2013 年 30 巻 p. 193-200
2013 年 30 巻 p. 193-200
A procedure is described for synthesizing cerium fluorcarbonate. This synthetic bastnaesite was characterized through X-Ray diffraction, chemical analysis and thermogravimetric studies. The solubility product of Ce-bastnaesite was determined to be 10−10.1. The stability constant pK CeHCO32+ complex was experimentally determined to be 3.04 ± 0.21. Based on the thermodynamic data available in the literature, a speciation diagram has been prepared for the synthetic cerium bastnaesite.
Owing to their unique magnetic and optical properties, the world demand for rare-earths has been steadily increasing in the latter half of the twentieth century and sharply since the 1990s. The discovery of high-temperature superconductivity in mixed oxides involving rare-earths opened up yet another market avenue for them. A recent report prepared by the US Department of Energy highlights the critical role of rare-earth metals in the clean energy economy1). The need to exploit all available rare-earth resources is expected to grow rapidly. Rare-earths occur in nature mainly as salt minerals, most commonly as complex fluocarbonates and phosphates. Bastnaesite, a fluocarbonate of the cerium group of rare-earth metals. (RE)FCO3, was first discovered in 1818 at Bastnas, Sweden2). The mineral contains about 75 percent light rare-earth (RE) oxides distributed as follows: 50.0% cerium, 34.0% lanthanum, 11.0% neodymium, 4.0% praseodymium, 0.5 % samarium, 0.2% gadolinium, 0.1% europium, and 0.2% others. The theoretical composition of bastnaesite (CeFCO3 synthetic or (Ce, La)FCO3 natural) is 74.77% Ce2O3, 20.17% CO3, and 8.73% F. The elemental analysis of natural bastnaesite is generally close to this composition.
Major occurrences of bastnaesite have been reported in several locations, but by far the two largest deposits known are those at Mountain Pass in California and in Inner Mongolia and Kanshu Province, China. The largest single producer of rare-earths was the Molycorp operation at Mountain Pass3–5) until low-cost Chinese production caused it to be shut down in the late 1990s. Extensive efforts are underway to reopen the Mountain Pass mine since today 97 percent of the world’s production comes from China.
Being a relatively rare mineral, little data are available in the literature about the various physico-chemical properties of bastnaesite. To provide information about pure particles for study of this important source of rare-earth metals, this paper summarizes some of our results on the synthesis and characterization of pure bastnaesite.
Bastnaesite belongs to a family of rare-earth/alkaline-earth fluocarbonates (those of the calcium and barium varieties) and is similar in structure to calcium fluorcarbonate. In 1956, Donnay and Donnay6) established the following isostructural series of calcium fluocarbonates, with different ratios of CeFCO3 to CaCO3:
| Bastnaesite | CeFCO3 | 100% CeFCO3 |
| Parisite | 2CeFCO3 · CaCO3 | 2/3 CeFCO3 |
| Rontgenite | 3CeFCO3 · 2CaCO3 | 3/5 CeFCO3 |
| Synchisite | CeFCO3 · CaCO3 | 1/2 CeFCO3 |
Semenov7) has suggested that additional members of this series with even higher calcium contents might exist:
| 2CeFCO3 · CaCO3 | 2/5 CeFCO3 |
| 2CeFCO3 · 2CaCO3 | 1/3 CeFCO3 |
| Vaterite | 0 % CeFCO3 |
McConnel7) later showed that calcite and vaterite are isostructural, vaterite being a special trigonal polymorphous modification of CaCO3 that differs from calcite. The minerals of this group have a characteristic layer structure, in which the layers of Ce and F ions are parallel to the (0001) plane and alternate with the layer of Ca ions. Plane CO3 triangles are arranged between the Ce-F and Ca layers. Details of the crystal structures of these minerals are available in the excellent paper by Donnay and Donnay6).
