylene glycols, fatty acids and fatty alcohols (Table 2)16). Unlike spray drying, spray chilling does not involve the evaporation of water around the core15). However, the resulting products are not water-soluble and therefore their application should not require this function. An advantage of spray cooling is the possibility to produce different particle sizes, ranging from about 10 to 1000 μm (Table 3)11). Wegmüller et al.95) investigated the triple fortification of salt by spray cooling a mixture of vitamin A, iron and iodine in a hydrogenated palm fat matrix. The active compounds were quite stable during storage but high losses were found during processing. This process is nevertheless not as common for the microencapsulation of nutraceuticals as spray drying.
3.7 Complex coacervationCoacervation is a liquid-liquid phase separation of an initial solution of one or more hydrocolloids into two aqueous phases: a hydrocolloid-rich phase (coacervate) and a hydrocolloid-lean phase16, 96). It is one of the oldest techniques of encapsulation and only a few studies have been published on this technology in the past years for micronutrients and nutraceuticals. Nevertheless, the production cost of particles by complex coacervation is still very high, and this technology is therefore only suitable for the microencapsulation of high-value active or for unstable compounds, e.g. polyphenols4). Fish oil coacervates are added to a number of food products including bread, yoghurt, milk and infant formulae, and it has been shown that they are as bioavailable and bioequivalent as standard fish oil soft-gel capsules97). Complex coacervates are typically made from an oil-in-water emulsion with oppositely charged proteins and polysaccharides, e.g. gelatin and gum arabic98), and the process is carried out in five steps involving polymer dissolution and hydration, emulsification (formation of the core), coacervation (formation of the shell), hardening and washing/separation96). Coacervate particles are often not perfectly spherical but have rather an oval shape4, 16). The slurry containing the coacervates can also be spray-dried or fluid-bed-dried to convert it into a dry powder, however, this might result in a loss of the initial structure16, 97, 98). Important process parameters include pH, ionic strength, temperature, molecular weight and concentration of the polymers that also determine the solubility of the resulting coacervates4, 96, 98, 99). Gum arabic can be replaced by other negatively charged molecules such as pectin, carrageenan or alginate and gelatin by whey proteins16, 100–102). Recently, hydrophobic nutraceuticals such as vitamin D2 or fish oil have been entrapped in electrostatically stable nanoparticles < 100 nm in size. The small nanocomplexes yield transparent solutions and can be used in clear acid beverages101, 103).
3.8 Gel-particle technologiesGel-particle technologies produce microparticles (or microbeads) composed of a biopolymer network that entraps the active compound16). The particle morphology can be either a homogeneous matrix or a core-shell structure (Table 3). The gel microparticles are typically produced in the presence of the active compound, but post-loading especially of coreshell structures is also possible. Sodium alginate – CaCl2 is the most commonly used gelling system (ionic gelation), and has been applied to a range of nutraceuticals, e.g. vitamin A palmitate104) or β-carotene66). Gel particles are typically produced by either so-called extrusion (dropping) or emulsion processes105). In the extrusion process, the size of the particles can be controlled by the droplet generation tool, e.g. vibrating nozzles or piezo effects, as well as by the viscoelasticity of the polymer solution. The generated droplets fall into a gelling bath containing, e.g. a CaCl2 solution and react into gel particles. This process has the advantage of producing rather spherical and monodisperse particles. The use of concentric nozzles (co-extrusion) allows the synthesis of core-shell microcapsules. In the emulsion process, particle formation occurs by the addition of either CaCl2 or an acid to an emulsion of alginate solution and the active compound in an oil, although several variations of the process exist16, 105). Smaller microparticles can be produced by this method compared to the extrusion process and the particle size can be adjusted by common emulsification process parameters.
