2013 年 30 巻 p. 181-192
2013 年 30 巻 p. 181-192
First we survey the scope and level of research activities in nanoparticle technology (NPT) in ASEAN by looking at relevant international journal publications (2006–2012) in terms of absolute numbers (AN), numbers of publications per million population (PMP) and per billion USD GDP (PBG). Obviously, the statistics show that, within ASEAN, the strength of Singapore covers a wide range of nanotechnology fields and applications, including carbon materials, biosensors, bioelectronics, and pharmaceutics. Malaysia places emphasis on alloys & compounds, carbon materials, and separation technology. Thailand’s emphasis is on molecular modeling, carbon materials, and biosensors. When classification is made on the morphology of nanoparticles mentioned in the titles, the topmost are nanotubes (including CNT, SWCNT, MWCNT) at 392 out of 612 (64.1%), followed by generic nanoparticle/nanopowder and nanofiber (including nanowire, nanorods). Next we look closer at 2 nanoparticle R&D organizations in Thailand, namely, CEPT (CU) and NANOTEC together with their alliances. The interest in nanotechnology of SCG (Siam Cement Group), a leading Thai industrial conglomerate, will also be briefly introduced. .
Being a subset of nanomaterials, nanoparticles or nanopowders here include nanospheres, nanotubes, nanofibers, nanorods, nanohorns, nano-onions, nanocapsules, nanoclusters, nanocrystals, and the like. Ultrafine particles or nanoparticles are generally sized between 1 and 100 nanometers. Nanoclusters have at least one dimension between 1 and 10 nanometers and a narrow size distribution. Nanopowders are agglomerates of nanoparticles or nanoclusters. Nanoparticles can be produced either by the top-down or bottom-up method. The former employs size reduction technique such as ball mill, planetary ball mill, etc. The latter employs laser ablation, CVD, arc discharge, plasma, sol-gel, spray pyrolysis, etc. Nanoparticle research is currently an area of intense scientific interest due to a wide variety of potential applications in nearly all industrial fields, including biomedical, optical and electronic.
ASAEN is currently composed of ten members (in alphabetical order): Brunei (B), Cambodia (C), Indonesia (I), Laos (L), Malaysia (M), Myanmar (MY), Philippines (P), Singapore (S), Thailand (T), and Vietnam (V). The top 3 most populous are Indonesia (233 million), Philippines (93.6M), and Vietnam (88.4M) whilst the 3 least populous are Brunei (0.41M), Singapore (5.14M), and Laos (6.44M). In terms of GDP per capita, the top 3 are Singapore (USD 43.3K), Brunei (USD 26.4K) and Malaysia (USD 8.52K) whilst the bottom 3 are Myanmar (USD 0.50K), Cambodia (0.80K) and Laos (USD 1.16K).
Table 1 lists the cumulative number of international journal publications (in decreasing order), 2010 GDP, Population, GDP per capita and relevant publication statistics of each ASEAN country.
| Number of Publications (AN) | Population (million) | GDP (billion USD) | GDP per captia (USD) | Publications per million population (PMP) | Publications per billion USD GDP(PBG) | |
| Singapore | 351 | 5.14 | 222.7 | 43,324 | 68.288 | 1.5761 |
| Malaysia | 113 | 27.91 | 237.8 | 8,519 | 4.049 | 0.4752 |
| Thailand | 97 | 68.14 | 318.8 | 4,679 | 1.424 | 0.3043 |
| Vietnam | 39 | 88.36 | 103.6 | 1,172 | 0.441 | 0.3764 |
| Indonesia | 7 | 232.52 | 706.6 | 3,039 | 0.030 | 0.0099 |
| Philippines | 4 | 93.62 | 199.6 | 2,132 | 0.043 | 0.0200 |
| Brunei | 1 | 0.41 | 10.7 | 26,367 | 2.439 | 0.0935 |
| Cambodia | 0 | 14.14 | 11.3 | 802 | 0 | 0 |
| Laos | 0 | 6.44 | 7.5 | 1,164 | 0 | 0 |
| Myanmar | 0 | 50.50 | 25.0 | 495 | 0 | 0 |
| Japan | 1,435 | 127.38 | 5458.8 | 42,854 | 11.266 | 0.2629 |
Source: Number of Publications (NP) from Science Direct (2006–Mar2012)
GDP and Population data from World Development Indicators database of World Bank (2010 except Brunei, Myanmar 2009)
The present article first surveys the scope and level of research activities in nanoparticle technology (NPT) in ASEAN and the major fields of national focuses. Next it looks closer at NPT R&D activities in Thailand as well as the interest in nanotechnology of a leading Thai industrial conglomerate, the Siam Cement Group. Specific reviews are given on recent researches carried out in the Center of Excellence in Particle Technology (CEPT) of Chulalongkorn Univ. (CU), the National Nanotechnology Center (NANOTEC), and their alliances.