The structural scheme of bastnaesite and related minerals described above was first predicted by Oftedal6) in 1931 and subsequently confirmed by the Donnays6). The structure of bastnaesite is trigonal with ao = 7.16A, co = 9.79A, with the co/ao ratio being 1.367. Cerium and fluorine ions alternate at the vertices of regular hexagons with carbonate groups interspersed between the Ce-F layers. There are six formula units of CeFCO3 per cell.
In our laboratory, we tried to separate some pure bastnaesite crystals from a sample of Mountain Pass ore through physical separation under ultra violet light5). However, this was done with only limited success and, therefore, for very precise characterization of this mineral, it was necessary to synthesize pure bastnaesite in the laboratory. Only one previous attempt in this direction was reported in the literature by Jansen et al.8), who were able to successfully synthesize a few milligrams of bastnaesite in the early 1950’s.
We modified the Jansen method in order to synthesize about one gram of bastnaesite in each run. The reaction involved in the synthesis is the following:
Cerium Bastnaesite:
| (1) |
Lanthanum Bastnaesite:
| (2) |
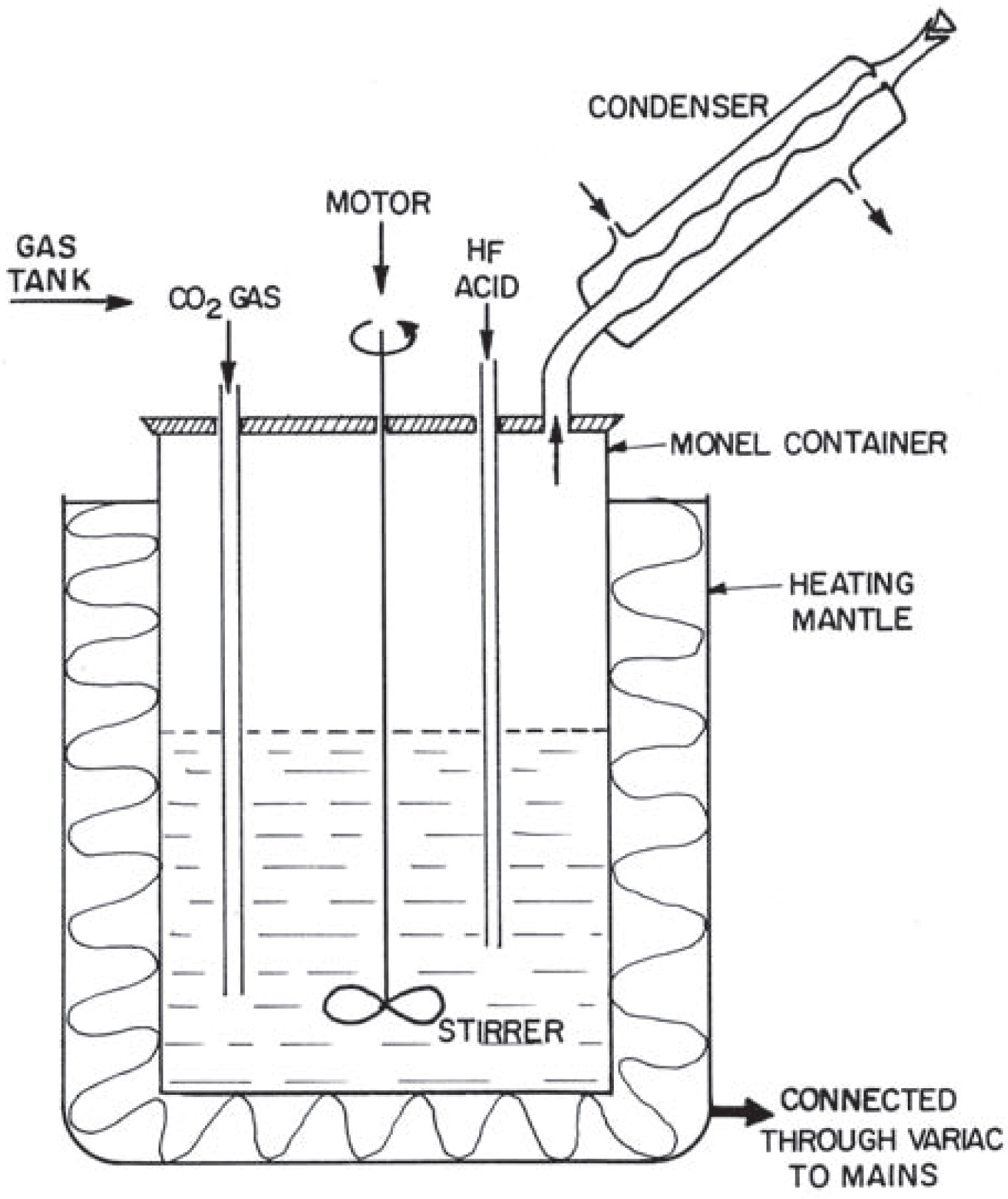
Schematic drawing of the apparatus used for the laboratory synthesis of bastnaesite in the laboratory.