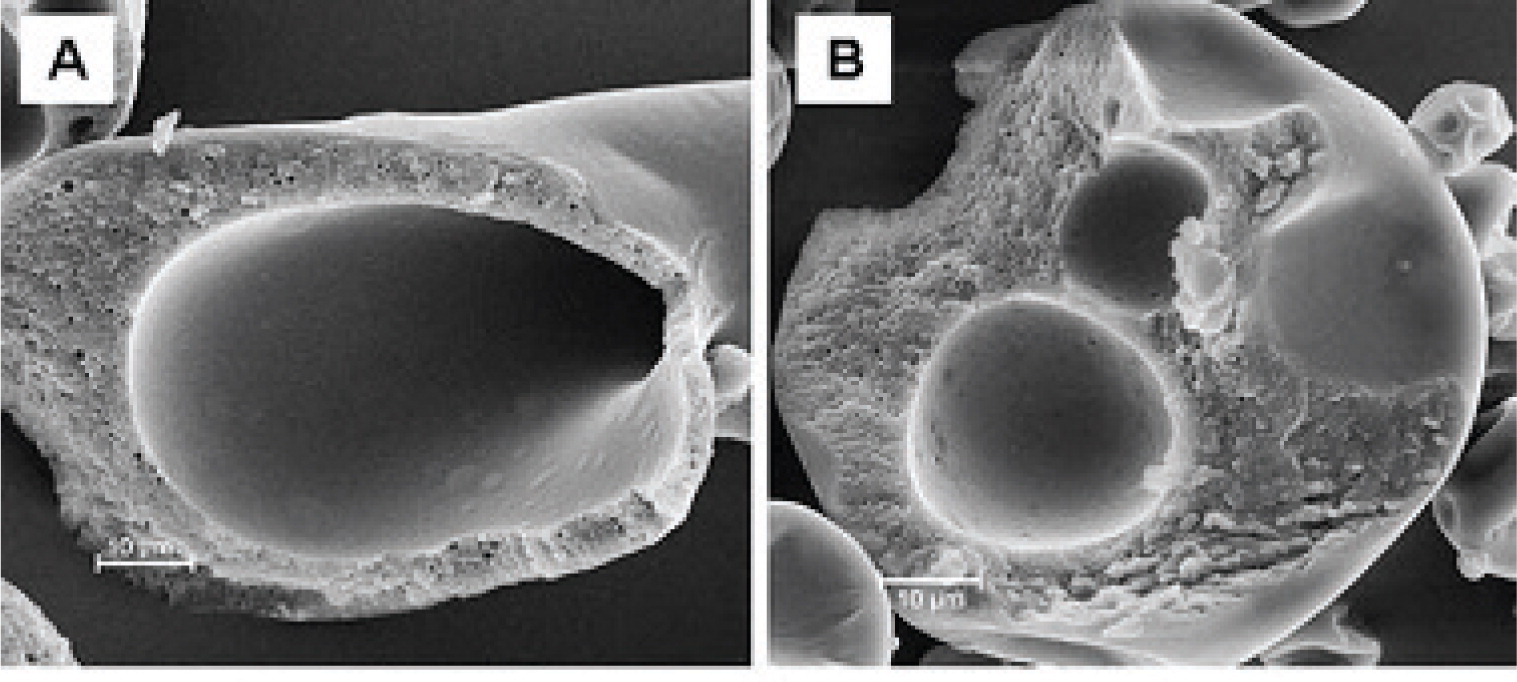
Novel material systems have been exploited for the generation of microbeads. Ca-pectinate beads have been investigated for the site-specific and sustained-release delivery of resveratrol106). Dropping solutions of water-soluble cellulose ethers such as methyl cellulose (MCE) or hydroxyl propyl methylcellulose (HPMC) into an aqueous solution of polyphenol (EGCG) results in the formation of spherical milky white beads with average diameters 600 – 2500 μm (Fig. 7)107). The beads have a core-shell structure with a porous shell (thickness 200 – 350 μm) and a hollow core with some unreacted polymer, and is formed due to the strong interactions of polymer and EGCG on the bead surface. The shell actually consists of a network of colloidal aggregates 200 – 500 nm in size107).