A comprehensive literature search was carried out in March 2012 to look for international journal articles with the keyword Nanoparticle or Nanopowder in their titles and with one of the authors’ affiliations located in an ASEAN country from Year 2006 onward using the ScienceDirect database. As expected, Singapore shows the highest number of articles (351) followed by Malaysia at 113, Thailand 97, Vietnam 39, Indonesia 7, Philippines 4, Brunei 1, Cambodia = Laos = Myanmar = 0, respectively. Since the ASEAN total is 612, Singapore alone is associated with 57.4% of the total with Malaysia’s and Thailand’s shares being 18.5% and 15.8%, respectively. Thus the top three countries are associated with nearly 92% of the whole ASEAN. Admittedly, the above search statistics could have a few double or triple counts when the authors’ affiliations of a single article belong to more than one ASEAN country. In any case, the statistics here reflect only the quantity, and not quality or citation impact, of the publications. In the meantime, nanostructural materials (including nanoporous) and ultrathin films (nanofilms, nanocoat) generally are not counted. As shown in Table 1, in terms of absolute publication numbers (AN), the numbers of publications per one-million population (PMP) and per 1-billion-USD GDP (PBG), the statistics show Singapore to be on top of all 3 categories, whilst Malaysia ranks 2nd in all 3 categories. Thailand, Brunei and Vietnam, respectively, rank 3rd in terms of AN, PMP, and PMG. For comparison, Table 1 shows that, having 1,435 publications, Japan is ahead of Singapore only in terms of AN but ranks 2nd after Singapore in term of PMP and 5th after Thailand in term of PBG.
The top 10 journals in terms of number of publications in the entire ASEAN are:
Singapore leads in all 10 journals above except J of Alloys and Compounds, which is led by Malaysia. Obviously, these statistics confirm that, within ASEAN, the strength of Singapore cover a wide range of nanotechnology and applications, including carbon materials, biosensors, bioelectronics, actuators, hydrogen energy, alloys and pharmaceutics. Malaysia places emphasis on alloys & compounds, carbon materials, applied surface science, and separation technology. Thailand’s emphasis is on molecular modeling, carbon materials, and biosensors. When classification is made on the morphology of nanoparticles mentioned in the titles, as shown in Table 2 the topmost are nanotubes (including CNT, SWCNT, MWCNT) at 392 out of 612 (64.1% of 612) followed by generic nanoparticle/nanopowder at 94 (15.4%) and nanofibers (including nanowire, nanorod) at 87 (14.2%). In short, the top 3 morphologies cover 93.7% with nanoporous being a distant 4th at 21 (3.43%) and fullerene/buckyballs 5th at 11 (1.80%). As expected, nanotubes especially CNT are the most popular morphology for R&D and applications.
| Singapore | Thailand | Malaysia | Vietnam | Indonesia | Philippines | Cambodia | Laos | Myanmar | Brunei | SUM | Japan | |
| nanotube, CNT, SWCNT, MWCNT | 222 | 53 | 88 | 24 | 2 | 3 | 0 | 0 | 0 | 0 | 392 | 833 |
| nanfiber, nanowire, nanorod | 65 | 7 | 10 | 4 | 1 | 0 | 0 | 0 | 0 | 0 | 87 | 130 |
| nanoparticle, nanopowder | 38 | 29 | 10 | 11 | 4 | 1 | 0 | 0 | 0 | 1 | 94 | 217 |
| nanoporous | 14 | 4 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 21 | 81 |
| fullerene, buckyball, Buckminsterfullerene | 10 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 11 | 151 |
| nanoencapsulation, nanocapsule | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 5 |
| nanocatalyst | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 |
| nanogold | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 4 |
| nanosilver | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| nanohorn, nanoonion, nanowhisker | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 12 |
| total | 351 | 97 | 113 | 39 | 7 | 4 | 0 | 0 | 0 | 1 | 612 | 1,435 |
Source: Science Direct (2006–Mar 2012)
Because of space limitation, the authors’ personal background and Thailand’s lower profile compared to Singapore and Malaysia, the present article will look closer at 2 nanoparticle R&D organizations in Thailand, namely, CEPT (CU) and NANOTEC together with their alliances. The interest in nanotechnology of SCG (Siam Cement Group), a leading Thai industrial conglomerate, will also be briefly introduced.
Designated a center of excellence by Chulalongkorn University, CEPT (Center of Excellence in Particle Technology) is committed to generating academic and technical outputs in various categories including young generation of high-caliber graduates, research papers, patents and technical services. The present focus of research activities is set on developing and improving alternatives for synthesis and applications of particulate materials and their derivatives in nano-scaled forms. More specifically, synthesis of selected metals and their compounds, such as TiO2, ZnO, Al2O3, ZnS and WO3, have been investigated using several alternative methods such as gas-phase reaction, solvothermal treatment, sol-gel system and micro-emulsion (Sahu et al. 2011). Similarly, synthesis of carbonaceous materials and their derivatives, including mesoporous and metal-hybridized nanocarbon are carried out using several methods, such as arc-discharge, pyrolysis, solvothermal and RF-gel formulation (Sano et al. 2009, Charinpanitkul et al. 2009a, 2009b, 2009c). In terms of applications, ongoing works on photocatalysis, solar concentrator, gas sensing, and pollution removal are conducted in collaboration with alliances in domestic industries and Thai or foreign institutions.