The synthesis of LaFCO3 would essentially involve the same procedure. Bastnaesite of the composition as found in nature (having both La and Ce as major constituents) can also be synthesized starting with the stoichiometric amounts of respective carbonates and hydrofluoric acid as reactants.
It is also important to point out that the limitation of this method for producing larger quantities of bastnaesite is due to the limited capacity (l liter) of the container. Since the solid/liquid ratio was found to affect the purity of the sample, it is impossible to work at higher concentrations.
Four different batches were selected for analysis and identification purposes. The only differences among these is the amount of cerium carbonate per 700 cc used in the synthesis, batches A and B starting with 0.5753 g, and batch C with 0.4602 g of cerium carbonate. Batch D had several hours of ultrasonic dispersion of the cerium carbonate before the HF was added. The chemical analyses, which were carried out at the Mountain Pass Research Laboratory of Molycorp (Molybdenum Corporation of America) are summarized in Table 1. A comparison of the chemical analysis of the synthetic products with the theoretical composition of bastnaesite indicates that the synthetic product is close to pure bastnaesite.
| Sample | % Re2O3 | % Ce or RE | % F |
| Theoretical CeFCO3 | 74.9 | 63.95 | 8.67 |
| Synthetic CeFCO3 | |||
| Batch A | 70.2 | 59.9 | 9.3 |
| Batch B | 71.3 | 60.9 | 9.0 |
| Batch C | 69.96 | 59.7 | 8.6 |
| Batch D | 63.15 | 8.83 | |
| Natural (Molycorp) bastnaesite crystal (Ce,La)FCO3 | 69.17 | 59.04 | 6.88 |
| Hand picked* (under UV light) Birthday Claims, Mountain Pass sample (Ce,La)FCO3 | 60.9 | 51.98 | - |
Using the multi-point BET method, the specific surface area of the powder was measured by the adsorption of nitrogen at liquid nitrogen temperature. It was found to be 9.27 m2/g, giving an average diameter of the particles of 0.14 μm.
X-Ray Diffraction StudiesA Philips diffractometer was used to obtain the x-ray diffraction patterns for the various synthetic samples of bastnaesite. The beam was Cu Ka with a Ni filter having a wavelength of 1.5418 Å. Table 2 compares the values of the d-spacings reported in the literature with those obtained for the synthetic bastnaesite from this work. The values are quite consistent with those of the bastnaesite (CeFCO3) sample synthesized by Jansen et al.8) and those of the natural bastnaesite from Mountain Pass.