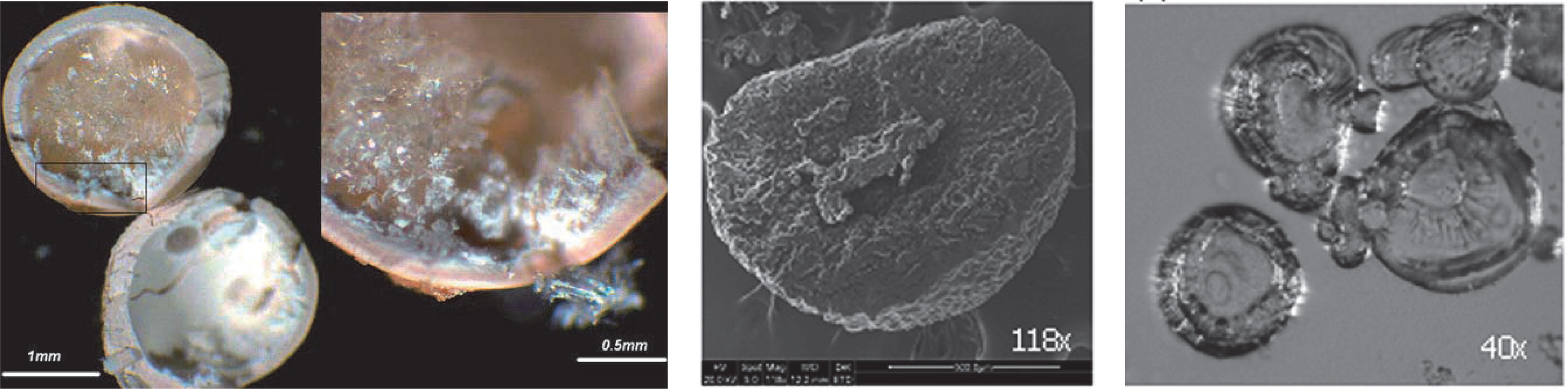
Additional coating layers are often applied to particles in order to improve the stability of the active compound. Vitamin A palmitate has been formulated into double-layer capsules: the vitamin A palmitate/chitosan core was surrounded by a chitosan-alginate membrane and an additional calcium-alginate outer shell (Fig. 7)104). The resulting capsules were 1000 – 1200 μm in size and exhibited a high vitamin A palmitate encapsulation efficiency (>90%) and loading (42.5%). This resulted in a significantly improved long-term stability of vitamin A palmitate at both refrigerator and ambient conditions104). EGCG-loaded gelatin nanoparticles about 200 - 300 nm in size have been coated layer by layer with shells of polyelectrolytes 5 - 20 nm thick108). This resulted in the sustained release of EGCG from the coated nanoparticles with a maximum EGCG concentration in solution after 8 h compared to 15 min from un-coated gelatin nanoparticles108).
A novel class of gel-like materials is constituted by the so-called aerogels that are highly porous (ε = 90 - 99%), lightweight (ρ = 0.07–0.46 g/cm3) and with a high surface area (Sa= 70 - 680 m2/g) resulting in enhanced active bioavailability and loading capacity109). Aerogels are obtained from wet gels (e.g. hydrogels) by using a suitable drying technology, usually a supercritical drying process, in order to preserve the porous texture. However, a drawback in the processing is the replacement of water present in the gel structure by a solvent (solvent exchange), forming an alcogel prior to the drying step.
Traditionally, this method focused on the synthesis of silica and carbon aerogels, however, recent research has led to the development of biodegradable and biocompatible polysaccharide-based aerogels that could also be of interest for food applications109–111). Aerogels have been produced from polysaccharides such as starch, pectin, alginate, κ -carrageenan, agar, chitosan and cellulose109). Alginate aerogel microspherical particles possess a high surface area of up to 680 m2/g, a pore volume of up to 4.0 cm3/g and mean particle diameters ranging from 25 μm to few hundred microns112). The structural properties of polysaccharide aerogels depend on their preparation method and the chemical nature of the gel phase109, 111). For example, an increase in temperature during gelation leads to densification of the nanoporous structure in starch aerogels (Fig. 8)109, 111). The loading of such aerogel structures still has to be demonstrated with micronutrients or nutraceuticals as research has so far focused on pharmaceutical actives.