In response to public interest in sustainability, development and regeneration of activated carbon from industrial waste, and production of glucose and its derivatives from agricultural wastes have been explored using specific techniques such as carbonization with pre-treatment, hydrothermal treatment and supercritical fluid process (Petchpradab et al. 2009, Charinpanitkul and Tanthapanichakoon 2011a, Yoshikawa et al. 2007). Innovative ideas on economical systems for particulate collection using spray nozzle and packed bed have been proposed and verified experimentally using actual conditions (Srinives et al. 2010, Charinpanitkul and Tanthapanichakoon 2011b). Progresses of representative works are elaborated as follows.
3.1.1 Nano-scaled metal particles and their derivativesResearch work on nanoscaled metal particles has been focused on synthesis and applications of specific metal oxides and their derivatives, i.e. TiO2, ZnO, Al2O3, WO3 Cu2O and ZnS. In CEPT, two alternatives involving either gas-phase or wet-chemistry synthesis are examined for making photocatalyst and sensors (Numpud et al, 2008, Klanwan et al. 2010, Charinpanitkul et al. 2011, Charnhattakorn et al. 2011). Viriya-empikul et al. (2008) reported that titanate nanotubes transformed from TiO2 nanoparticles would possess higher surface area and short tube-length. Morphology change of the titanates from nanosheet, nanotube and nanowire was affected by an increase in the reaction temperature. The surface area of the samples corresponded well with the amount of hollow titanate nanotube and non-hollow nanowire, whilst the length of titanate nanotube became longer when ultrasonication pretreatment was employed.
For applications, TiO2, Ni, Co and Fe impregnated on activated carbon were prepared for phenol removal from industrial waste water using three-phase fluidized bed. It was found that the use of Co nanocatalyst with the presence of O3 led to the best removal condition in which phenol was completely decomposed within 10 min (pseudo-1st order rate constant k = 0.1944 min−1). In contrast, the use of TiO2 without O3 resulted in the least decomposition of phenol (k = 0.0066 min−1) (Mungmart et al. 2011). Contributions of adsorption on the porous supporting materials, carbon gel or silica gel, were also examined and deducted from those of the metal catalysts. As a typical example, uniform dispersion of metal nanoparticles in the porous carbon gel is illustrated in Fig. 1. At present works are proceeding on new types of hybridized materials which incorporate metal-compound nanoparticles into materials such as carbon in order to gain synergetic effect of their characteristics for applications to chemical sensing, electron-transferring and catalysis.
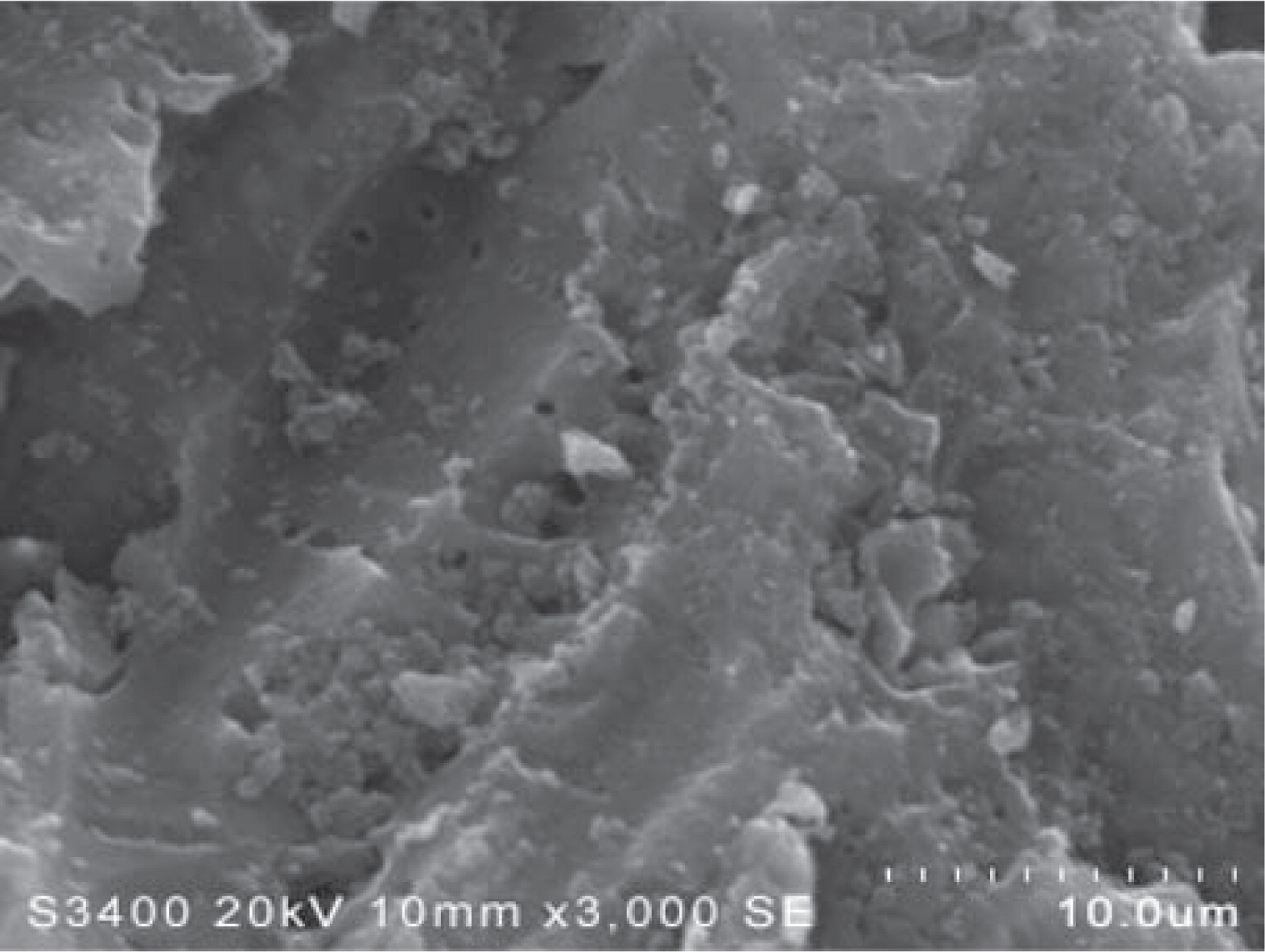
Typical SEM micrograph of Fe2O3 on the surface of carbon gel particle.