| Jansen’s Synthetic | Natural Bastnaesite | Synthetic CeFCO3 (this work) | |||||
| CeFCO3 | (Ce, La)FCO3 (Molycorp) | Batch A | Batch B | Batch C | Batch D | ||
| d, Å | I | d, Å | I | d, Å | d, Å | d, Å | d, Å |
| 4.96 | 70 | 4.88 | 4.874 | 4.874 | 4.874 | 4.901 | |
| 3.52 | 100 | 3.564 | 70 | 3.548 | 3.562 | 3.562 | 3.568 |
| 2.92 | 100 | 2.879 | 100 | 2.867 | 2.876 | 2.876 | 2.881 |
| 2.46 | 9 | 2.445 | 10 | 2.442 | 2.442 | 2.442 | 2.446 |
| 2.29 | 2 | 2.273 | 3 | - | - | - | - |
| 2.238 | 3 | - | - | - | - | ||
| 2.09 | 50 | 2.057 | 40 | 2.049 | 2.053 | 2.953 | 2.050 |
| 2.03 | 50 | 2.016 | 40 | 2.006 | 2.014 | 2.014 | 2.019 |
| 1.92 | 35 | 1.898 | 40 | 1.892 | 1.895 | 1.895 | 1.849 |
| 1.81 | 6 | 1.783 | 9 | 1.778 | 1.778 | 1.778 | 1.784 |
| 1.70 | 18 | 1.674 | 21 | 1.670 | 1.670 | 1.670 | 1.676 |
| 1.64 | 2 | 1.629 | 1 | - | - | - | - |
| 1.59 | 13 | 1.573 | 15 | 1.568 | 1.570 | 1.573 | 1.573 |
| 1.49 | 9 | 1.481 | 9 | 1.478 | 1.482 | 1.481 | 1.482 |
| 1.46 | 9 | 1.439 | 11 | 1.435 | 1.437 | 1.439 | 1.439 |
| 1.37 | 4 | 1.347 | 7 | 1.344 | 1.344 | 1.344 | 1.345 |
| 1.32 | 15 | 1.298 | 15 | 1.283 | 1.296 | 1.296 | 1.297 |
| 1.29 | 4 | 1.277 | 7 | 1.274 | 1.276 | 1.277 | - |
It is also apparent from the data that the methods employed for synthesis did indeed yield bastnaesite mineral particles. The intensities of the peaks (not shown here) obtained with the synthetic bastnaesite samples also compared quite well with the reported literature values.
Thermogravimetric Studies on BastnaesiteA Perkin-EImer TGS-2 Thermogravimetric System was used to obtain the weight loss as a function of the temperature of heating. The corresponding first derivative of this curve was also recorded. The heating rate was programmed to be 50 °C per minute through a temperature range of 30 to 900 °C. Since nitrogen was being flushed through the system during heating, the oxygen concentrations must have been minimal. The heating curve as well as its first derivative is plotted in Fig. 2a and b, respectively. When heated in a nitrogen atmosphere, cerium carbonate exhibits two plateaus with the final weight loss at 900 °C asymptotically approaching 30 %, whereas cerium fluocarbonates show an asymptotic weight loss of 20 % at 900 °C. This total weight loss can be understood in terms of the following reactions in a nitrogen atmosphere.

Thermogravimetric analysis results for cerium carbonate, synthetic bastnaesite and natural bastnaesite (top figure) and the respective differential curves (bottom figure).
| (3) |
| (4) |
| (5) |
Since the weight loss obtained for synthetic cerium fluocarbonate was very close to this value, the above reaction is very probable.
Note that here the final products of decomposition are the corresponding oxides and fluorides. In an oxygen environment, cerium can also be oxidized to its tetravalent state since CeO2 is the most stable oxide product. Then the reaction could be
| (6) |
| (7) |
The decomposition peak for bastnaesite from Mountain Pass was found to occur between 500 to 675°C. Vlasov7) reported a decomposition peak between 420 and 600 °C for a bastnaesite from Mongolia. X-ray analysis of its calcination product also showed a cubic phase with a = 5.555 Å which was in fact a solid solution of lanthanide sesquioxides CeLaO3.5.