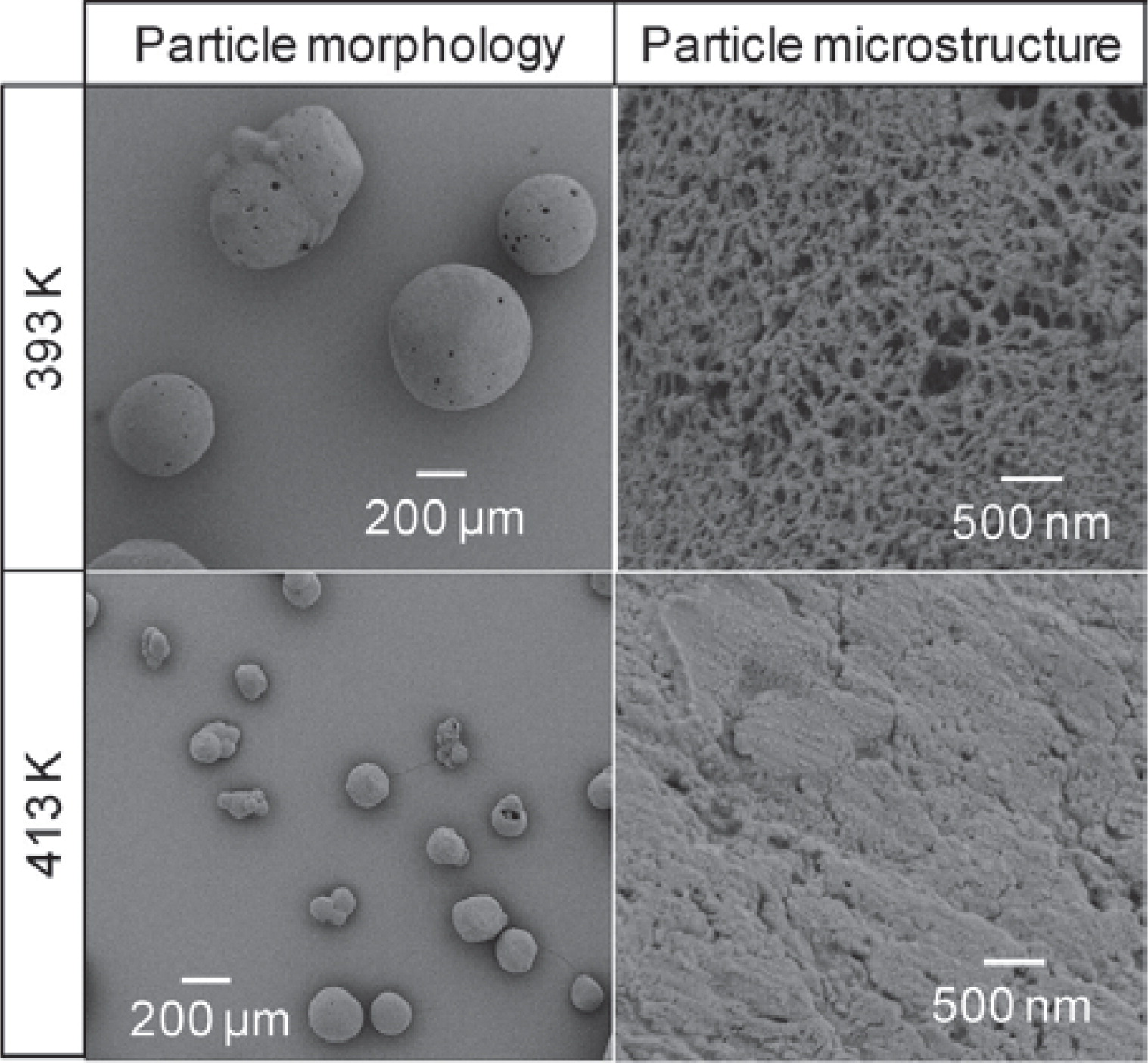
Effect of gelatinization temperature on the particle morphology and microstructure of starch aerogels109).