Nanocarbonaceous materials are potential candidates for numerous applications, such as drug delivery, electron capacitor or molecular separation. Synthesis of multi-walled carbon nanotubes (MWCNTs) and multi-shelled carbon nanocapsules (MSCNCs) has been examined in a simple system of pyrolyzing reactor (Charinpanitkul et al. 2009b). It has been proved that this pyrolysis method could provide a new type of carbon nanoparticles with magnetism due to the presence of iron nanoparticles encapsulated between graphene layers. Schematic diagram for describing the formation of those nanoparticles is shown in Fig. 2. Recently, a new method to prepare single-walled carbon nanohorns (SWCNHs), incorporated with Pd or Pt nanoparticles via simple gas-injection arc-in-water, was proposed (Poonjarernsilp et al. 2011). They reported that the size of Pd nanoparticles in a range of 3–6 nm could be controlled by using different sizes of Pd wire inserted into the carbon anode. According to thermogravimetric analyses, the weight fraction of Pd nanoparticles in SWCNHs could be increased by increasing the Pd wire diameter whilst the yield of Pd nanoparticles decreased. SWCNHs hybridized with dispersed Pd nanoparticles exhibited strong anti-oxidation resistance with a highly graphitic structure. In the meantime, a simple strategy of matching the electrode materials employed for arc discharge in water or liquid nitrogen could also provide hybrid materials of multi-walled carbon nanotubes and Fe or Cu nanoparticles (Charinpanitkul et al. 2009c). These hybrid materials could have potential applications to dye-sensitized solar cells that yield suitably high solar conversion efficiency. In addition, gas sensors made of composite of MWCNT and PMMA have been developed for detecting certain toxic hydrocarbon gases, such as toluene and hexane (Srisurichan et al. 2009). Ice-crystal templating of MWCNTs in macroporous foams by freeze drying was also prepared and tested as gas sensor (Thongprachan et al. 2008).
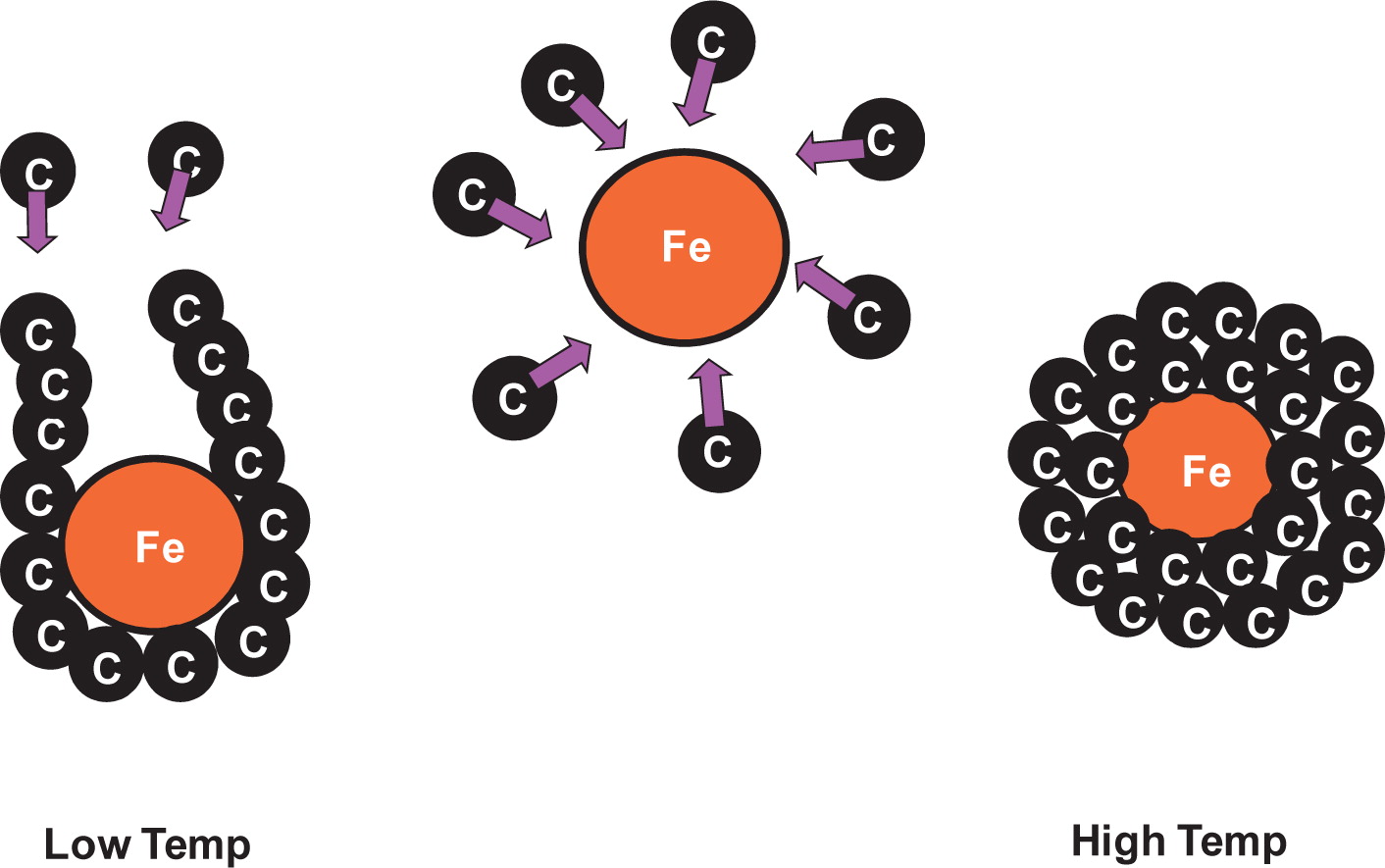
Schematic of formation mechanism of carbon nanoparticles induced by Fe particles via pyrolysis method.
Abundant in Thailand, biomaterials such as curcumin, menthol, and capsaicin as well as certain industrial and household wastes could be used and transformed by supercritical fluid or hydrothermal techniques to obtain nanostructure and other useful forms, such as glucose and their derivatives. For instance, poly(DL-lactic-glycolic acid) or PLGA, which can be employed for drug delivery applications, could be transformed into ultrathin film encapsulating fine particles via rapid expansion of supercritical CO2 (SCC) (Kongsombut et al. 2008, 2009). Thin film morphology could be controlled by the operating pressure and temperature of polymer dissolving in SCC. Interestingly, supercritical water could be employed for regenerating activated carbon saturated with phenol or pyridine, thereby demonstrating its usefulness to tackle environmental issues of waste reduction (Charinpanitkul and Tanthapanichakoon 2011a). Meanwhile, certain waste materials, such as naphthalene or glycerol, could be transformed by pyrolysis to provide carbon nanoparticles with controlled characteristics as mentioned earlier in section 3.1.2. An alternative to convert biomaterials, such as rubber wood or jatropha seed residue, into glucose or 5-HMF could be achieved by hydrothermal treatment in an autoclave (Petchpradab et al. 2009). Interestingly, solid residues of the hydrothermal process could be a potential source of nanoparticles containing amorphous carbonaceous networks.