This is due to the dissociation of the mineral involving the loss of CO2 and possibly F in an oxygenated environment. Our results also show a shift in the decomposition peak from 600 °C for natural bastnaesite to about 500 °C in the case of synthetic bastnaesite. With the limited work conducted on this system, it is difficult to explain this shift except to note that these two bastnaesites have different rare-earth compositions and that in the case of natural bastnaesite, small amounts of calcite and barite are also present. The presence of impurities apparently stabilizes the fluocarbonate such that decomposition is delayed by 100 °C. It is interesting to note that the final products are the same since the final weight losses are identical. Perhaps the enthalpies of the decomposition reactions in the two cases are different.
Similar to such minerals as apatite, barite and calcite, bastnaesite is a sparingly soluble salt mineral. The soluble species resulting from dissolution of the mineral form ion complexes in the solution as well as at the water-solid interface. Ionic complexes may participate in interfacial reactions and play a significant role in subsequent processing. The synthetic Ce-bastnaesite was therefore subjected to detailed investigation of its solution chemistry.
Thermodynamic CalculationsFor all minerals containing carbonate as lattice anions, the solubility and the corresponding solution behavior is primarily controlled by the partial pressure of CO2. For a system open to the atmosphere, the activity of carbonate species in water will be determined by the aqueous solubility of CO2 and the mineral. In addition, Ce-bastnaesite (CeFCO3) will contribute Ce3+ ions, which will undergo hydrolysis and form a series of hydroxo complexes in solution10–13). Similarly, fluoride ions can form such complexes as HF, HF2, CeF2+. Since fluoride and cerium ions in solution result from the dissolution of bastnaesite, their total concentration will be identical.
Based on reported data in the literature, the following charged species are expected to exist in aqueous solution in equilibrium with bastnaesite through the reactions listed in Table 3: Ce3+, HCO3−, CO32−, F−, CeOH2+, Ce(OH)2+, Ce(OH)4−, Ce3(OH)5−, HF2−, CeF2+, CeHCO32+. Except for the solubility product of CeFCO3, KSO, and the solubility constant of CeHCO32+, KCeHC, (which were determined experimentally in this work) all the values of equilibrium constants were taken from the published literature.
| Reference | |||
| [1] | CeFCO3 = Ce3+ + F− + CO32− | KSO = 10−16.1 | (this work) |
| [2] | HCO3− + H2O = H2CO3 + OH− | K1 = 10−7.65 | 15) |
| [3] | CO32− + H2O = HCO3− + OH− | K2 = 10−3.67 | 15) |
| [4] | CO2 + H2O = H2CO3 | KH = 10−1.47 | 15) |
| [5] | Ce3+ + OH− = CeOH2+ | K3 = 105.9 | 10,16) |
| [6] | Ce3+ + 2OH− = Ce(OH)2+ | K4 = 10−2.3 | 10,16) |
| [7] | Ce3+ + 3OH− = Ce(OH)3o[aq] | K5 = 10−12.0 | 10,16) |
| [8] | Ce3+ + 3OH− = Ce(OH)3[s] | K6 = 1019.7 | 17,18) |
| [9] | Ce(OH)3o[aq] = Ce(OH)3[s] | K7 = 1031.8 | * |
| [10] | Ce3+ + 4OH− = Ce(OH)4− | K8 = 10−24.0 | 10,16) |
| [11] | 3Ce3+ + 4OH− = Ce3(OH)54+ | K9 = 10−18.8 | 10,16) |
| [12] | Ce3+ + HCO3− = CeHCO32+ | K10 = 103.4 | (this work) |
| [13] | Ce3+ + F− = CeF2+ | K11 = 103.99 | 13) |
| [14] | F− +H+ = HF | K12 = 102.91 | 13) |
| [15] | 2F− +H+ = HF2− | K13 = 103.48 | 13) |
The solubility product of Ce-bastnaesite is defined as follows:
| (8) |
| (9) |
Fig. 3 shows the measured total equilibrium concentration of Ce3+, F− and CO32− as a function of pH at a constant partial pressure of 10−3.5 atm. CO2 in an open system. Based on these solubility data and the thermodynamic data available in the literature for reactions given in Table 3, the molar concentrations of various ionic species in solution were computed as a function of pH19). The ionic strength of solutions under these conditions was calculated using these results and the corresponding activity coefficients for Ce3+, F− and CO32− ions were computed with the extended Debye-Huckel Law17–18). Knowing the activity coefficients and the free ion concentrations, the solubility product of bastnaesite was estimated as a function of ionic strength as plotted in Fig. 4. The value of pKSO for Ce-bastnaesite is thus 16.1, on extrapolation to zero ionic strength of the solution.