The extrusion process has mostly been used to encapsulate active compounds in a carbohydrate melt (melt extrusion)16). Most commonly, the carbohydrate melts are processed and mixed with active compounds in twin-screw extruders and are pressed downstream through a die to shape the product particles. Cutters can be mounted directly at the die to obtain pellets, or post-processing shaping equipment such as mills or spheronizers can be used12, 16). The particles produced by extrusion techniques are typically dense, spherical and 0.5 to 2 mm in size12). Most studies on melt extrusion have focused on the encapsulation of flavor or pharmaceutical compounds rather than micronutrients or nutraceuticals. Chang et al. encapsulated ascorbic acid in an amorphous maltodextrin matrix by extrusion113). Control of the water content was the most important process parameter to assure that the product extrudates had glass transition temperatures above room temperature113). Vitamin K1 and vitamin D3 have been encapsulated in matrices consisting of mannitol, sorbitol, glucono-lactone and maltodextrin by melt extrusion114). The encapsulation of oil in a starch matrix in the presence of low-molecular-weight emulsifiers by extrusion has also been demonstrated115). Li et al. developed a cold-forming extrusion process for the agglomeration and microencapsulation of ferrous fumarate in dough matrices116). The extruded particles were 300 – 700 μm in size and had a higher density, lower porosity and smoother surface texture compared to particles made by fluidized-bed agglomeration. It was possible to apply a coating to the extruded particles and they were then suitable for fortification of iodized salt117).
In the preceding sections, particle technologies for the synthesis and formulation of micronutrients and nutraceuticals have been briefly presented. A thorough characterization of the as-obtained micronutrient- or nutraceutical-loaded particles is essential, including physicochemical properties such as particle size, size distribution, morphology, density, porosity, surface charge, shell thickness, mechanical strength, glass transition temperature, degree of crystallinity, flowability and compressibility16). Luykx et al. recently published a comprehensive review on analytical methods including separation (e.g. chromatography), imaging (e.g. TEM, SEM, atomic force microscopy) and characterization (e.g. photo correlation spectroscopy, NMR spectroscopy, X-ray diffraction, small-angle X-ray scattering) techniques for nano delivery systems in food118). The particle’s physicochemical characteristics will in turn govern its interaction with other molecular species and determine the bulk physicochemical properties of the overall food system (e.g. appearance, texture and stability)119). The choice of technology, control and understanding of the resulting particle properties is therefore crucial to achieve the desired performance in target end-use applications; several examples have already been cited in the preceding paragraphs. Readers are also kindly referred to the reviews on colloidal delivery systems for micronutrients and nutraceuticals by Velikov and Pelan8) as well as on properties of biopolymer particles by McClements and coworkers119, 120).
Particle size is probably the most important property in the design of micronutrient or nutraceutical delivery systems. The recent trend has been a ‘down-sizing’ from micro- to nano-sized delivery systems25). Nanoparticles provide a greater surface area compared to micro-sized particles and can therefore increase solubility and bioavailability3, 8). This has been demonstrated, e.g. for flame-made Fe and Fe/Zn compounds43) and precipitated resveratrol74). However, although the primary particle size might be small, aggregation during post-processing steps such as freeze drying can reduce the effective surface area exposed to the dissolution medium and thereby reduce the dissolution rate74). The particle crystal structure also has an influence on solubility42, 74). It has been demonstrated that the surface area has a limited influence on the solubility of amorphous compounds (as it is mostly high already), while a high specific surface area is required to ensure a high solubility of crystalline compounds41).
Particle size also has a pronounced effect on optical properties. This is exploited in the development of carotenoids as natural colorants turning from yellow through orange to red by changing the particle size121). Particles ideally < 50 nm that do not scatter light are required in transparent, low-turbidity beverages enriched with nutraceuticals (Fig. 9)101, 103, 119). In contrast, large particles (> 500 nm) are used in opaque products. Alternatively, the particle refractive index or the concentration can also be modulated to alter the optical properties119). The particle composition itself can play a role as for example iron, which can be delivered in different molecular forms to avoid color and sensory changes when used to fortify food41). Small particle sizes (< 400 nm) are also favorable for product stability to avoid creaming or sedimentation in food applications8, 57). Particle shape and morphology affects flowability, miscibility with other food ingredients, appearance, rheology, mouth feel and release characteristics120).