3.2 Nanoparticle R&D Activities in NANOTEC and its AlliancesThe trends of nanoparticle production of NANOTEC and its alliances have moved toward a facile system which incorporates multicomponents into hybrid or composite nanoparticles with the aim of enhancing performance in specific applications. Metal/metal oxide nanoparticles have been applied to energy and petrochemical, while photocatalysts, metal-doped TiO2 and ZnO in particular, have extensively been used in textile and environmental fields. The fabrication of composites based on carbon nanotubes shows good performance as electrochemical devices. In addition, organic-based nanoparticles, including chitosan-, gelatin-, and nisin-bonded polymers have been developed for (bio)medical, electronic and food industry.
3.2.1 Metal and metal oxide nanoparticlesNanoparticles of metal and metal oxides have played a crucial role in various industries such as petrochemical, energy, electronic, and environmental industries. Metal nanoparticles could generally be obtained through the reduction of metal-containing compounds with reducing agents in either gas or liquid phase. Gold nanoparticles provide novel optical and electronic properties while their Ag counterparts are widely employed in a variety of products which require anti-bacterial activity. Metal oxides are extensively used as catalyst supports for metal nanoparticles. In addition, the synthesis and application of photocatalysts, especially ZnO and TiO2 as well as its modified forms, have actively been studied in recently years to push them toward final products in the market.
The flame spray pyrolysis (FSP) has been generally employed for synthesis of various oxides including alumina and zirconia for catalytic reaction in petro-chemical processes. More recently, FSP is developed as one-step synthesis of supported metal catalysts. The flame-made Pt/Al2O3 showed an improved turnover frequency in the hydrogenation of ethyl pyruvate compared to conventional porous catalysts. Recent studies show that Pd/SiO2 synthesized by FSP method exhibited higher hydrogenation activity in the liquid-phase selective hydrogenation of 1-heptyne compared to the ones prepared by conventional impregnation (Mekasuwandumrong et al. 2009). Al2O3-supported Pt-based bi- and tri-metallic catalysts were developed for dehydrogenation of propane to propylene (Pisduangdaw et al. 2009, 2011). Stronger metal–support interaction in the flame-made nanoparticles is found to produce highly beneficial effects in such reactions. A typical TEM image of the flame-made nanoparticles is shown in Fig. 3. Moreover, the FSP technique has been used for single-step production of unloaded ZnO and loaded Nb/ZnO nanoparticles (Kruefu et al. 2011). FSP yielded small Nb particles attached to the surface of the supporting ZnO nanoparticles, while they showed a high potential for use as NO2 sensing material.