The pH dependence of total cerium, total fluorine and total carbonate concentrations in aqueous solutions in equilibrium with air.
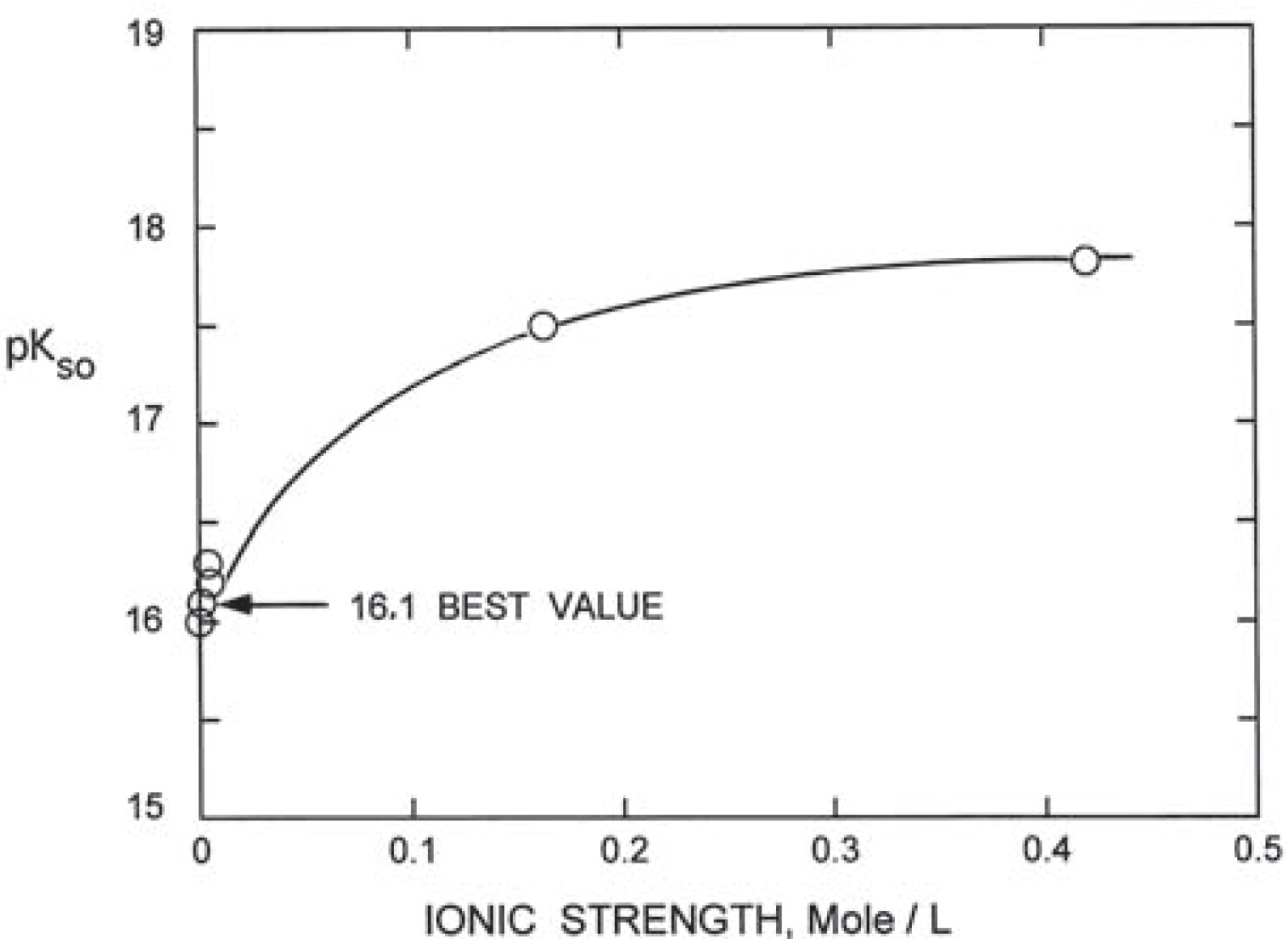
The solubility product of synthetic cerium bastnaesite in aqueous solutions as a function of ionic strength.