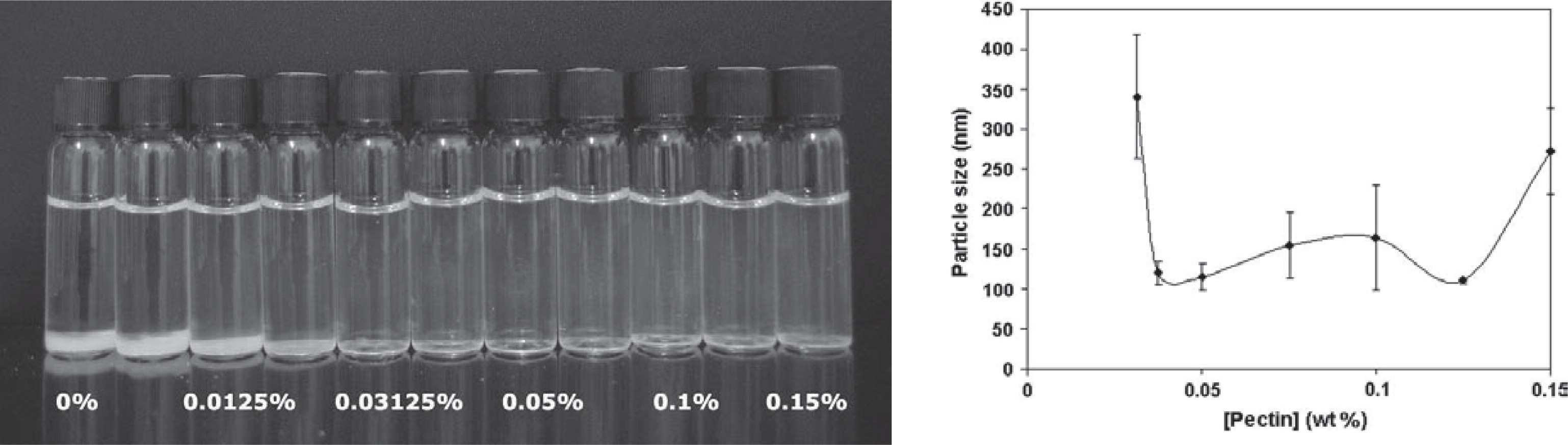
Colloidally stable nanocomplexes of DHA-loaded β-lactoglobulin and increasing concentration of low methoxyl pectin. A precipitate is visible in the first four vials while the dispersions in the other vials are transparent. The visual observation correlates well with the mean particle size of the complexes as a function of the pectin concentration. Transparent, stable solutions had average particle sizes of ∼100 nm103).
Probably one of the most challenging tasks in the formulation of micronutrients and nutraceuticals is to control the release rate while still assuring protection of the active compound during processing, transport and storage. In general, the release of an entrapped compound from a dense particle is slower than from a highly porous structure which also inversely impacts the stability of the active compound78, 120). Release of active ingredients from particles dispersed in a liquid medium is often measured to determine the release kinetics and effective diffusion coefficients. These will depend on, e.g. diffusion of the active compound through the matrix, the size and morphology of the particles, composition, transfer from the matrix to the environment and degradation or dissolution of the matrix material16). Four release mechanisms are typically distinguished for the dissolution of a particle: diffusion, erosion, fragmentation and swelling/ shrinking119). The difference in lutein release profiles shown for SLN and NLC in Fig. 10 can be related to the particle matrix composition and internal structure. This on the other hand can also be used as means to improve the stability of active compounds, e.g. as in the case of compounds entrapped in extruded hydrophilic glassy matrices that provide an almost impermeable barrier against oxygen11, 113, 122) .