Typical TEM image of nanoparticles of Pt/Al2O3 prepared by flame spray pyrolysis technique.
One novel approach to disperse metal nanoparticles on oxide supports has been proposed as crystalline phase separation of parent complex oxides during reduction process (Faungnawakij et al. 2009, Shimoda et al. 2010). It has been demonstrated that Cu nanoparticles can be formed on the iron oxide (Fe3O4) host from original copper-iron spinel oxide (CuFe2O4). The size of the copper nanoparticles depends strongly on the reduction temperature, time and reducing species. Beyond that, the reconstruction of the parent spinel phase can be achieved via solid state formation by oxidation at high temperature. This feature leads to ultimate recycle/reuse of the catalyst nanomaterials.
Photocatalysts based on TiO2 and ZnO have extensively been studied in various applications. Metal doping of ZnO and TiO2 is a common method to enhance their visible light absorption by narrowing the bandgap energy. It was demonstrated that manganese-doped ZnO photocatalysts synthesized via wet-chemical technique could create tail states within the band gap of ZnO (Ullah and Dutta 2008). The doped photocatalysts can effectively degrade organic contaminants with just visible light irradiation. In addition, solvothermal technique has been employed to synthesize zinc oxide nanoparticles and nanorods (Yiamsawas et al. 2011). It was found that the concentration ratio of sodium hydroxide to zinc acetate has the greatest effect on ZnO nanocrystal growth. Since TiO2 is well known as an active photocatalyst, the synthesis of Fe(III)-doped TiO2 by solvothermal method has been done in the presence of isopropyl alcohol using PEG as a template (Wantala et al. 2010). Red shift phenomenon due to substitution of Fe(III) in the TiO2 crystal lattices was observed, with the improved activity under visible light irradiation. Because a major concern of the use of nanoparticles catalysts in liquid phase processing is catalyst separation from the reaction system, Kangwansupamonkon et al. (2010) have proposed the concept of TiO2-polymer composite for photocatalytic water treatment. TiO2/poly[acrylamide-co-(acrylic acid)] composite hydrogel was synthesized by polymerization of TiO2-containing monomer solution. The composite hydrogel is a good dye adsorbent with self-photodegradability and it can also be easily separated from treated water by simple filtration.
The potential use of nanosilver-decorated titanium dioxide nanofibers for toxin decomposition with antimicrobial and self-cleaning features was recently proposed and investigated (Srisitthiratkul et al. 2011). The nanofibers were prepared through sol–gel reaction followed by an electrospinning process. Following the Japan Industrial Standard protocol, decompositions of nitrogen oxides (NOx) and volatile organic compound (VOC) by the TiO2 nanofibers suggested that they were capable of air treatment. To further enhance their anti-microbial activity, silver nanoparticles were decorated onto the TiO2 nanofibers via photoreduction of silver ion in a suspension of the nanofibers. Nanostructure of the Ag-TiO2 fibers was reported and the possibility of using these hybrid nanofibers in environmental and hygienic nanofiltration was proposed, in which the self-cleaning characteristics was expected to facilitate maintenance work.
3.2.2 Carbon nanotubesSawatsuk et al. (2009) have developed dye-sensitized solar cells based on TiO2–MWCNTs composite electrodes. The incorporation of MWCNTs into a TiO2 active layer contributes to a significant improvement in the energy conversion efficiency of dye-sensitized solar cells. The improvement is correlated with not only increased photocurrent and electrical double layer capacitance but also decreased electrolyte-electrode interfacial resistance and Warburg impedance. A unidirectional-freezing technique for preparation of freeze-dried solid foams of carbon nanotubes has been proposed and the materials were applied to gas diffusion layers of a proton exchange membrane fuel cell (Nakagawa et al. 2011a). Freeze-dried macroporous solid foams were prepared from aqueous suspensions of MWCNTs dispersed with chitosan. It was suggested that the cell performance was closely linked to the interconnected carbon networks formed during the freezing step.
Wisitsoraat et al. (2010) have developed a flow-injection microfluidic device with electrochemical sensor based on functionalized carbon nanotubes for fast cholesterol detection. CNTs working electrode, silver reference and platinum counter electrode layers were fabricated on the chip by sputtering and low-temperature chemical vapor deposition methods. The proposed system is promising for clinical diagnosis of cholesterol with high-speed real-time detection capability, very low sample consumption, high sensitivity, low interference and good stability.
3.2.3 Polymer-based nanoparticlesPrombutara et al. (2012) have produced nisin-loaded solid lipid nanoparticles by high-pressure homogenization at 1500 bars for sustained antimicrobial activity. Nisin is a natural antimicrobial agent that is used as a preservative in heat processed and low pH foods. The antibacterial activity of nisin-loaded solid lipid nanoparticles against Listeria monocytogenes DMST 2871 and Lactobacillus plantarum TISTR 850 was evident for up to 20 and 15 days, respectively, compared to only one and three days, respectively, for free nisin. More recently, a potential use of niosomes for encapsulation of nisin and EDTA nanoparticles was proposed as an alternative approach to nisin encapsulation as good as or better than liposomes (Kopermsub et al. 2011).