Titration of H2CO3 in aqueous solution in the presence of Ce(NO3)3 was carried out in order to determine the stability constant of the complex cation CeHCO32+. For a pure aqueous, air-saturated solution, the pH is defined by the charge balance:
| (10) |
Using the stability constant thus determined with Equation 10 and the thermodynamic data given in Table 3, the relative concentrations of various solution spcies in equilibrium with synthetic cerium fluocarbonate in a system open to atmosphere at 25 °C, were computed. The results ace plotted in Fig. 5. The main charged species in a solution in equilibrium with synthetic cerium bastnaesite in a system open to atmosphere are Ce3+, CeF2+, HCO3−, CO32−, F−, CeOH2+, Ce(OH)2+ and CeHCO3+ in addition to H+ and OH−. As shown in Fig. 3, the total species concentration is minimum in the pH range of 7.2 - 7.6.
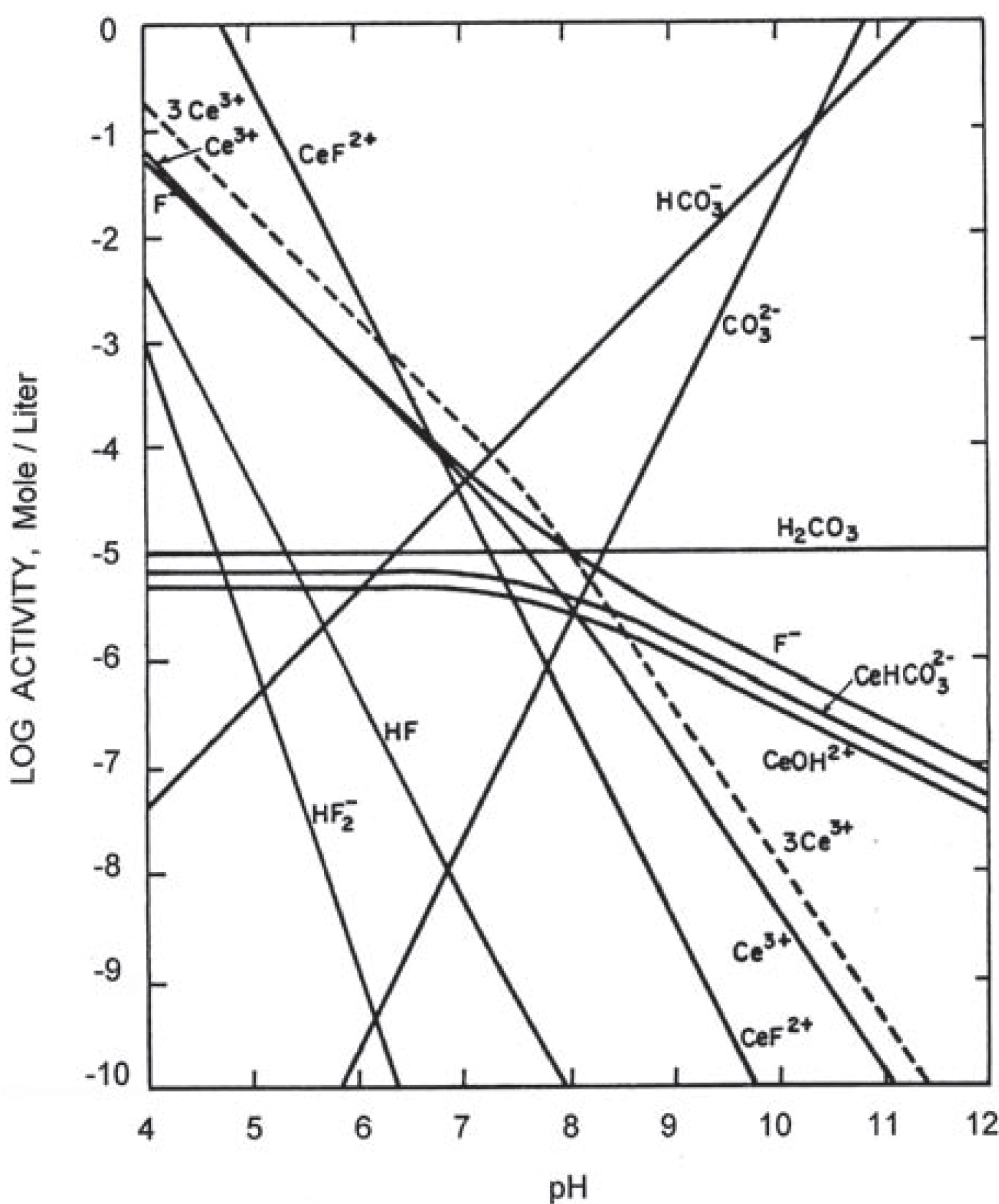
Equilibria in the synthetic cerium bastnaesite/water system open to the atmosphere at 25 °C.
A sample of cerium f1uocarbonate (CeFCO3) was synthesized in the laboratory and characterized through X-ray diffraction and chemical analysis. Thermogravimetric studies on the synthetic bastnaesite exhibited a decomposition peak at 500 °C as compared to 600 °C in the case of natural bastnaesite obtained from Mountain Pass, California. The total weight loss when heated to 900 °C was 20 percent, consistent with the decomposition reaction for the mineral.
The solubility product KSO as well as the stability constant KCeHC were experimentally determined through solubility and titration measurements. Using the thermodynamic data available in the literature as well as the constants, a speciation diagram for cerium fluocarbonate has been presented.