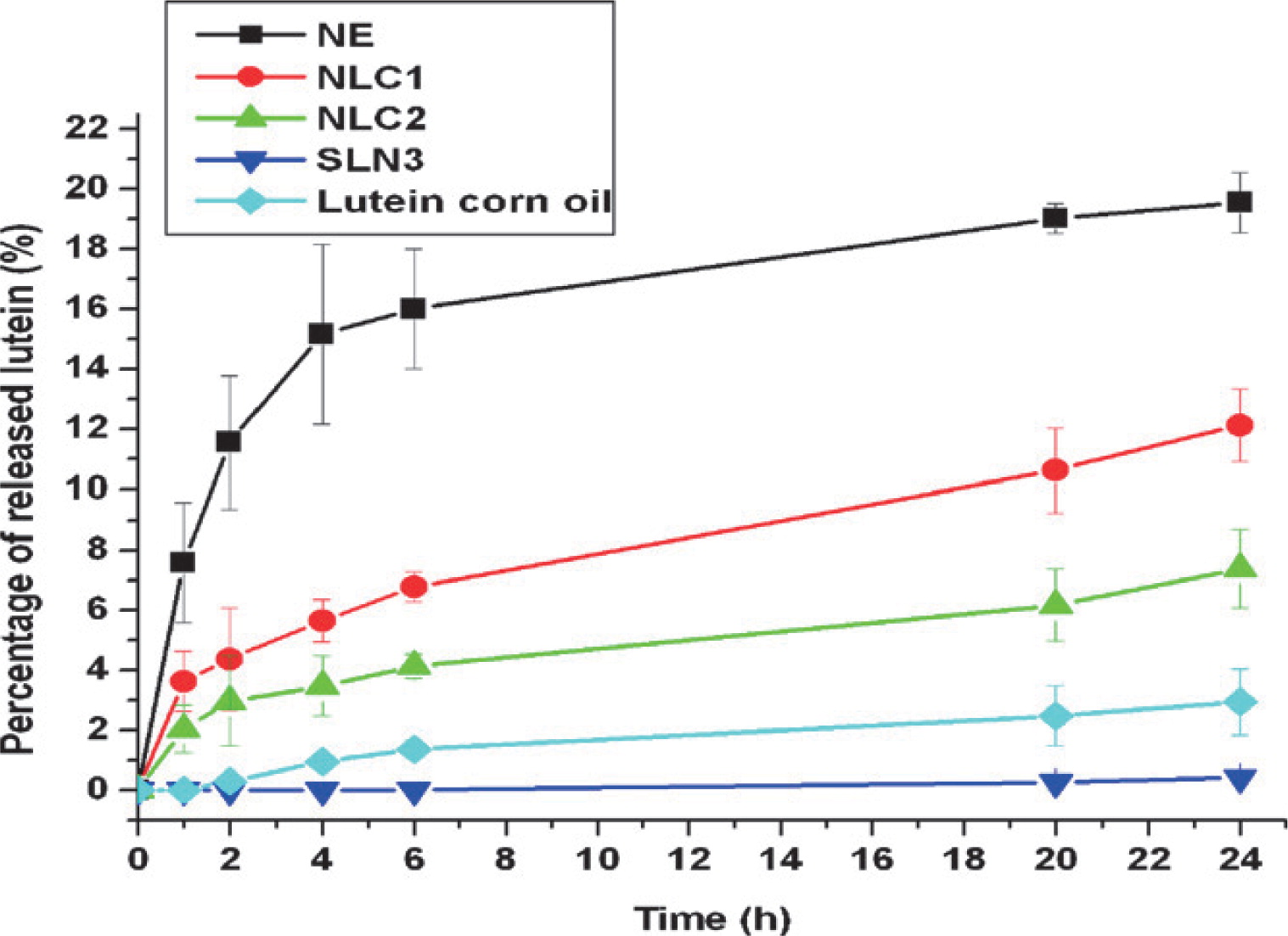
Release profiles of lutein at pH 5.5 from lipid nano delivery systems. NE: nanoemulsion, NLC: nanostructured lipid carriers 1 with glyceryl tripalmitate and 2 with carnauba wax, SLN3: solid lipid nanoparticles with carnauba wax. The release profiles depend on the lipid matrix compositions, active load and mode of incorporation in the matrix and surfactant type54).
One hundred years of vitamins – the “history of vitamins” has still not come to an end! Triggered by new technologies and building on new science, a renaissance in vitamin research started. We see a constantly increasing number of publications and studies which open up new and improved applications. We learn about nutrient-gene interactions and with the discovery of polymorphism, specific requirements for vitamins are identified. In addition we still have to assure that everyone all over the globe has access to vitamins according to recommendations. This can only be achieved by industrial production providing vitamins in the desired quality and bioavailability delivered via product forms tailored to the targeted application (e.g. acceptable sensory characteristics, storage stability, ease of handling).