Nanoparticles of N,N,N-Trimethyl and chitosan have been developed for the delivery of monoclonal antibodies against hepatocellular carcinoma cells (Vongchan et al. 2011). The chitosan-based nanocomplexes appeared safe and could potentially enhance the half-life of added antibodies. In addition, the core–shell nanoparticles possessing poly(methyl methacrylate) core coated with chitosan (CS), polyethyleneimine (PEI), or chitosan-mixed-polyethyleneimine (CS/PEI) shell were developed using a synthesis method of the emulsifier-free emulsion polymerization triggered by a redox initiating system (Inphonlek et al. 2010). All nanoparticles were spherical in shape with uniform size distribution. The introduction of PEI to CS nanoparticles induced grafting efficiency and percentage comparable with neat CS nanoparticles while it brought about higher colloidal stability of the nanoparticles as evidenced by zeta-potential measurement. The nanoparticles exhibited a promising antibacterial activity against Staphylococcus aureus and Escherichia coli.
Nakagawa et al. (2011b) have studied a formation of unidirectionally frozen poly(epsilon-caprolactone) nanocapsules stabilized by gelatin. The prepared sample had different dispersion characteristics at different positions in the dried bulk sample, and this heterogeneity depended on the freezing protocol. The gel network formation would be advantageous for producing excellent nanocapsule dispersion characteristics after drying. Another synthesis technique of polymer-based nanoparticle is the use of the miniemulsion polymerization technique (Polpanich et al. 2011). With this technique, self-colored nanoparticles containing naphthalene-bisimide derivatives can be synthesized with uniform size distribution. The good colloidal stability of the self-colored nanoparticles has potential application in biomedical areas.
3.3 Industrial applications of particle technologyThe Siam Cement Group (SCG) is one of the largest industrial conglomerates in Thailand with leading market positions in each of its core businesses which include, though not limited to, Cement, Chemicals, Construction Materials and Paper. SCG Chemicals (SCGCh) is one of the five core business units of SCG which contributes the highest share to SCG’s consolidated revenues in 2011. What follow are some examples of nanoparticle technology applications in SCGCh.
The Research and Development Unit of SCGCh takes on the challenges of employing new technology in various steps of invention and improvement of capabilities. Raising the demand of commodity plastics through novel high-value applications means that new properties must be introduced to common polyolefins like polyethylene and polypropylene. A key development strategy is the application of nanotechnology, including nanoparticle technology. SCGCh has invested resources into research on the use of nanotechnology in various aspects, ranging from synthesis of nanoparticles to improvement of material performance. In recent years, SCGCh has delved into polymer nanocomposites development involving carbon nanomaterials, layered nano-silicates, and novel nano-nucleating agents, which result in improvements to the bulk polyethylene and polypropylene as well as the finished products. Apart from the rather obvious use of nanoparticles to modify polymer matrix, other areas of petrochemical industry can benefit from nanotechnology as well, such as nanoporous support for catalyst system and adsorption media.
SCGCh not only targets development of new products but also aims to build up knowledge in nanotechnology. Recently, SCGCh scientists with collaboration of Chulalongkorn University published a paper on polypropylene/multi-walled carbon nanotubes composites to tackle the issue of scaling up nanocomposite production in the real world. What was found is an interesting regime of operating window with highly predictive manner (Polrut et al. 2011).
Meanwhile, sensitive issues about safety in NPT handling as well as health and environmental impacts cannot be overlooked by industrial companies which would have to fill in the gaps of knowledge. In fact this has been the Achilles’ heel of nanotechnology implementation in SCGCh. To deal effectively with these key issues, SCGCh is actively working with regional and world-class universities as well as leading research institutes in pinning down the directions to proceed in the nanotechnology world and in developing new technology, new material system and applications. The aim is to launch nano-enabled products in the near future, thereby creating unique benefits for their customers and maintaining SCGCh’s leading position in the market.
Singapore is the undisputed leader of nanoparticle technology in ASEAN, followed by Malaysia and Thailand, respectively. Because of Thailand’s lower international profile compared to Singapore and Malaysia, the present article is focused at 2 nanoparticle R&D organizations in Thailand, namely, CEPT (CU) and NANOTEC together with their alliances. The interest in nanotechnology of SCG (Siam Cement Group), a leading Thai industrial conglomerate, has also been introduced.