Pradip
After graduating from the Indian Institute of Technology in Kanpur, Pradip entered the University of California at Berkeley to continue graduate studies in mineral processing. He obtained his M.S. degree in 1977 and Ph.D. degree in 1981. After serving as Scientific Officer, Ore Dressing Section, at the Bhabha Atomic Research Centre, he joined the Tata Research Design and Development Centre where he currently is Chief Scientist and Head of the Process Innovation Laboratory.
Charles C.H. Li
After graduating from Central South Institute of Mining and Metallurgy in Changsha, Charles Li entered the University of California at Berkeley to pursue graduate studies in mineral processing, and obtained his M.S. degree in 1982 and his Ph.D. degree in 1986. He then joined CRA (now Rio Tinto) in Australia, where he worked as a research engineer on mineral processing problems until his retirement.
Douglas W. Fuerstenau
After receiving his Sc.D. degree at MIT, Dr. Fuerstenau spent a six-year period teaching at MIT and working in industry, after which time he joined the faculty of the University of California. At Berkeley he established an extensive program of teaching and research in mineral processing, applied surface chemistry and particle technology. With his graduate students and post doctoral researchers, he has published a wide range of seminal papers in these fields. He currently is P. Malozemoff Professor Emeritus of Mineral Engineering in the University of California.