Table 3 summarizes the synthesis and formulation technologies for the dry powder delivery systems of micronutrients and nutraceuticals described in this review. A broad choice of technologies exist covering particles from nano- through micro- to macro-sizes with morphologies ranging from core-shell to matrix-type. The industrially most relevant particle formation technology is spray drying. The influence of process parameters on the resulting particle characteristics such as size and morphology is quite well understood. Nevertheless, only a few studies discuss the importance of particle shape for processing in the food industry, e.g. the influence of surface irregularities and particle morphology on flowability and coating123). Novel techniques can quantify and classify the particle shape (e.g. elongation, circularity, compactness and convexity) with advanced image analysis techniques and software algorithms124). Novel developments are mostly made in the field of nanotechnology, as the nanostructures can provide unprecedented properties. However, the scalability of these upcoming nanotechnologies still has to be proven in many cases. Another challenge is the compatibility of some technologies with legislatively approved, food-grade raw materials without the use of solvents during processing. Furthermore, technologies developed in other industries, e.g. the pharmaceutical industry, might be too costly for processing food ingredients and need to be adapted to reduce cost.
The thorough characterization of particulate delivery systems described in this review is of great importance to understand and design their behavior in end-use applications. Physical, mechanical, structural and mass transfer properties need to be considered16). Often a compromise has to be made to ensure the protection of the active compound during processing and storage while still controlling the release at the desired location and time. Analysis of the systems is often complicated by their multicomponent nature including bio-polymers whose properties might even vary from batch to batch. A range of powerful characterization techniques are nowadays available for the delivery systems of micronutrients and nutraceuticals. For example, the mechanical stability of microcapsules can be studied with a combination of fluorescence and atomic force microscopy to help understand the release behavior and deformation properties125, 126). The observed mechanical properties have to be explained by and related to the structural properties on a molecular level inside the microcapsules. Current research therefore tries to identify the complex relationship between these properties by advanced analysis methods often combining several techniques and modeling. Innovative methods that are in development include, e.g. moisture sorption isotherms127), positron annihilation lifetime spectroscopy to determine free volume21, 128), coupled with exact measurements of the glass transition temperature of the product as a function of water activity and composition.
Consumer awareness towards a healthy and well-balanced diet and therefore the demand for functional, high-quality food products and nutritional supplements steadily increases. Formulation scientists in industry and academia will therefore continue to enhance the delivery systems of micronutrients and nutraceuticals to provide the best performance and quality for the costumers’ needs.
Alexandra Teleki
Alexandra Teleki received her Master of Science in chemical engineering from the Royal Institute of Technology, Stockholm (Sweden) in 2003 and her Ph.D. from the Department of Mechanical and Process Engineering at ETH Zurich (Switzerland) in 2008. She worked as a lecturer and research associate at the particle technology laboratory at ETH before she took up her current position as a scientist at DSM Nutritional Products in Basel (Switzerland). Her research interests are formulation and microencapsulation technologies of micronutrients and nutraceuticals for application in foods or nutritional supplements.
Andrea Hitzfeld
Andrea Hitzfeld received her diploma degree (Dipl. Ing.) in food technology from the Technical University of Munich (Germany) in 2005. She is currently working at the research and development center of DSM Nutritional Products in Basel (Switzerland) as an associate scientist in the research area of vitamin formulation.
Manfred Eggersdorfer
Manfred Eggersdorfer studied chemistry at the Technical University Munich and did his PhD in organic chemistry in the field of the synthesis and characterization of unusual amino acids. He was post-doc at Stanford University, California, working with Carl Djerassi on the isolation and characterization of sterols of marine origin. He joined Roche in 1999 as head of R+D Vitamins and Fine Chemicals which was acquired by DSM. Prior to Roche, Manfred Eggersdorfer was working for BASF, Ludwigshafen, in different positions including head of research and development fine chemicals. Manfred Eggersdorfer is active as a member of the advisory board of the John Hopkins Bloomberg School of Public Health, of the strategy board of the Institute of Food Science University Hamburg, and has published many papers in the field of nutrition.