The present focus of research activities of CEPT is set on developing and improving alternatives for synthesis and applications of particulate materials and their derivatives in nano-scaled forms. More specifically, using several alternative methods such as gas-phase reaction, solvothermal treatment, sol-gel system and micro-emulsion, syntheses of selected metals and their compounds such as TiO2, ZnO, Al2O3, ZnS and WO3 have been investigated. Similarly, synthesis of carbonaceous materials and their derivatives, including mesoporous and metal-hybridized nano-carbon was carried out using several methods, such as arc-discharge, pyrolysis, solvothermal and RF-gel formation. The trends of nanoparticle production of NANOTEC and its alliances have moved toward a facile system which incorporates multicomponents into hybrid or composite nanoparticles with the aim of enhancing performance in specific applications. Metal/metal oxide nanoparticles have been applied to energy and petrochemical, while photocatalysts, metal-doped TiO2 and ZnO in particular, have extensively been used in textile and environment fields. The fabrication of composites based on carbon nano-tubes shows good performance as electrochemical devices. In addition, organic-based nanoparticles, including chitosan-, gelatin-, and nisin-bonded polymers have been developed for (bio)medical, electronic and food industry. The Research and Development Unit of SCG Chemicals (SCGCh) takes on the challenges of employing new technology in various steps of invention and improvement of their capabilities. A key development strategy is the application of nanotechnology, including nanoparticle technology. In recent years, SCGCh has delved into polymer nanocomposites development involving carbon nanomaterials, layered nanosilicates, and novel nano-nucleating agents which result in improvements to the bulk PE and PP as well as the finished products.
Wiwut Tanthapanichakoon
Wiwut Tanthapanichakoon is concurrently a professor of chemical engineering (ChE), Graduate School of Science & Engineering, Tokyo Institute of Technology, an adjunct professor of Kyoto Univ. and Distinguished Scholar in Particle Technology, Chulalongkorn University. He received his B.Eng. (ChE) from Kyoto University and PhD (ChE) from University of Texas at Austin. In the recent past, he was Senior Research Advisor, SCG Chemicals Co., Ltd.; founding Executive Director, National Nanotechnology Center (NANOTEC); President, Technology Promotion Assoc. (Thailand-Japan); President, Thai Institute of Chemical Engineering and Applied Chemistry (TIChE); etc. His research interest covers (nano-) particle tech, aerosol engineering, process simulation, heat and mass transfer operations, and energy technology. Wiwut has received numerous research awards and honors: ASEAN Outstanding Engineering Achievements Award 2010; Japan Society for Promotion of Science (JSPS) Fellow; Senior Research Scholar/Team Research Award, Thailand Research Fund (TRF); S&T Award, Thailand Toray Science Foundation; Outstanding Faculty Award in S&T (Univ. Level), Chulalongkorn University. In recognition of his dedication, he was bestowed several Royal Decorations, the highest being the Knight Grand Cordon (Special Class) of the Most Exalted Order of the White Elephant in 2004.
Tawatchai Charinpanitkul
Tawatchai Charinpanitkul is an Associate Professor of Chemical Engineering in Faculty of Engineering, Deputy Director of Energy Research Institute (ERI) and Director of Center of Excellence in Particle Technology (CEPT) affiliated with Chulalongkorn University. He received his Bachelor in Chemical Engineering from Chulalongkorn University in 1986, and Master and Doctor in Chemical System Engineering from University of Tokyo in 1989 and 1992, respectively. His present research interest is focused on Synthesis and Application of Nanomaterials, in particular, Carbonaceous Nanoparticles and Zinc Nanostructure, and Biomass Technology. With his attempt in academic contributions, TCR has published 110 technical journal papers related to the abovementioned research interest. In recognition of his dedication to Chulalongkorn University, he was bestowed several Royal Decorations, the highest being the Knight Grand Cordon (Special Class) of the Most Exalted Order of the White Elephant.
Suracha Udomsak
Dr. Suracha Udomsak received his degree in Chemical Engineering from Chulalongkorn University in 1989. He briefly worked for SCG Building Materials Co., Ltd. before continuing his education in the United States. In 1995, he was awarded Ph.D. degree in Chemical Engineering from Texas A&M University. He re-joined SCG in the technical department of polyethylene and polypropylene production company in SCG Chemicals Co., Ltd. Since then, he had built his successful career in the corporation by holding managing director positions for 3 different subsidiary companies in SCG Chemicals Co., Ltd. Currently, he is the Head of Technology Business for SCG Chemicals Co., Ltd. and the managing director of Texplore Co., Ltd. He is taking the lead of building SCG Chemicals Co., Ltd. towards forefront of technological and innovative business.
Butra Boonliang
Dr. Butra Boonliang is currently working for SCG Chemicals Co. Ltd., as Technology Intelligence Manager. He obtained his degree in Materials Science and Technology and master degree in Science and Engineering of Materials in The University of Birmingham and was awarded Ph.D. in Manufacturing and Mechanical Engineering with a focus on Micro Engineering and Nanotechnology in 2007. He joined SCG Chemicals Co., Ltd. as a researcher in polymer nanocomposite group. He was then assigned to lead a product development team for fiber, PE wax and master-batch applications and moved to Technology Intelligence Department in 2011. His current responsibility are technical and commercial evaluation of SCG Chemicals’ intellectual properties and scouting for emerging technologies with an aim to expand company’s portfolio of products and network.
Kajornsak Faungnawakij
Dr. Kajornsak Faungnawakij is a senior researcher and lab-head of Nanomaterials for Energy and Catalysis Laboratory at National Nanotechnology Center, National Science and Technology Development Agency (Thailand). He received his B.Eng. (First-class honor) and D.Eng. in Chemical Engineering from Prince of Songkla University and Chulalongkorn University, respectively. He was a researcher at Japan Science and Technology Agency (JST), and a postdoctoral fellow at Kyoto University (Japan) during 2005–2007. Currently, he has actively worked in research fields of heterogeneous nanocatalysis and biofuels.